摘要/Abstract
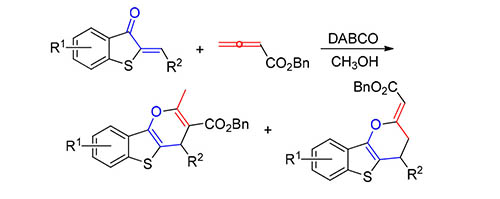
发展了1,4-二氮杂二环[2.2.2]辛烷(DABCO)催化硫代橙酮类似物与联烯酸酯之间的串联环化反应,以甲醇为溶剂,以较好的选择性和收率获得两类苯并噻吩并吡喃衍生物.同时,该反应条件温和、底物适应性广,吸电子基或供电子基取代的硫代橙酮类似物都能以较好的选择性和收率生成目标产物.该反应为苯并噻吩并吡喃衍生物的合成提供了一种便利的方法.
关键词: 串联反应, 联烯酸酯, 苯并噻吩衍生物
A 1,4-diazabicyclo[2.2.2]octane (DABCO)-catalyzed[4+2] annulation reaction between 2-alkylidenebenzothio phene-3(2H)-ones and allenoate has been developed. The substrate scope includes both electron-withdrawing and electron-donating groups on the benzothiophene moiety. This method can be carried out under mild conditions and gives a wide range of highly functionalized benzothiophene-fused γ-pyran derivatives in good yields with moderate selectivity.
Key words: domino reaction, allenoate, benzothiophene derivatives
PDF全文下载地址:
点我下载PDF
