

浙江工业大学 生物工程学院,浙江 杭州 310014
收稿日期:2020-12-01;接收日期:2021-03-02
基金项目:国家重点研发计划(No. 2019YFA0905300), 浙江省博士后择优资助项目(No. zj2018076)资助
摘要:甾醇是一种环戊烷多氢菲衍生物,是真核细胞的重要生物膜组分,具有多种生物学活性,是食品、制药等行业的重要原料。酿酒酵母具有天然的麦角甾醇合成途径,是实现甾醇类衍生物从头合成的理想底盘细胞。近年来,细胞器的理性改造技术为实现目标产物的定向存储、转运并最终为产物的合成强化提供了新的理性改造策略。文中重点阐述了酿酒酵母细胞在不同生理胁迫下甾醇合成及转运的反馈应激表型及相关的分子作用机理,指出构建酵母细胞中甾醇的定向存储、转运及胞外分泌途径的人工强化途径是突破酵母细胞甾醇合成极限的新策略。
关键词:酿酒酵母甾醇存储与转运脂滴理性改造
Reinforcement of sterols production through directed storage and transportation in yeast: a review
Xia Ke, Yi Shen, Lisha Cao, Bo Zhang, Zhiqiang Liu


College of Biotechnology and Bioengineering, Zhejiang University of Technology, Hangzhou 310014, Zhejiang, China
Received: December 1, 2020; Accepted: March 2, 2021
Supported by: National Key Research and Development Program of China (No. 2019YFA0905300), Preferred Fund of Postdoctoral Scientific Research Project of Zhejiang, China(No. zj2018076)
Corresponding author: Zhiqiang Liu. Tel: +86-571-88320379; E-mail: microliu@zjut.edu.cn.
Abstract: Sterols, a class of cyclopentane poly-hydrophenanthrene derivatives, are the predominant membrane constituent of eukaryotes. These substances have a variety of biological activities and have been widely used in food and pharmaceutical industries. The presence of endogenous ergosterol biosynthetic pathway in Saccharomyces cerevisiae cells make it an ideal chassis for the de novo synthesis of sterol and its derivatives. Most recently, the rational modification of organelles provides a novel strategy for the directed transportation and storage of target products and the ultimate enhanced product synthesis. This review summarizes the phenotypic responses of S. cerevisiae cells upon different physiological stimulations and the underlying molecular mechanisms. Reinforcement of sterol production through directed storage, transportation, and excretion of sterols offers a novel strategy for breaking the limitation of de novo biosynthesis of sterols in yeast.
Keywords: Saccharomyces cerevisiaesterolstorage and transportationlipid dropletsrational modification
甾醇(Sterol) 又称固醇,是一类由3个己烷环及1个环戊烷稠合而成的环戊烷多氢菲衍生物,母核结构中含有3′-羟基,是真核细胞膜的重要脂类成分,在维持细胞的完整性、膜的流动性、以及细胞物质运输等过程中起重要作用。目前已鉴定甾醇大致可分为动物细胞来源的胆甾醇(Cholesterol),植物细胞来源的豆甾醇(Stigmasterol)、谷甾醇(Sitosterol)、菜油甾醇(Campesterol)、菜籽甾醇(Brassicasterol) 以及真菌来源的麦角甾醇(Ergosterol) 等,典型结构如图 1所示[1]。
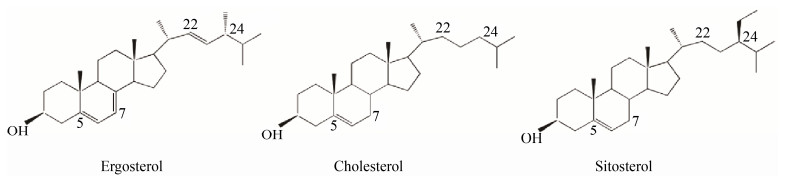 |
| 图 1 典型甾醇的化学结构[1] Fig. 1 Chemical structures of typical sterols[1]. |
| 图选项 |
甾醇类物质具有多种生物学活性,在食品和制药行业中有着广泛的应用。如植物甾醇具有良好的抗氧化性,可作为食品添加剂,具有促进人体胆甾醇代谢、伤口愈合、抗衰老等多种功效[2-3]。麦角甾醇可增强人体抗疾病能力,是重要的脂溶性维生素D2的来源[4]。胆甾醇是维生素D3、胆汁酸和甾体激素(包括皮质激素和性激素) 等多种活性化合物的合成前体[5-6]。
甾醇类药物如维生素D3、胆甾醇类衍生物、可的松等结构复杂,来源受限。传统合成方法以薯蓣属黄姜等植物为原料基础提取甾体母核,通过多步化学反应及微生物转化过程获得甾醇药物中间体,该合成路线资源依赖度高、原料提取和合成效率较低,三废排放量大[7-8]。近年来,建立在代谢工程、基因组学、系统生物学等学科基础上的合成生物学领域的相关技术快速推进了天然产物类药物的异源从头合成[9]。酿酒酵母Saccharomyces cerevisiae具有内源性的甲羟戊酸途径和麦角甾醇合成途径,能够稳定提供前体异戊烯焦磷酸(Isopentenyl pyrophosphate,IPP)、二磷酸二甲基烯丙酯(Dimethylallyl diphosphate,DMAPP)和2, 3-环氧角鲨烯(2, 3-oxidosqualene),其完整内膜系统和翻译后修饰有利于环化酶和P450酶的活性表达,借助其完善的遗传操作平台,已成为萜类化合物等天然产物合成的重要底盘[10]。目前紫杉醇前体紫杉二烯[11]、青蒿酸[12],法呢烯[13]以及甾体类药物睾丸激素[14]等萜类化合物及其衍生物已实现在酵母细胞中的从头合成。此外,通过敲除erg5/erg6,可重构麦角甾醇的从头合成途径,实现7-脱氢胆甾醇[15]、维生素D3关键中间体胆甾-5, 7, 24-三烯醇[16]等活性甾醇类化合物的有效累积(图 2)。随着高密度发酵控制技术的成熟与强化,酿酒酵母对麦角甾醇合成能力显著提高,达到1.5 g/L以上[17],为甾醇类药物及其中间体的从头合成及高效生产奠定了基础。
