摘要/Abstract
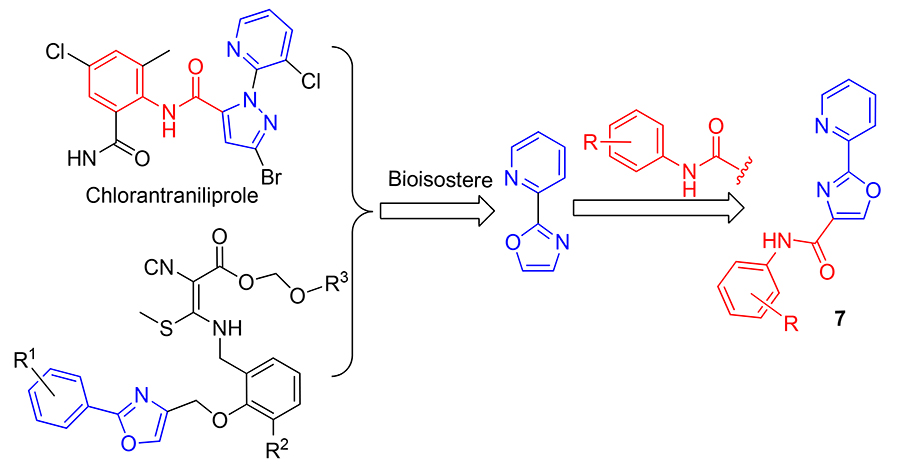
以吡啶-2-甲醛为起始原料, 经环合、氧化、取代、水解、氯化和胺解反应, 采用中间体衍生化法合成了13个新型的吡啶联噁唑酰胺类化合物, 其结构经1H NMR和高分辨质谱表征. 初步的杀菌活性测试结果显示, 在100 mg/L浓度下有7个化合物对黄瓜灰霉病菌(Botrytis cinerea)的抑制率为100%, 其中N-4-氟苯基-2-(吡啶-2-基)-噁唑-4-甲酰胺(7b)对水稻纹枯病菌(Rhizoctonia solani)的抑制率也为100%, 进一步的杀菌活性测试结果表明, 部分目标化合物杀菌活性优于嘧菌酯, 值得进一步研究.
关键词: 噁唑, 酰胺, 吡啶, 杀菌活性
Thirteen novel pyridyl oxazoamide compounds were synthesized using pyridine-2-formaldehyde as the starting material through cyclization, oxidation, substitution, hydrolysis, chlorination and ammonolysis reactions via the disclosed intermediate derivatization method (IDM). The structures of title compounds were characterized by 1H NMR and HRMS. Preliminary bioassay results indicated that at 100 mg/L, 7 compounds exhibited 100% fungicidal activities against Botrytis cinerea, and N-(4-fluorophenyl)-2-(pyridin-2-yl)oxazole-4-carboxamide (7b) exhibited 100% fungicidal activities against Rhizoctonia solani. Further fungicidal activity test results suggested that some of the target compounds had better fungicidal activity than azoxystrobin, which was worthy of further study.
Key words: oxazole, amide, pyridine, fungicide activity
PDF全文下载地址:
点我下载PDF
