摘要/Abstract
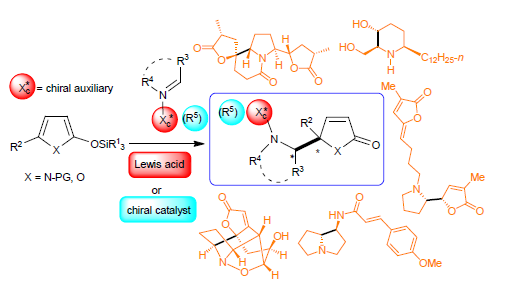
基于杂环(α,β/β,γ-不饱和γ-内酯,α,β-不饱和γ-内酰胺)的插烯Mannich反应在构建新C-C键的同时形成含邻氨基醇片段的α,β-不饱和γ-内酯和含邻二胺片段的α,β-不饱和γ-内酰胺结构单元,是构建含氧、含氮杂环及合成生物碱的重要合成砌块,具有广泛的应用价值.对2011年以来基于杂环的不对称插烯Mannich反应研究进展进行综述,涵盖手性辅助基诱导的插烯Mannich反应、金属-手性配体络合物和有机小分子催化的不对称插烯Mannich反应及其在复杂生物碱合成中的应用.文中也对相关方法的局限进行了分析.
关键词: 插烯Mannich反应, 呋喃硅醚, 吡咯硅醚, 亚胺, 手性辅助基, 不对称催化, 生物碱, 不对称全合成
Heterocycles (α,β/β,γ-unsaturated-γ-lactones, α,β-unsaturated-γ-lactams)-based vinylogous Mannich reactions (VMR) constitute a class of effective C-C bond formation approach to install vicinal aminol-containing α,β-unsaturated-lactones and vicinal diamine-containing α,β-unsaturated-γ-lactams. Possessing multiple functionalities, the latters are versatile building blocks for the synthesis of O-heterocycles, N-heterocycles and the synthesis of alkaloids. The progresses of the asymmetric vinylogous Mannich reactions of silyloxy pyrroles and silyloxy furans from 2011 to mid-2018 are summarized. The methods are organized according to chiral auxiliary-induced asymmetric VMRs, asymmetric VMRs catalyzed by metal-chiral ligand complex or organocatalyst, and the applications of the aymmetric VMRs to the syntheses of complex alkaloids. Some limitations of the developed heterocycles-based VMRs are also briefly discussed.
Key words: vinylogous Mannich reaction, silyloxy pyrrole, silyloxy furan, imine, chiral auxiliary, asymmetric catalysis, alkaloid, asymmetric total synthesis
PDF全文下载地址:
点我下载PDF
