摘要/Abstract
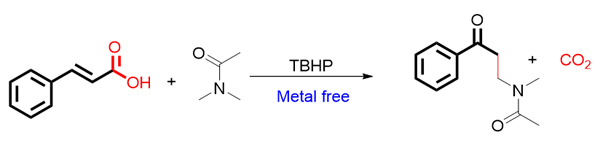
碳-碳键的构建是有机反应中最常见的一类反应, 也是构建有机化合物骨架最常用的手段. 近些年, 通过脱羧反应来构建碳-碳键, 碳-杂原子键得到了广泛而深入的研究. 肉桂酸类化合物的脱羧偶联反应也得到了较多的关注. 这类反应一般包括两个过程, 自由基加成和羧基的脱去, 从而得到新的有机化合物. 这类反应的特点是用氧化剂产生自由基, 在反应过程产生二氧化碳和水为副产物, 相比使用卤代试剂或者有机金属试剂来说, 更为绿色. 作者在之前的研究过程基础上发现, 在无需任何金属催化剂的条件下, 只用过氧叔丁醇(有机溶剂)作为氧化剂, 肉桂酸类衍生物和酰胺类能够发生脱羧氧化偶联反应, 实现C(sp3)―C(sp3)键的生成. 该反应特点是没有用过渡金属盐作为催化剂, 符合绿色化学的发展要求.
关键词: C―H键官能团化, 脱羧, 氧化偶联, 酰胺
Traditional coupling reactions generally employ metal organic compounds, boron reagents or halogenated compounds as reaction starting materials under the condition of noble metal-catalyst, such as Suzuki reaction and Heck reaction. Although these reactions have been widely used in synthesis, due to the need for prefunctionalization of reactants, they do not have high atomic economy and step economy. Especially for some substrates that are not easy to introduce functional groups, the application of such reactions is limited. In recent years, with the development of organic chemistry, direct C―H bond functionalization has become a research hotspot in organic synthesis. This kind of reaction can overcome the deficiency of traditional coupling reaction, making this kind of reaction more attractive. Amides are very important compounds, which are widely found in natural products and drugs. Through literature investigation, it was found that some functionalization reactions of C(sp3)―H bond adjacent to the nitrogen atom of amides had already reported. Due to the facile synthesis, stability and low toxicity of cinnamic acid compounds, they are also used in organic synthesis to obtain desired product. When we studied the decarboxylation reaction of cinnamic acid compounds, we found that the decarboxylative coupling product of cinnamic acid with amides could be obtained by using amide as solvent, tert-butyl hydroperoxide (TBHP) as oxidant and cinnamic acid as starting material without the use of metal catalyst. The reaction mechanism was also proposed. First, homolysis of tert-butyl alcohol peroxide generated tertiary butyl oxygen free radical and hydroxyl free radicals, the oxidation of C(sp3)―H bonds adjacent to the nitrogen atom of N,N-dimethylacetamide offered carbon free radical, which adds to the α-position of the double bond of cinnamic acid to produce intermediate A. A hydroxyl radical, generated from homolysis of TBHP, then combined with A to generate B, and B was further oxidized to C. Finally, C decarboxylated easily to generate the desired product.
Key words: C―H bond functionalization, decarboxylation, oxidative cross-coupling, amide
PDF全文下载地址:
点我下载PDF
