摘要/Abstract
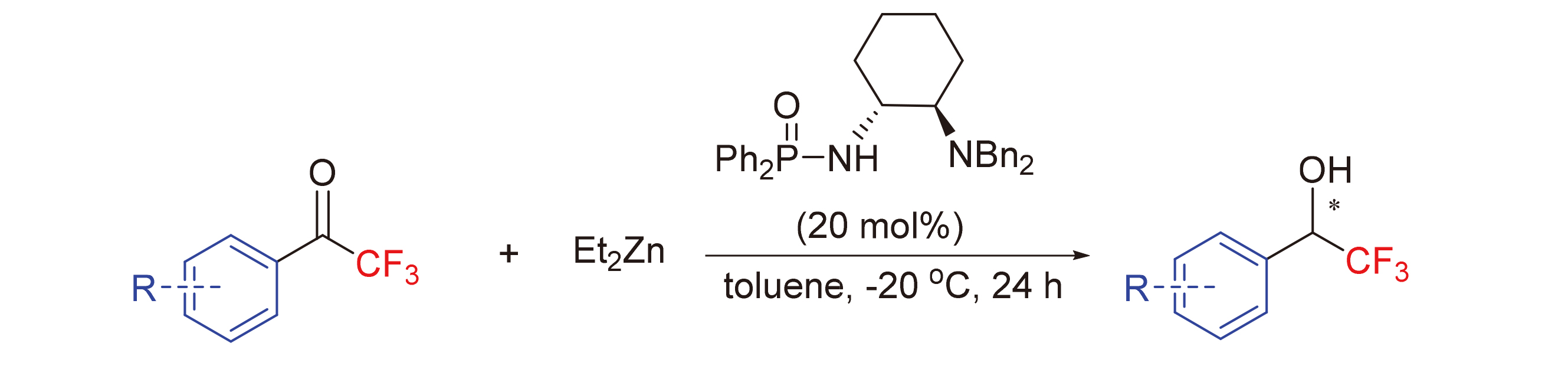
手性三氟甲基类化合物拥有重要生理活性, 为了获得该类化合物, 以磷酰胺为配体, 乙基锌、三氟甲基芳香醛及其衍生物为底物合成出一系列的三氟甲基类化合物, 所使用的原料廉价易得, 催化效率较高. 在最优条件下, 可以高收率、高ee值地合成相应的手性三氟甲基类化合物. 尽管配体使用量较高, 但其可回收利用. 最后, 对可能的反应机理进行了合理推测, 认为反应的高立体选择性主要归因于催化过程中形成的两个六元环过渡态及空间位阻作用.
关键词: 磷酰胺配体, 不对称催化, 手性三氟甲基类化合物
In order to obtain trifluoromethylated organic compounds with important biological activity, using phosphoramide as catalyst, diethylzinc and trifluoromethyl aromatic aldehyde as reactants, chiral trifluoromethylated organic compounds were synthesized through catalytic asymmetricβ-H transfer reduction reaction. The raw materials are cheap and easy to obtain, and the catalytic efficiency is high. The yield and ee value can be guaranteed to be highly and simultaneously under the optimized reaction conditions. Despite the large amount of catalyst, the ligand is very convenient to recycle and reuse in this system. At the same time, the reaction mechanism was speculated, and it was considered that the high stereoselectivity of the reaction was due to the formation of two six membered ring transition states and steric hindrance.
Key words: phosphoramide ligand, asymmetric catalysis, chiral trifluoromethylated compound
PDF全文下载地址:
点我下载PDF
