摘要/Abstract
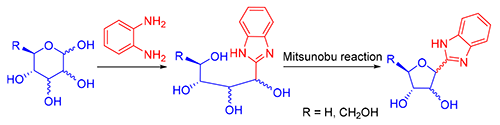
以不保护的五、六元单糖与邻苯二胺反应,得到中间体多羟基链苯并咪唑.进一步,利用Mitsunobu反应,分子内脱水合成了构型翻转和构型保持的呋喃糖基苯并咪唑C-核苷.Mitsunobu反应良好的区域选择性,为呋喃糖基苯并咪唑C-核苷的合成提供一个有效方法.
关键词: C-糖苷, 呋喃糖基苯并咪唑, Mitsunobu反应, 区域选择性
Tetri/pentitolyl benzimidazoles were prepared by using the unprotected monosaccharides and o-phenylenediamine as the starting materials. Intramolecular dehydration of the oligotoltyl benzimidazoles afforded two furanosyl benzimidazole C-nucleosides (α/β isomers) through Mitsunoble reaction. One isomer was the configuration-retension product, the other was the configuration-inversion one. The regioselectivity of Mitsunobu reaction is good, which provides an effective protocol for the synthesis of furanosyl benzimidazole C-nucleosides.
Key words: C-nucleoside, furanosyl benzimidazole, Mitsunobu reaction, regioselectivity
PDF全文下载地址:
点我下载PDF
