摘要/Abstract
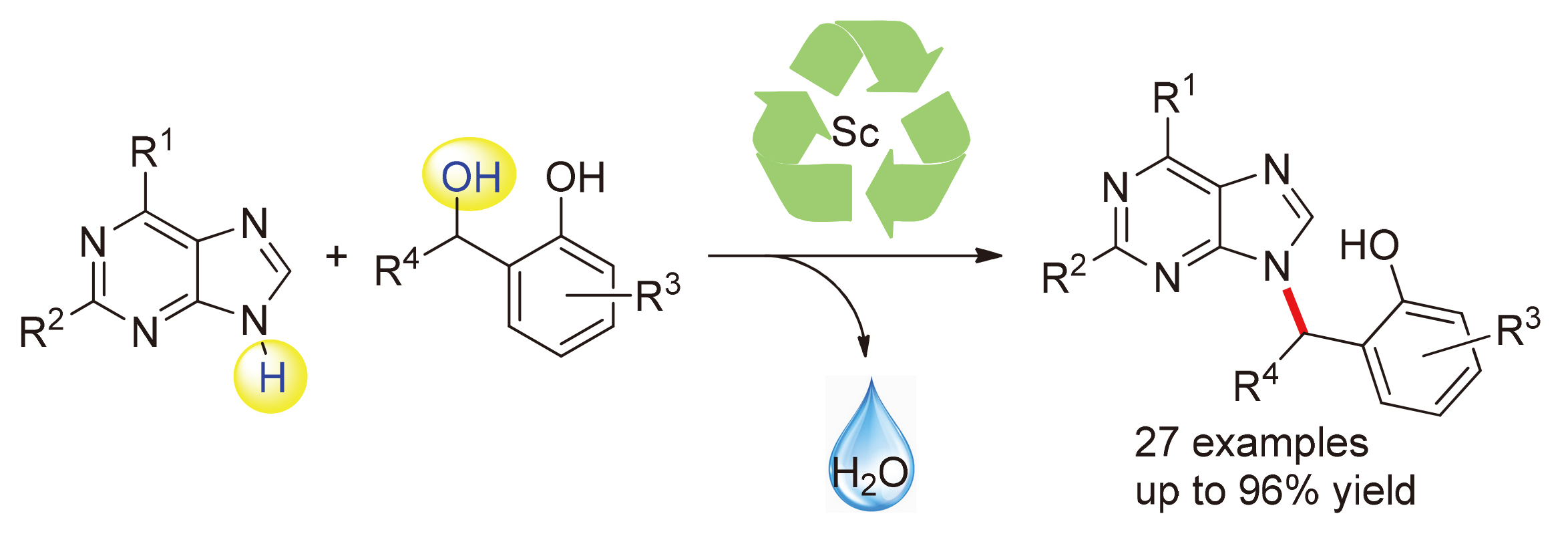
通过三氟甲磺酸钪催化, 在温和条件下实现了邻羟基苄醇与嘌呤的烷基化反应. 该C—N键形成的过程可能是通过邻亚甲基苯醌中间体进行的. 以优秀的产率(最高可达96%)高效地合成了一系列非环核苷类似物, 底物范围广. 反应规模放大后, 产率保持不变.
关键词: 嘌呤非环核苷, 邻亚甲基苯醌, 钪催化, 烷基化反应
Efficient alkylation of purines with ortho-hydroxybenzyl alcohols under mild condition has been achieved by Sc(OTf)3 catalysis. This C—N bond formation process is proposed to proceed through an ortho-quinone methide intermediate. The reaction allows for efficient synthesis of various acyclic nucleoside analogs, with excellent yields of up to 96%, across a broad range of substrates and the yields were maintained as the reactions were scaled up.
Key words: purine acyclic nucleosides, ortho-quinone methide, scandium catalysis, alkylation reaction
PDF全文下载地址:
点我下载PDF
