摘要/Abstract
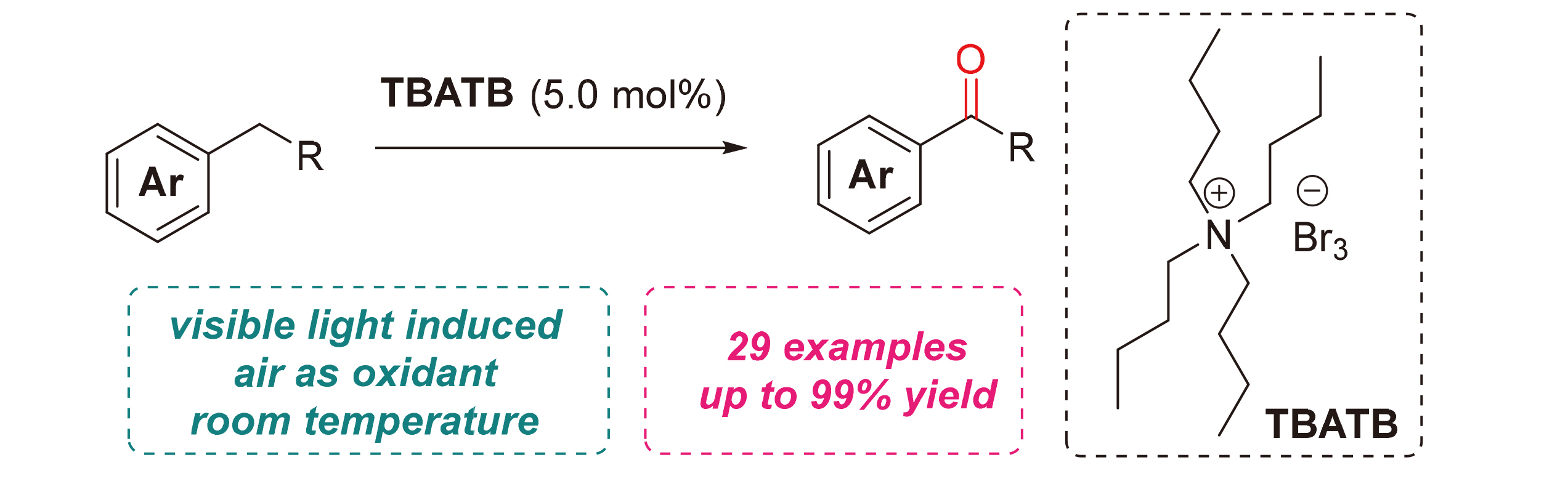
芳烃苄位碳氢键的直接氧化官能团化可以实现羰基化合物的快速合成, 在有机合成中具有重要意义. 光照条件下, 利用相对绿色、易得的氧气作为氧化剂实现这一反应, 在过去十几年中获得了快速的发展. 将一种市售、廉价的化学试剂四丁基三溴化铵用于催化苄位碳氢键的光氧化反应. 在可见光照射下, 以空气作为氧化剂, 使用催化量的四丁基三溴化铵, 即可完成烷基芳烃至芳基酮的有效转化. 该反应条件温和, 不需使用添加剂, 底物适用范围广, 可作为目前苄位光氧化反应体系的有效补充.
关键词: 氧化反应, 可见光催化, 烷基芳烃, 四丁基三溴化铵
The direct oxidative functionalization of the benzylic C—H bonds of alkyl arenes paves a way for the quick synthesis of carbonyl compounds, which is of great significance in organic synthesis. In the past decades, the photooxidative pathways to achieve this reaction have developed rapidly, using relatively green and readily available oxygen as the terminal oxidant under light irradiation. In this paper, a commercially available and inexpensive chemical reagent, tetrabutylammonium tribromide (TBATB), was used to catalyze the photooxidation of the benzylic C—H bonds. Under visible light irradiation and air atmosphere, a catalytic amount of TBATB was utilized to achieve the efficient conversion of alkyl arenes to the corresponding aryl ketones. The reaction is feasible under mild conditions and without any additives, leading to a broad scope of ketone substrates. This method can be an effective supplement to the current benzylic photooxidation reaction systems.
Key words: oxygenation reaction, visible-light photocatalysis, alkyl arenes, tetrabutylammonium tribromide
PDF全文下载地址:
点我下载PDF