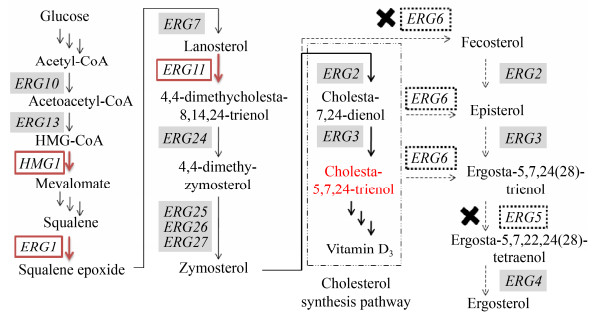 |
| 图 2 酿酒酵母麦角甾醇代谢通路改造合成胆甾醇类前体激素 Fig. 2 Metabolic engineering of the ergosterol pathway in S. cerevisiae for biosynthesis of cholesterol precursors. |
| 图选项 |
麦角甾醇合成途径涉及30步反应,包括甲羟戊酸、焦磷酸法尼酯和麦角甾醇3个生物合成模块。其中麦角甾醇生物合成模块涉及多个细胞色素P450酶催化的脱氢、去甲基及羟化反应[7, 13],是甾醇合成的关键模块[9]。该类酶多定位于内质网膜,部分依赖特定的折叠蛋白辅助形成功能性的多酶复合物才能发挥催化功能,如甾醇C-4脱甲基多酶体系(ERG25/26/27)[18]。内质网膜等细胞器组分为功能酶及相关电子传递系统提供特定生化反应的场所,在稳定蛋白、提升催化效率等方面具有重要作用[19]。此外,酵母细胞中游离甾醇的合成、转运、酯化、水解等过程都受到内膜系统的动态精确调控,并成为影响单位细胞甾醇产率的关键因素[20]。由于胞内甾醇合成及存储空间有限,单一强化合成途径的关键限速酶活力仍无法突破细胞甾醇合成的极限,累积的甾醇中间体不仅直接影响细胞活性,也会产生产物抑制效应[21],从而显著限制单位细胞的最终产率。
近年来,细胞器理性改造技术成为继强化目标产物代谢通量之外的另一种理性强化策略,尤其对缓解疏水性酯类化合物的胞内蓄积并提升单位细胞合成效率具有重要意义[22-23]。本文梳理了影响酵母细胞中甾醇存储与转运的关键作用分子,并结合酯类化合物定向合成的细胞理性改造相关研究进展,探讨了构建酵母细胞中甾醇定向存储、转运及胞外分泌途径的细胞理性改造策略,为从根本上突破酵母细胞甾醇合成极限提供新的研究思路。
1 甾醇的存储路径甾醇作为真核生物细胞膜的重要成分,参与包括囊泡形成、蛋白质分选、内吞作用、同型膜融合、跨膜蛋白(酶) 的活性、蛋白质和脂质之间的侧向聚集[20]等多种生物功能。甾醇在细胞中的浓度及分布受到严格控制。甾醇通常以游离甾醇(Free sterol,FS) 与甾醇酯(Sterol ester,SE)两种形式存在,甾醇酯为甾醇的储存形式,甾醇的酯化与甾醇酯的水解可以缓冲游离甾醇的过量与缺乏,使其达到稳态[1]。此外,麦角甾醇前体如羊毛甾醇等也可以酯化的形式存储,并可根据细胞生理代谢需求,作为合成前体迅速转化为麦角甾醇[24]。酵母中存在两种乙酰辅酶A甾醇酰基转移酶(Acyl-CoA sterol acyl transferase,ACAT),分别为Are1p与Are2p,作为膜蛋白定位于内质网膜上,以乙酰辅酶A为共底物,催化甾醇的3′-乙酰化反应[25-26]。Are1p与Are2p的序列同源性为49%,且与人源ACATs具有24%同源性,后者在维持不同组织中胆甾醇的浓度平衡发挥了重要的调节作用[27]。经研究发现,Are1p主要在厌氧状态下介导酵母中的酯化,且底物倾向于选择麦角甾醇的前体,尤其是羊毛甾醇,Are2p更倾向于选择终产物麦角甾醇为底物[27]。
甾醇酯储存于细胞器脂滴(Lipid droplets,LDs) 中。脂滴以中性脂质为核心,由单层磷脂包裹,允许细胞在特定的隔室中储存非极性分子,使得非极性分子与细胞的水环境相隔离,是酯类物质的存储场所,在酯类和能量代谢中起到了核心作用。脂滴的形成来自于内质网,其结构在进化中高度保守,主要存储甘油三酯、甾醇等酯类物质;在正常培养条件下,酿酒酵母脂滴中甾醇的质量占比约为44%[28],但在有限氮源或外源添加亚油酸等长链脂肪酸为碳源的培养条件下,两者的比例、脂滴的数量及体积会受到显著影响,细胞通过动态响应细胞能量代谢需求,调控其储存的脂类分子——甘油三酯、胆甾醇酯和少量维生素[29]。图 3描述了酿酒酵母中脂滴的形成过程,涉及多种具有跨膜序列及门腔结构域的关键作用蛋白,对积累中性脂质发挥关键作用[30]。具体如下:(1) 磷脂二酰甘油酰基转移酶(Phospholipid:diacylglycerol acyltransferase,PDAT),酵母的同源蛋白为Lro1p,在有丝分裂时期主要负责三酰甘油(TAG) 的合成;(2) 二酰甘油酰基转移酶(Diacylglycerol acyltransferase,Dga1p),催化二酰甘油(DAG) 加入第3个酰基分子,定位于脂滴(平稳期) 和内质网(生长期),但Dga1p在内质网上的活性似乎更强;(3) Seipin蛋白,具有维持脂滴形态,调节脂肪细胞和神经元分化等功能,在酵母中的同源蛋白为SEI1p,可介导内质网脂滴间的接触,促进蛋白和脂质体从内质网膜转移至脂滴中,并具有调控脂滴大小的功能;(4) 脂肪储存诱导蛋白(Fat storage inducing proteins,FIT),诱导内质网上的磷脂单层凸起以便于最终形成脂滴;(5) Are1p与Are2p介导甾醇的酰化形成甾醇酯与TAG共同组成脂滴的脂质内核。
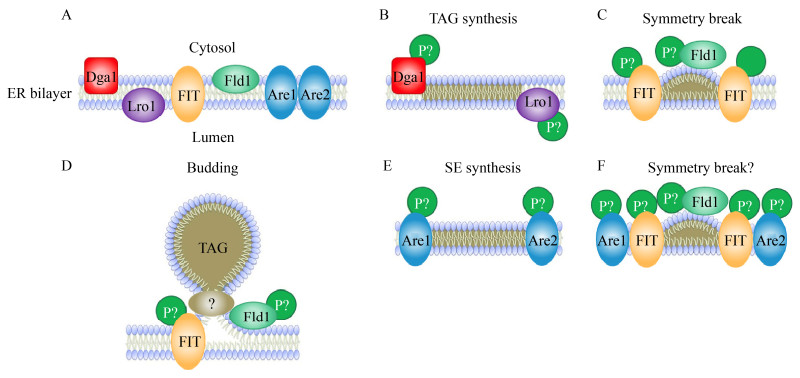 |
| 图 3 酿酒酵母细胞从内质网中形成脂滴的过程[30] Fig. 3 Lipid droplets formation from endoplasmic reticulum of S. cerevisiae[30]. A schematic diagram of endoplasmic reticulum (ER) structure related to lipid droplet formation. (A) Key factors involved in lipid droplet biogenesis located in ER bilayer. (B) Both Dga1p and Lro1p can convert DAG to TAG, but Dga1p uses free acyl-CoA whereas Lro1p cleaves acyl-CoA from phospholipids. (C) A lens of neutral lipids forms on the cytosolic side of the ER bilayer. FITps may sequester neutral lipids in puncta so that the lens can form. SEI1p limits the formation of normal size droplets in yeast. (D) Once the lens grows in size, the neutral lipids assume a spherical structure surrounded by the outer leaflet of the ER bilayer. (E) The coupling mechanism between SE and TAG synthesis is unclear. (F) It is unclear if the same factors that aid in TAG droplet formation also promote SE droplet formation. The phosphorylation states of the above factors (indicated by "P") are largely unknown[30]. |
