摘要/Abstract
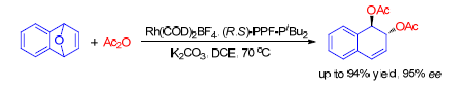
过渡金属催化的氧杂苯并降冰片烯的不对称开环反应是合成含有四氢化萘骨架结构的有效方法之一,许多传统的亲核试剂被用于该反应中,并取得了一系列优异的结果.酸酐作为常用的酰基化试剂,其对氧杂苯并降冰片烯的不对称开环反应可以合成许多重要的手性化合物,而该反应目前尚无报道.通过研究发现,离子型Rh(COD)2BF4与手性二茂铁类配体(R,S)-PPF-PtBu2的配合物可以高效地催化酸酐对氧杂苯并降冰片烯的不对称开环反应.在经过一系列的条件优化后,该铑催化体系表现出优秀的催化活性,能够催化含有多种取代基的氧杂苯并降冰片烯与乙酸酐和丙酸酐的不对称开环反应,并取得了最高94%的收率和对映体过量值为95%ee的结果.
关键词: 铑, 催化, 氧杂苯并降冰片烯, 酸酐, 不对称开环反应
Transition metal catalyzed asymmetric ring-opening reaction (ARO) of oxabenzonorbornadienes is a useful tool in the formation of carbon-carbon bond or carbon-heteroatom bond, which have attracted extensive study and received great achievements over the past decades. A series of efficient catalysts have been established and the high-level control of enantioselectivity for these reactions have been realized. A wide range of nucleophiles including carbon and heteroatom nucleophiles have been used in this reaction. Anhydride is a common acylation reagent and there has been no report about the ARO reaction of oxabenzonorbornadienes with anhydride, which can generate some useful chiral compounds. In this work, Rh-catalyzed ARO of oxabenzonorbornadienes with acetic anhydride and propionic anhydride have been developed. The developed protocol could result in ARO product in high yield (up to 94%) and excellent enantioselectivity (up to 95% ee).
Key words: rhodium, catalysis, oxabenzonorbornadiene, anhydride, asymmetric ring-opening reaction
PDF全文下载地址:
点我下载PDF
