摘要/Abstract
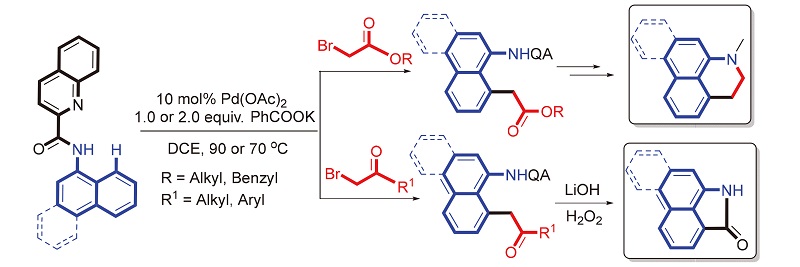
报道了一种钯催化1-萘酰胺的8位碳氢键烷基化反应. 在该反应中,喹啉甲酰胺作为N,N-双齿螯合基团, 各种取代的α-溴乙酸烷基酯以及α-溴代苯乙酮作为烷基化试剂, 高效、高区域选择性地合成了8-烷基-1-萘胺衍生物. 最后, 将含酯基和酮基的烷基化产物分别通过相应的衍生化反应合成具有多种生物活性的阿朴菲和马兜铃内酰胺类生物碱结构单元.
关键词: 钯催化, 烷基化, 1-萘酰胺, 阿朴菲类生物碱, 马兜铃内酰胺类生物碱
A practical methodology for the palladium-catalyzed regioselective alkylation of 8-C—H bonds in 1-naphthyl- amides containing a quinolinamide moiety as bidentate directing group with functionalized alkyl halides is reported. Various functionalized alkyl halides includingα-bromo esters and ketones can be employed as coupling partners, providing exclusively 8-alkyl-1-naphthylamine derivatives. In particular, the alkylated products with these ester and carbonyl groups can readily be further converted into the core structures of aporphine and aristolactam alkaloids respectively.
Key words: palladium-catalyzed, alkylation, 1-naphthylamides, aporphine alkaloids, aristolactam alkaloids
PDF全文下载地址:
点我下载PDF
