摘要/Abstract
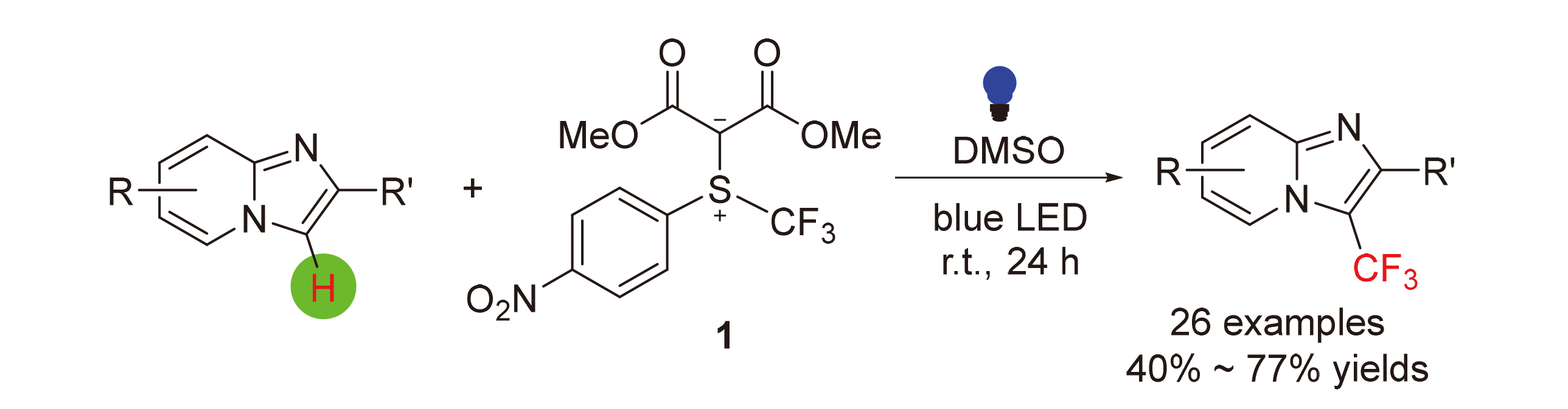
发展了一个无光敏剂参与的可见光促进的咪唑并[1,2-a]吡啶衍生物的三氟甲基化新反应. 该反应利用本课题组此前发展的基于硫叶立德骨架的亲电三氟甲基化试剂作为三氟甲基自由基源, 反应条件温和, 底物普适性好, 同时兼容常见的官能团. 机理研究表明该反应可被自由基捕获剂阻断. 对两个反应底物及它们的1∶1混合物的紫外-可见光吸收光谱进行了研究, 研究表明三氟甲基化试剂与咪唑并[1,2-a]吡啶之间形成了一个Donor-Acceptor加合物. 在此基础上提出了一个合理的机理, 即该加合物在光照条件下被激发, 发生硫-三氟甲基键均裂生成三氟甲基自由基, 该自由基与咪唑并[1,2-a]吡啶发生自由基取代反应. 同时也成功地将该方法应用于治疗胃溃疡药物佐利米定的三氟甲基化衍生物的合成.
关键词: 三氟甲基, 可见光, 无光敏剂, 咪唑并[1,2-a]吡啶
A photosensitizer-free visible-light-promoted method for direct trifluoromethylation of imidazo[1,2-a]pyridine derivatives using an electrophilic trifluoromethylating reagent based on sulfonium ylide skeleton was described. The reaction occurred in broad substrate scope under mild conditions and tolerant various functional groups. Initial mechanistic experiments including reactions in the presence of radical scavengers and UV absorption spectroscopic studies were conducted. Inhibition by the radical scavengers were observed. In addition, an extra-absorption peak between 500~510 nm in UV-Vis absorption spectrum was observed. These observations led us to propose a working mechanism. Finally, application of the current method for the preparation of trifluoromethylated derivative of gastroproctive drug zolimidine was demonstrated.
Key words: trifluoromethyl, visible light, photosensitizer free, imidazo[1,2-a]pyridine
PDF全文下载地址:
点我下载PDF