| 图选项 |
2 酵母甾醇的转运机制2.1 转运路径在正常生理状态下,酿酒酵母细胞中游离甾醇(Free sterol) 的合成、转运及酯化过程及其在不同细胞膜结构上的定位及浓度都受到严格调控。作为亲脂性化合物,游离麦角甾醇浓度在不同细胞器中的维持需依赖细胞器之间的接触及脂质交换过程,或通过非囊泡途径直接与甾醇结合蛋白结合增强其可溶性来实现。典型的细胞器接触为细胞核与囊泡的接触以及内质网与线粒体的接触[31-32]。游离甾醇在其合成场所内质网膜上的浓度最低,如在CHO-K1细胞中,内质网膜上胆固醇含量仅占脂质总量的5 mol%[33],其浓度随着甾醇在细胞内膜转运路径逐渐提高,并在质膜中达到最高,游离甾醇在质膜上占脂质总量的30 mol%[34-38],占甾醇总质量的90%以上。该运输过程涉及囊泡运输与非囊泡转运过程。麦角甾醇以每秒约105分子速率进出质膜上的甾醇池,当细胞处于分裂期时,速度提高10倍[39-40]。非囊泡依赖的甾醇转运路径依赖氧化甾醇结合蛋白相关蛋白(Oxysterol-binding protein-related proteins,ORPs) 为关键作用分子,介导不同细胞器之间脂质交换过程。脂质交换蛋白连接不同细胞器的膜组分,从供体膜上获取甾醇后提供给受体膜。酵母中含有Osh1p-Osh7p七种该家族蛋白,蛋白序列中含有约400个残基的脂质结合口袋[41-44]。以Osh4p为例,Osh4p具有β-桶状的疏水通道结构,可容纳一个3′-羟基朝向通道封闭侧的甾醇分子。通道的另一端由一个铰接盖封闭,当甾醇结合时,铰接盖关闭,从而保护疏水内部不受水性环境的影响[45]。该结构表明Oshp家族分子可作为连接细胞器并传输甾醇的转运蛋白。Alfaro等发现Osh4p可能作为酵母胞内极化囊泡运输甾醇依赖调节因子,在质膜上膜对接的最后步骤影响囊外复合物的组装[46]。此外,在酵母细胞中,锚定在细胞膜接触位点的脂交换家族蛋白(Lipid transfer proteins anchored at the membrane contact sites,LAM) Lam1p-Lam6p具有酯交换结构域(StARTkin domain),其疏水性口袋能够特异性识别多种甾醇化合物,在介导甾醇非囊泡依赖的物质转运过程中也起到重要的功能[47]。近期,Sokolov等综述了麦角甾醇在酿酒酵母细胞中的合成及转运路径的转换过程,突出了Oshp与Lamp在促进甾醇非囊泡转运途径的重要功能[48]。
2.2 外源摄取麦角甾醇的合成途径涉及多步氧化还原反应,分子氧作为最终电子受体。有氧条件下,酵母存在“甾醇外排”现象(Sterol export effect),即内源性麦角甾醇的合成能够满足酵母生长需求,酵母细胞不具备摄取外源甾醇及其前体的能力。在缺氧条件下(O2 < 1%),由于缺乏有效的电子传递路径,麦角甾醇合成代谢的级联氧化还原过程被阻断,此时酵母成为甾醇缺陷型菌株,依赖摄取外源甾醇而存活[49]。另一方面,酵母在缺氧应激状态下上调了缺氧效应分子Sut1p、UPC2p、ECM22p等全局调控转录因子的表达水平,从而激活了下游靶基因编码的定位于细胞质膜甾醇转运蛋白(ATP-binding cassette,ABC) 的表达[20, 48]。该家族成员如Aus1p与Pdr11p等参与完成甾醇的外源摄取[50],而对应的缺失体无法在厌氧状态下摄取外源甾醇,且对外源甾醇的酯化能力也被阻断[51]。进入酵母的外源甾醇会通过非囊泡转运的“逆转运过程”由质膜转运至内质网,随后被甾醇酯化酶Are1p/Are2p酯化,其运输/酯化速率与甾醇对质膜上脂筏结构的亲和力相关[51]。而后与新合成的甾醇一起,由ATP依赖的非囊泡途径从内质网到达质膜,如此形成一个循环。当细胞中的游离甾醇总量过高时,甾醇会被酯化储存至脂滴。脂滴中的甾醇被再次使用前,需要由3种甾醇酯水解酶:Yeh1p、Yeh2p和Tgl1p将其催化水解并释放出游离甾醇,通过以上过程将胞内游离甾醇浓度控制在稳定状态[52-54],具体过程如图 4所示[20]。
 |
| 图 4 酵母麦角甾醇转运的关键作用分子及途径[20] Fig. 4 The key functional molecules and pathways for ergosterol transport in yeast[20]. |
| 图选项 |
2.3 胞外分泌有氧状态下,当甾醇供应充足时,酵母细胞会将胞内过量的甾醇通过分泌途径转运至胞外以达到胞内甾醇的稳态。Tiwari等首次发现酵母细胞通过对甾醇3′-羟基的乙酰化-去乙酰化修饰来调控甾醇的胞外分泌[55]。甾醇的乙酰化标签可被视作胞外分泌的信号,该循环由醇乙酰转移酶(Alcohol acetyltransferase 2,Atf2p) 与去乙酰酶(Steryl deacetylase 1,Say1p) 完成:若甾醇需留在胞内,则由Say1p催化甾醇去乙酰化;若需要排出体外,则由Atf2p将其乙酰化后分泌出胞[55]。Nagasawa等通过多拷贝质粒在酿酒酵母中实现了Atf2p的过表达,总酶活力较出发菌株提高了2.4倍[56]。此外,由于孕酮等甾体结构类似物能够与甾醇C8–C7异构酶高度亲和,因此Atf2p可能与外排作用协同,以清除酵母细胞中因麦角甾醇合成缺陷而产生的3β-羟基甾体对细胞生长的抑制作用[57-58]。此外,酵母在分泌甾醇的过程中,还会产生一种病原相关酵母蛋白(Pathogen-related yeast proteins,Pryp),该蛋白为一类甾醇结合蛋白的保守蛋白家族,属于CAP (Cysteine-rich secretory proteins,antigen 5,and pathogenesis-related 1 proteins) 超家族蛋白,与甾醇结合后能够显著提高其水溶性[59-60]。Choudhary等用[14C]胆甾醇标记Δsay1血红素缺陷细胞的分泌过程,结果显示pry1基因的缺失导致细胞外胆甾醇乙酸酯的水平降低了约40%;同时敲除pry1及pry2基因后,双突变体的胆甾醇乙酸酯的输出被完全阻断,这表明Pry1p、Pry2p对乙酰化甾醇的分泌出胞发挥了决定作用[61]。
3 强化甾醇定向存储与转运的理性改造策略近年来,随着对酵母等真核细胞膜转运蛋白的序列、结构、底物和转运机理研究的不断深入,强化疏水性酯类物质的定向运输已成为提高目的产物单位细胞产量的新策略,代表性实例见表 1。麦角甾醇虽为酵母中不可或缺的细胞膜组分,但作为亲脂性疏水化合物,当其在细胞中大量积累时,会造成细胞毒性[20],从而导致依赖传统代谢工程改造相关的合成途径强化技术提高产物的合成效率及产物累积浓度变得困难。因此,强化酵母对甾醇类化合物的耐受与蓄积能力对提高单位酵母细胞的甾醇合成能力有重要的提升作用[62]。
