摘要/Abstract
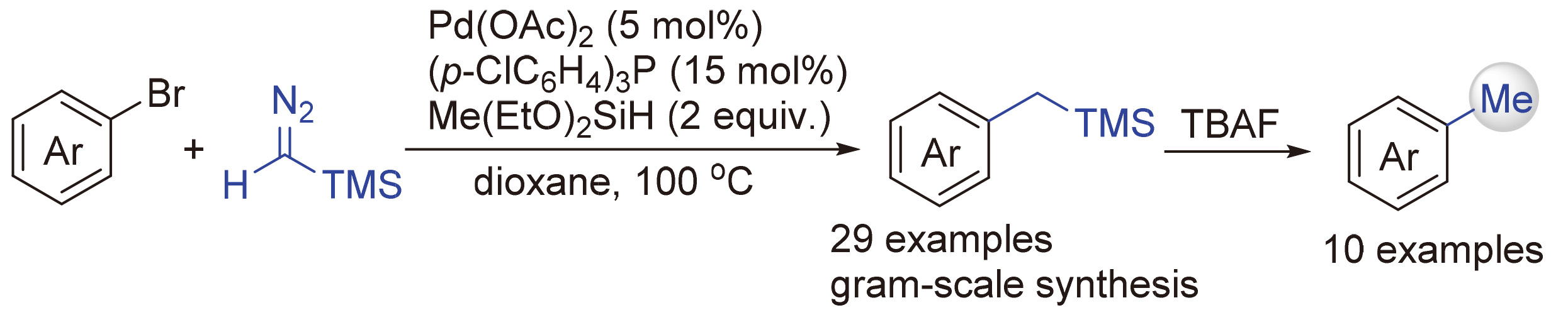
芳香化合物的甲基化反应是一类重要的转化.其中,由芳香卤化物出发的转化是在芳香体系中引入甲基基团的有效策略.已有的方法多需要使用预先制备的甲基金属试剂或高毒性的甲基亲电试剂作为甲基化试剂.本研究发展了一种利用三甲基硅基重氮化合物和芳香溴化物在钯催化下的还原偶联反应生成苄基硅化合物,再经过脱硅质子化过程实现的甲基化方法.此方法具有良好的官能团兼容性,是一种具有潜在应用价值的对芳香卤化物进行甲基化的新方法.同时,该方法也可应用于在有机分子中引入硅甲基基团.
关键词: 甲基化, 三甲基硅基重氮甲烷, 金属卡宾, 转移插入, 还原偶联
The introduction of methyl group into aromatic compounds is a valuable transformation. A large number of known methods use organohalides as the starting materials. However, those methods require pre-synthesized methyl metal reagents or toxic methyl electrophiles. Herein, a palladium-catalyzed reductive coupling reaction between aryl bromides and trimethylsilyl-diazomethane is developed, and the following desilicification process can afford the methylated products. This transformation has broad functional group tolerance and allows methylation of (hetero)aryl halides in moderate to good yields. Thus, it has the potential to be an attractive approach for methylation of organic. In addition, this reductive coupling can also serve as an efficient way for the introduction of silylmethyl group.
Key words: methylation, trimethylsilyldiazomethane, metal carbene, migratory insertion, reductive coupling
PDF全文下载地址:
点我下载PDF
