摘要/Abstract
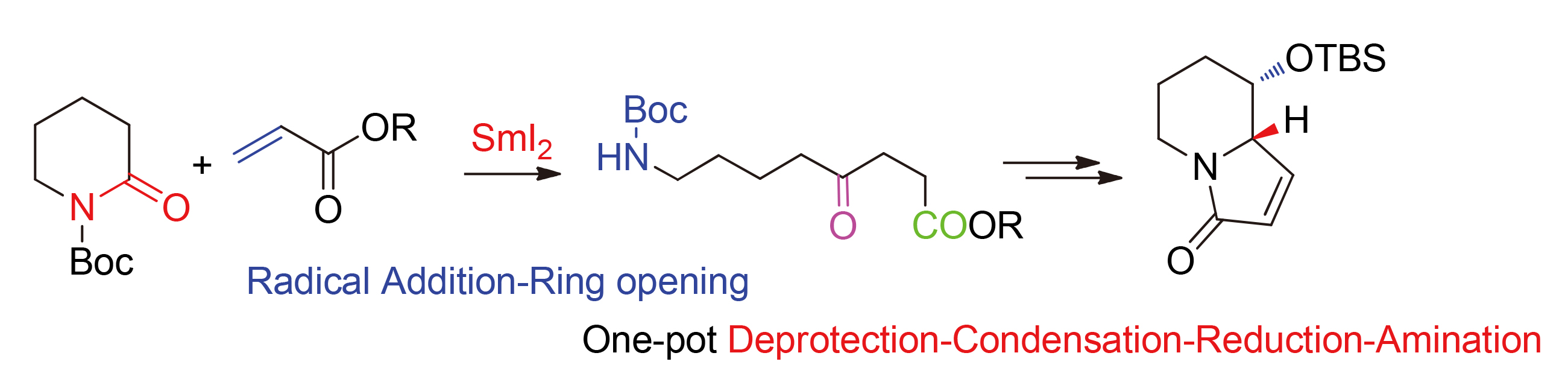
以tBuOH作为质子供体, 基于SmI2促进2-哌啶酮与α,β-不饱和酸酯进行自由基加成-开环反应, 以43%~89%的收率制备了一系列含N-Boc氨基酮化合物3a~3l. 以(S)-8-[(叔丁氧基羰基)氨基]-5-[(叔丁基二甲基甲硅烷基)氧基]-4-氧代辛酸甲酯(3e)为关键中间体, 经一锅去保护-环合-还原-胺解等反应建立了吲哚里西啶骨架(6)的合成方法.
关键词: 二碘化钐, 2-哌啶酮, 自由基加成-开环反应, 吲哚里西啶
A convenient approach to N-Boc amino ketones 3a~3l has been developed, which features a SmI2 prompted one-pot radical addition-ring opening process of 2-piperidinone with α,β-unsaturated esters. Moreover, the indolizidine skeleton (6) has been successfully synthesized from the key methyl (S)-8-((tert-butoxycarbonyl)amino)-5-((tert-butyl- dimethylsilyl)oxy)-4-oxooctanoate (3e), which undergoes one-pot deprotection-condensation-reduction-amination process.
Key words: samarium diiodide, 2-piperidinone, radical addition-ring opening, indolizidine
PDF全文下载地址:
点我下载PDF