表 1 疏水性脂类化合物合成强化的理性改造策略Table 1 Rational modification strategies for enhanced production of hydrophobic lipids
| Compounds | Strains | Genes | Yield | Improvement | References |
| Lipids | S. cerevisiae | AtDGAT1, Δtgl3, Atclo1, Ald6-SEACSL641P, ACC1ser659ala, ser1157ala mutation | 307.0 mg/L | 3.60 folds | [65] |
| TAG | S. cerevisiae | ACC1ser659ala, ser1157ala, DGA1, PAH1, Δtgl3/4/5, Δare1Δerd1, PLIN3 | 201.0 mg/g DCW | 2.38 folds | [66] |
| Campesterol | S. cerevisiae | PO1f-Δmfe1, ERG5-, DHCR7+, DHCR7+ | 837.0 mg/L | 3.70 folds | [67] |
| Lycopene | S. cerevisiae | Δsei1:: HphMX_PPGK1-, OLE1 | 70.5 mg/g DCW | 25% | [23] |
| Squalene | S. cerevisiae | Δecm33 | 50.0 mg/g DCW | 12% | [68] |
| β-carotene | S. cerevisiae | ATCC 8739, dxs: : M1-37, idi: : M1-46, Crt: : M1-93, SucAB: : M1-46, sdh: : M1-46, talB: : M1-46, ispG-mRSL-4, ispH-mRSL-14, crtEYIB: : Ptrc-crtEYIB, ΔnlpI, ΔtolR | 44.8 mg/g DCW | 61% | [22] |
| Carotenoids | Rhodosporidiumtoruloides | Pdr10 | 1.8.0 μg/mg | 5.00 folds | [69] |
| Monoterpenoid indole alkaloid | S. cerevisiae | VmTPT2/VmABCG1 | –/– | Extracellular secretion | [70] |
| Hydrocortisone | S. cerevisiae | CLCDR4 | 268.0 mg/(L·d) | 20% | [71] |
表选项
3.1 脂滴的理性改造策略脂滴作为酵母细胞内重要亲脂性化合物的储存结构,增加其在胞质溶胶中的体积占比是增加甾醇胞内蓄积的主要策略。Ta等首次发现ERG1突变所导致的转化前体角鲨烯在胞内累积与酵母脂滴聚集的突变表型相关[63],Δdga1Δlro1Δare1Δare2导致脂滴形成障碍,从而直接影响角鲨烯的胞内累积并严重地抑制了细胞生长及对药物特比萘芬的耐受能力[64]。Peng等通过DGAT1过表达及ΔTgl3策略,显著提高了酿酒酵母细胞的脂滴含量,在进一步强化脂肪酸合成代谢后,通过细胞生长和脂肪酸合成分阶段培养的方法,显著提了升细胞脂肪酸产量,达到307 mg/L,提升了4.6倍[65]。同样地,Teixeira等通过调控脂滴的结构和生成,有效提高了TAG的胞内累积[66]。Qian等利用解脂耶氏酵母麦角甾醇合成途径改造生产菜油甾醇时,发现产物合成与细胞脂质水平呈显著的正相关性,通过敲除多能β-氧化蛋白(Multifunctional β-oxidation protein 1,Mfe1p) 及过氧化物酶体生成因子(Peroxisomal biogenesis factor 10,Pex10p) 显著提高了菜油甾醇与脂质体在脂滴中的存储含量,最终在5 L发酵罐中实现了837 mg/L菜油甾醇的累积,较出发菌提高了3.7倍[67]。Fei等发现酿酒酵母中SEI1基因的缺失会增加脂质水平[68]。在SEI1缺失的细胞中,Sorger等观察到比正常体积大50倍的“超大型”脂滴[69]。Ma等以番茄红素(Lycopene) 为目标产物,将重组酵母细胞的SEI1基因敲除,最终使其产量提高了25%[23]。考虑到脂滴结构是番茄红素与麦角甾醇等亲脂性化合物共同的存储场所,后续可尝试该策略提高酵母对甾醇的蓄积能力。
3.2 细胞膜/细胞壁的结构改性细胞膜/细胞壁作为酵母单位细胞的结构支撑,决定了细胞在生长分裂过程中形态的变化及完整性的维持,同时也为细胞提供了对外界理化损伤的抵抗能力;反之,甾醇类物质作为酵母细胞重要的膜组分,其结构及含量的差异会显著影响细胞质膜的流动性及硬度。如何平衡甾醇类产物累积及其对细胞壁/细胞膜结构稳定性的影响对总体产量的提高至关重要。酵母细胞自身具有保持细胞壁完整性(Cell wall integrity,CWI) 的调节能力以维持自身胞内稳态[72],同时CWI也是维持细胞脂质平衡信号通路MAPK的结构基础[73]。Kim等构建了一种角鲨烯高产酵母菌株SQ[74],在该菌株中,角鲨烯的大量积累使得细胞膜硬度下降,细胞因此变得更容易裂解。对此,他们对CWI途径进行了改造,通过敲除ECM33基因上调了几丁质合成相关酶的表达水平,显著提升了胞内几丁质含量,增强了细胞膜的硬度,从而加强了细胞壁对高压环境的抵抗能力,并使角鲨烯产量比亲本SQ菌株提高了约12%[68]。Wu等构建了一种新型的人工膜囊泡转运系统,实现了大肠杆菌细胞从头合成类胡萝卜素的胞外分泌,摇瓶发酵产量从27.7 mg/g DCW提高至44.8 mg/g DCW,其中产物胞外分泌的含量从0.5 mg/g DCW提高至12.7 mg/g DCW[22],为后续真核细胞人工膜转运系统的构建提供了借鉴。
