摘要/Abstract
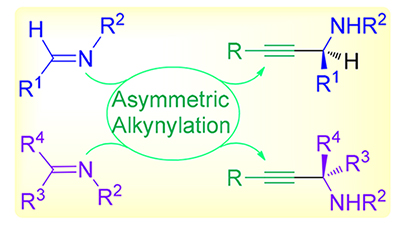
手性炔丙胺是天然产物和药物活性分子不对称全合成中常用的关键中间体, 亚胺及其类似物的不对称炔基化反应可以为该砌块提供高效高对映选择性的合成路径; 此外通过合理的底物和反应设计, 亚胺的不对称炔基化反应还能作为一系列串联反应的起点, 来合成多种结构新颖的含氮杂环化合物. 因此, 亚胺及其类似物的高效高对映选择性炔基化反应得到合成化学家们持续关注. 按照底物类型, 主要分为醛亚胺的不对称炔基化和酮亚胺的不对称炔基化两大部分, 介绍了亚胺及其类似物的不对称炔基化反应在过去十年中的研究进展. 对这些反应的机理、优势与不足之处以及该反应在合成中的应用进行简要讨论, 从而为拓展该反应在合成中的应用提供一些有益参考和借鉴.
关键词: 手性炔丙胺, 不对称炔基化, 醛亚胺, 酮亚胺, 含氮杂环化合物
Chiral propargyl amines are key intermediates in the asymmetric total synthesis of natural products and bioactive compounds. The asymmetric alkynylation of imine and its analogues can provide a highly efficient and enantioselective synthetic route for these chiral building blocks. In addition, through rational substrate and reaction design, the asymmetric alkynylation of imines can be used as the starting point of a series of cascade reactions to synthesize a variety of novel nitrogen-heterocyclic compounds. Therefore, highly efficient and enantioselective alkynylation of imines and their analogues has attracted the continuous attention of synthetic chemists. According to the types of substrate, the research progress in asymmetric alkynylation of imines and their analogues over the past decade is introduced, which is divided into two parts: asymmetric alkynylation of aldimines and asymmetric alkynylation of ketoimines. The reaction mechanism and their advantages and disadvantages, together their synthetic applications will be briefly introduced, hoping to provide some useful inspiration for expanding the application of this reaction in synthesis.
Key words: chiral propargyl amine, asymmetric alkynylation, aldimine, ketoimine, nitrogen-heterocyclic compound
PDF全文下载地址:
点我下载PDF
