摘要/Abstract
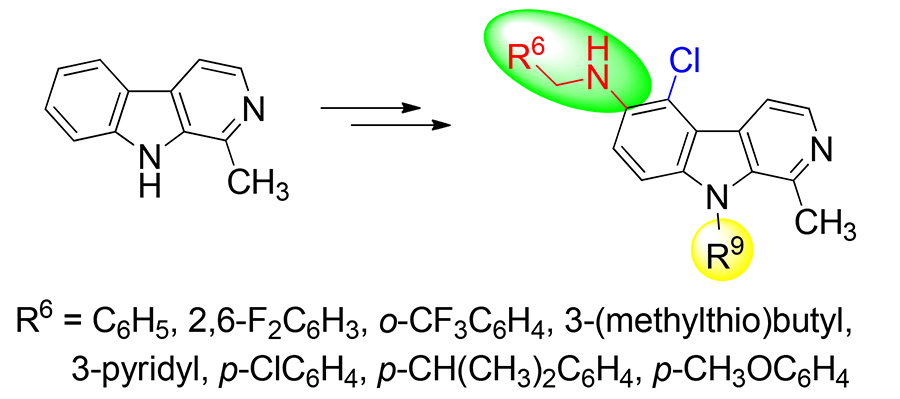
以1-甲基- β-咔啉为原料, 经过硝化、 N 9-烷基化反应和还原胺化反应等步骤, 合成了一系列新型的5-氯- β-咔啉衍生物, 目标化合物的结构经 1H NMR, 13C NMR以及HRMS确证, 并利用单晶X射线衍射分析了 N-(吡啶-3-基)甲基-5-氯-1,9-二甲基- β-咔啉-6-胺(5e)的精确结构. 采用噻唑蓝(MTT)法测试了目标化合物对肺癌细胞A549、胃癌细胞BGC-823、结肠癌细胞CT-26、肝癌细胞Bel-7402和乳腺癌细胞MCF-7的细胞增殖抑制作用. 实验结果表明, 部分化合物具有较好的抗肿瘤活性, 其中 N-(2,6-二氟苄基)-1-甲基-5-氯-9-(2,3,4,5,6-五氟苄基)- β-咔啉-6-胺(5j)和 N-(吡啶-3-基甲基)-1-甲基-5-氯-9-(2,3,4,5,6-五氟苄基)- β-咔啉-6-胺(5m)对所测试的4种肿瘤细胞株均有较高的抑制活性, IC50值均小于10 µmol•L –1.
关键词: 5-氯-β-咔啉, 合成, 抗肿瘤, 构效关系
Sixteen novel 5-chloro- β-carboline derivatives were synthesized from harmane in four steps: N 9-alkylation, nitration, reduction, and Borch reduction. The structures of target compounds were confirmed by 1H NMR, 13C NMR, and HRMS. A single crystal of 5-chloro-1,9-dimethyl- N-(pyridin-3-ylmethyl)- β-carboline (5e) was cultured, and its single crystal structure was determined by X-ray diffraction study. The in vitro antiproliferative activities were evaluated in a panel of cancer cell lines (A549, BGC-823, CT-26, Bel-7402, and MCF-7) via methyl thiazolyl tetrazolium (MTT) assay. The results indicated that some compounds had good activities, and especially N-(2,6-difluorobenzyl)-1-methyl-5-chloro-9-(2,3,4,5,6-pentafluorobenzyl)- β- carboline-6-amine (5j) and N-(pyridin-3-ylmethyl)-1-methyl-5-chloro-9-(2,3,4,5,6-pentafluorobenzyl)- β-carboline-6-amine (5m) showed considerable antitumor activity with IC50 values lower than 10 μmol•L –1 against four cancer cell lines.
Key words: 5-chloro-β-carboline, synthesis, antitumor, structure-activity relationship
PDF全文下载地址:
点我下载PDF