3.3 甾醇出胞途径的构建建立有效的甾醇胞外分泌途径,可彻底解除甾醇终产物在胞内的蓄积,是突破酵母甾醇人工合成极限的最有效策略。提高细胞质膜上ABC转运蛋白的表达水平是强化产物胞外转运的主要策略,其中ABC家族蛋白中参与酵母多药耐药外排的转运蛋白(Pleiotropic drug resistance,PDR)是目前提高疏水性化合物胞外转运的关键调控分子[75]。Mahé等首次鉴定了Pdr5p and Snq2p介导雌二醇在酵母细胞中的分泌外排[76]。Lee等在两相体系合成类胡萝卜素的圆红冬孢酵母中,通过过表达Pdr11p实现了类胡萝卜素的原位分泌并转运至油相,有效解除了产物累积,最终产率达到1.8 μg/mg,较出发菌株提高了5倍,并有效解决了产物分离问题[69]。最近,Bu等研究了粟酒裂殖酵母Schizosaccharomyces pombe细胞中ABC转运蛋白(Pdr5p、Pdr10p、Snq2p、Yor1p和Yol075cp)的过表达对β-胡萝卜素的胞外分泌能力的影响,其中Snq2p的诱导型过表达实现了7.04 mg/L产物的分泌,较出发菌株提高4倍左右,并且胞内产物的累积量也提高至116.3 mg/L[77]。在利用脂肪酸合成代谢改造实现1-烯烃从头合成的酿酒酵母细胞中,Zhou等通过动态调控策略控制产物合成及转运过程的耦合,产物终产量达35.3 mg/L,其中80%以分泌形式排出细胞外,合成能力较出发菌株提高了10倍以上[78]。Demessie等通过在酿酒酵母细胞中异源表达小蔓长春花Vinca minor来源的ABC转运蛋白VmTpt2p/VmAbcg1p,成功实现了单萜吲哚生物碱的胞外分泌[70]。Chen等通过挖掘编码月状旋孢腔菌Cochliobolus lunatus来源的转运蛋白基因ClCDR4,其编码蛋白的氨基酸序列与酿酒酵母内源的Pdr5p具有48%的同源性,该基因串联启动子PGK1在酿酒酵母菌株HC017的过表达显著促进了底物乙酰-11-脱氧皮甾醇的胞内转运及胞外分泌,终产物氢化可的松的产率提升约20%,达到268 mg/(L·d)[71]。
基于前文所述的酵母甾醇乙酰化/去乙酰化调控的分子机理,设想尝试通过乙酰化酶Atf2p的过表达及去乙酰基酶Say1p的敲除可实现乙酰化甾醇的胞外分泌,且同时考察胞外分泌途径的构建对酵母细胞甾醇从头合成能力的影响。为进一步提高分泌能力,可考虑异源表达同源蛋白CRISP2 (哺乳动物来源)[59]。该类蛋白是CAP家族成员,负责与乙酰化甾醇结合,并以分泌的形式转运出胞。前期研究提示CRISP2的表达缓解了Δpry1、Δpry2双敲除突变株的脂质输出阻滞[61]。此外,基于已报道的病原体相关酵母蛋白Pry1的晶体结构(PDB No. 5ETE)[79],通过分子改造策略提高Pry蛋白与甾醇的亲和力,可进一步提升酵母对甾醇的胞外分泌能力。甾醇类化合物的高效胞外分泌途径的构建,不仅能够突破单位酵母细胞的甾醇合成极限,也为进一步实现甾体类化合物的从头合成路线奠定了基础。如图 5所示,利用酿酒酵母麦角甾醇合成通路的重构及胞外分泌途径的强化,可实现胆甾醇等甾醇类化合物的从头合成并实现有效外排;该途径进一步耦和以分枝杆菌生物转化实现甾醇的侧链降解,通过串联两阶段的生物转化体系,构建以简单碳源如葡萄糖等为底物,实现4-雄烯二酮等甾体激素的从头合成。
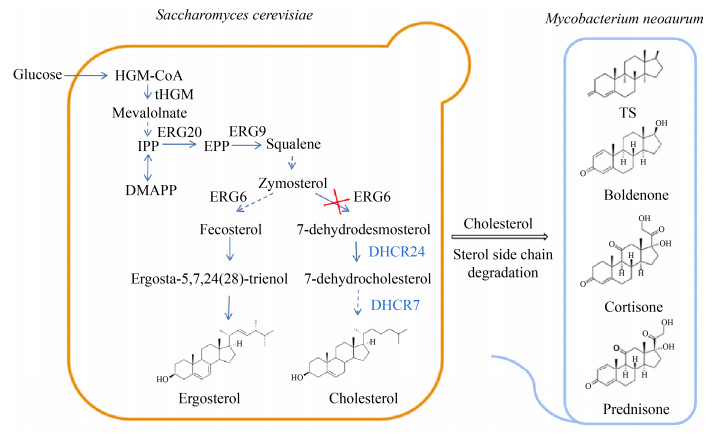 |
| 图 5 细胞共培养体系甾体激素从头合成路线设计 Fig. 5 De novo synthesis of steroid hormone in a mixed cell culture system. |
| 图选项 |
4 总结与展望甾体化合物作为重要的细胞膜组分及关键信号分子,其重要的应用价值及高效合成技术日益受关注。利用酵母细胞天然的麦角甾醇从头合成途径能够从根本上解决甾醇类药物及相关衍生物生产的资源及环境制约。本文归纳了影响酵母单位细胞甾醇定向存储与转运的关键作用分子,相信随着对酵母细胞内膜结构及其对特定底物的转运载体蛋白及相关分子转运机理研究的深入,将有助于理性调控甾醇类疏水性分子在细胞内的定向转运及蓄积,并为构建甾醇特异性的胞外分泌通道提供理论基础。结合图 6所示,本文提出了酵母细胞甾醇从头合成后胞内定向转运的理性改造策略及未来展望,拟通过提高酵母的脂滴含量及强化甾醇酯化过程提升酵母对甾醇的胞内蓄积能力,并基于酵母甾醇乙酰化/去乙酰化调控机理尝试构建甾醇定向胞外分泌途径,从根本上突破酵母细胞的甾醇从头合成极限。
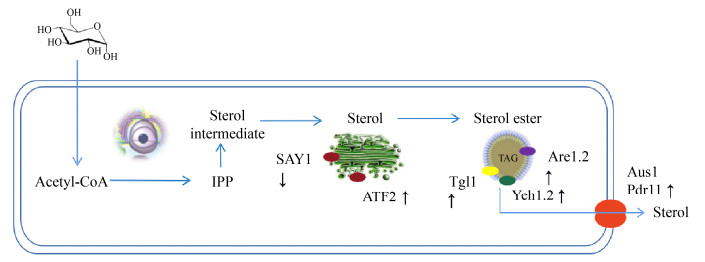 |
| 图 6 酵母甾醇定向转运的理性改造策略构想 Fig. 6 Strategies for rational engineering of yeast to achieve directed sterol transport. |
| 图选项 |
参考文献
| [1] | Korber M, Klein I, Daum G. Steryl ester synthesis, storage and hydrolysis: a contribution to sterol homeostasis. Biochim Biophys Acta Mol Cell Biol Lipids, 2017, 1862(12): 1534-1545. |
| [2] | Tolve R, Condelli N, Caruso MC, et al. Preparation and characterization of microencapsulated phytosterols for the formulation of functional foods: scale up from laboratory to semi-technical production. Food Res Int, 2019, 116: 1274-1281. DOI:10.1016/j.foodres.2018.10.016 |
| [3] | Luo X, Su P, Zhang W. Advances in microalgae-derived phytosterols for functional food and pharmaceutical applications. Mar Drugs, 2015, 13(7): 4231-4254. DOI:10.3390/md13074231 |
| [4] | Prasad R, Shah AH, Rawal MK. Antifungals: mechanism of action and drug resistance. Adv Exp Med Biol, 2016, 892: 327-349. |
| [5] | Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev, 2004, 25(6): 947-970. DOI:10.1210/er.2003-0030 |
| [6] | Hanukoglu I. Steroidogenic enzymes: structure, function, and role in regulation of steroid hormone biosynthesis. J Steroid Biochem Mol Biol, 1992, 43(8): 779-804. DOI:10.1016/0960-0760(92)90307-5 |
| [7] | Zhu YL, Huang W, Ni JR, et al. Production of diosgenin from Dioscorea zingiberensis tubers through enzymatic saccharification and microbial transformation. Appl Microbiol Biotechnol, 2010, 85(5): 1409-1416. DOI:10.1007/s00253-009-2200-8 |
| [8] | Ren Y, Chen Y, Hu BH, et al. Microwave-assisted extraction and a new determination method for total steroid saponins from Dioscorea zingiberensis C.H. Wright. Steroids, 2015, 104: 145-152. DOI:10.1016/j.steroids.2015.09.008 |
| [9] | Cravens A, Payne J, Smolke CD. Synthetic biology strategies for microbial biosynthesis of plant natural products. Nat Commun, 2019, 10: 2142. DOI:10.1038/s41467-019-09848-w |
| [10] | Xu SH, Li YR. Yeast as a promising heterologous host for steroid bioproduction. J Ind Microbiol Biotechnol, 2020, 47(9/10): 829-843. DOI:10.1007/s10295-020-02291-7 |
| [11] | Ajikumar PK, Xiao WH, Tyo KEJ, et al. Isoprenoid pathway optimization for taxol precursor overproduction in Escherichia coli. Science, 2010, 330(6000): 70-74. DOI:10.1126/science.1191652 |
| [12] | Paddon CJ, Westfall PJ, Pitera DJ, et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature, 2013, 496(7446): 528-532. DOI:10.1038/nature12051 |
| [13] | Meadows AL, Hawkins KM, Tsegaye Y, et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production. Nature, 2016, 537(7622): 694-697. DOI:10.1038/nature19769 |
| [14] | Shao ML, Zhang X, Rao ZM, et al. Efficient testosterone production by engineered Pichia pastoris co-expressing human 17β-hydroxysteroid dehydrogenase type 3 and Saccharomyces cerevisiae glucose 6-phosphate dehydrogenase with NADPH regeneration. Green Chem, 2016, 18(6): 1774-1784. DOI:10.1039/C5GC02353J |
| [15] | Guo XJ, Xiao WH, Wang Y, et al. Metabolic engineering of Saccharomyces cerevisiae for 7-dehydrocholesterol overproduction. Biotechnol Biofuels, 2018, 11: 192. DOI:10.1186/s13068-018-1194-9 |
| [16] | Ma BX, Ke X, Tang XL, et al. Rate-limiting steps in the Saccharomyces cerevisiae ergosterol pathway: towards improved ergosta-5, 7-dien-3β-ol accumulation by metabolic engineering. World J Microbiol Biotechnol, 2018, 34(4): 55. DOI:10.1007/s11274-018-2440-9 |
| [17] | Shang F, Wen S H, Wang X, et al. High-cell-density fermentation for ergosterol production by Saccharomyces cerevisiae. J Biosci Bioeng, 2006, 101(1): 38-41. DOI:10.1263/jbb.101.38 |
| [18] | Mo C, Valachovic M, Randall SK, et al. Protein-protein interactions among C-4 demethylation enzymes involved in yeast sterol biosynthesis. Proc Natl Acad Sci USA, 2002, 99(15): 9739-9744. DOI:10.1073/pnas.112202799 |
| [19] | Nes WD. Biosynthesis of cholesterol and other sterols. Chem Rev, 2011, 111(10): 6423-6451. DOI:10.1021/cr200021m |
| [20] | Jacquier N, Schneiter R. Mechanisms of sterol uptake and transport in yeast. J Steroid Biochem Mol Biol, 2012, 129(1/2): 70-78. |
| [21] | Liu JF, Xia JJ, Nie KL, et al. Outline of the biosynthesis and regulation of ergosterol in yeast. World J Microbiol Biotechnol, 2019, 35(7): 98. DOI:10.1007/s11274-019-2673-2 |
| [22] | Wu T, Li SW, Ye LJ, et al. Engineering an artificial membrane vesicle trafficking system (AMVTS) for the excretion of β-carotene in Escherichia coli. ACS Synth Biol, 2019, 8(5): 1037-1046. DOI:10.1021/acssynbio.8b00472 |
| [23] | Ma T, Shi B, Ye ZL, et al. Lipid engineering combined with systematic metabolic engineering of Saccharomyces cerevisiae for high-yield production of lycopene. Metab Eng, 2019, 52: 134-142. DOI:10.1016/j.ymben.2018.11.009 |
| [24] | Wagner A, Grillitsch K, Leitner E, et al. Mobilization of steryl esters from lipid particles of the yeast Saccharomyces cerevisiae. Biochim Biophys Acta Mol Cell Biol Lipids, 2009, 1791(2): 118-124. |
| [25] | Yu C, Kennedy NJ, Chang CC, et al. Molecular cloning and characterization of two isoforms of Saccharomyces cerevisiae acyl-CoA: sterol acyltransferase. J Biol Chem, 1996, 271(39): 24157-24163. DOI:10.1074/jbc.271.39.24157 |
| [26] | Yang HY, Bard M, Bruner DA, et al. Sterol esterification in yeast: a two-gene process. Science, 1996, 272(5266): 1353-1356. DOI:10.1126/science.272.5266.1353 |
| [27] | Zweytick D, Leitner E, Kohlwein SD, et al. Contribution of Are1p and Are2p to steryl ester synthesis in the yeast Saccharomyces cerevisiae. Eur J Biochem, 2000, 267(4): 1075-1082. DOI:10.1046/j.1432-1327.2000.01103.x |
| [28] | Grillitsch K, Connerth M, K?feler H, et al. Lipid particles/droplets of the yeast Saccharomyces cerevisiae revisited: lipidome meets proteome. Biochim Biophys Acta Mol Cell Biol Lipids, 2011, 1811(12): 1165-1176. |
| [29] | Gajdo? P, Ledesma-Amaro R, Nicaud JM, et al. Overexpression of diacylglycerol acyltransferase in Yarrowia lipolytica affects lipid body size, number and distribution. FEMS Yeast Res, 2016, 16(6): fow062. DOI:10.1093/femsyr/fow062 |
| [30] | Meyers A, Weiskittel TM, Dalhaimer P. Lipid droplets: formation to breakdown. Lipids, 2017, 52(6): 465-475. DOI:10.1007/s11745-017-4263-0 |
| [31] | Kornmann B, Currie E, Collins SR, et al. An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science, 2009, 325(5939): 477-481. DOI:10.1126/science.1175088 |
| [32] | Kvam E, Goldfarb DS. Nucleus-vacuole junctions and piecemeal microautophagy of the nucleus in S. cerevisiae. Autophagy, 2007, 3(2): 85-92. DOI:10.4161/auto.3586 |
| [33] | Radhakrishnan A, Goldstein JL, McDonald JG, et al. Switch-like control of SREBP-2 transport triggered by small changes in ER cholesterol: a delicate balance. Cell Metab, 2008, 8(6): 512-521. DOI:10.1016/j.cmet.2008.10.008 |
| [34] | Mesmin B, Maxfield FR. Intracellular sterol dynamics. Biochim Biophys Acta Mol Cell Biol Lipids, 2009, 1791(7): 636-645. |
| [35] | Klemm RW, Ejsing CS, Surma MA, et al. Segregation of sphingolipids and sterols during formation of secretory vesicles at the trans-Golgi network. J Cell Biol, 2009, 185(4): 601-612. DOI:10.1083/jcb.200901145 |
| [36] | Brügger B, Sandhoff R, Wegehingel S, et al. Evidence for segregation of sphingomyelin and cholesterol during formation of COPI-coated vesicles. J Cell Biol, 2000, 151(3): 507-518. DOI:10.1083/jcb.151.3.507 |
| [37] | Schneiter R, Brügger B, Sandhoff R, et al. Electrospray ionization tandem mass spectrometry (ESI-MS/MS) analysis of the lipid molecular species composition of yeast subcellular membranes reveals acyl chain-based sorting/remodeling of distinct molecular species en route to the plasma membrane. J Cell Biol, 1999, 146(4): 741-754. DOI:10.1083/jcb.146.4.741 |
| [38] | Bretscher MS, Munro S. Cholesterol and the Golgi apparatus. Science, 1993, 261(5126): 1280-1281. DOI:10.1126/science.8362242 |
| [39] | Sullivan DP, Ohvo-Rekil? H, Baumann NA, et al. Sterol trafficking between the endoplasmic reticulum and plasma membrane in yeast. Biochem Soc Trans, 2006, 34(3): 356-358. DOI:10.1042/BST0340356 |
| [40] | Baumann NA, Sullivan DP, Ohvo-Rekil? H, et al. Transport of newly synthesized sterol to the sterol-enriched plasma membrane occurs via nonvesicular equilibration. Biochemistry, 2005, 44(15): 5816-5826. DOI:10.1021/bi048296z |
| [41] | Raychaudhuri S, Prinz WA. The diverse functions of oxysterol-binding proteins. Annu Rev Cell Dev Biol, 2010, 26: 157-177. DOI:10.1146/annurev.cellbio.042308.113334 |
| [42] | Schulz TA, Prinz WA. Sterol transport in yeast and the oxysterol binding protein homologue (OSH) family. Biochim Biophys Acta, 2007, 1771(6): 769-780. DOI:10.1016/j.bbalip.2007.03.003 |
| [43] | Beh CT, Rine J. A role for yeast oxysterol-binding protein homologs in endocytosis and in the maintenance of intracellular sterol-lipid distribution. J Cell Sci, 2004, 117(Pt 14): 2983-2996. |
| [44] | Beh CT, Cool LG, Phillips JW, et al. Overlapping functions of the yeast oxysterol-binding protein homologs. Genetics, 2001, 157(3): 1117-1140. DOI:10.1093/genetics/157.3.1117 |
| [45] | Im YJ, Raychaudhuri S, Prinz WA, et al. Structural mechanism for sterol sensing and transport by OSBP-related proteins. Nature, 2005, 437(7055): 154-158. DOI:10.1038/nature03923 |
| [46] | Alfaro G, Johansen J, Dighe SA, et al. The sterol-binding protein Kes1/Osh4p is a regulator of polarized exocytosis. Traffic, 2011, 12(11): 1521-1536. DOI:10.1111/j.1600-0854.2011.01265.x |
| [47] | Tong J, Manik MK, Im YJ. Structural basis of sterol recognition and nonvesicular transport by lipid transfer proteins anchored at membrane contact sites. Proc Natl Acad Sci USA, 2018, 115(5): E856-E865. DOI:10.1073/pnas.1719709115 |
| [48] | Sokolov SS, Trushina NI, Severin FF, et al. Ergosterol turnover in yeast: an interplay between biosynthesis and transport. Biochemistry (Mosc), 2019, 84(4): 346-357. DOI:10.1134/S0006297919040023 |
| [49] | Lewis TA, Taylor FR, Parks LW. Involvement of heme biosynthesis in control of sterol uptake by Saccharomyces cerevisiae. J Bacteriol, 1985, 163(1): 199-207. DOI:10.1128/jb.163.1.199-207.1985 |
| [50] | Wilcox LJ, Balderes DA, Wharton B, et al. Transcriptional profiling identifies two members of the ATP-binding cassette transporter superfamily required for sterol uptake in yeast. J Biol Chem, 2002, 277(36): 32466-32472. DOI:10.1074/jbc.M204707200 |
| [51] | Li YF, Prinz WA. ATP-binding cassette (ABC) transporters mediate nonvesicular, raft-modulated sterol movement from the plasma membrane to the endoplasmic reticulum. J Biol Chem, 2004, 279(43): 45226-45234. DOI:10.1074/jbc.M407600200 |
| [52] | Müllner H, Deutsch G, Leitner E, et al. YEH2/YLR020c encodes a novel steryl ester hydrolase of the yeast Saccharomyces cerevisiae. J Biol Chem, 2005, 280(14): 13321-13328. DOI:10.1074/jbc.M409914200 |
| [53] | Koffel R, Tiwari R, Falquet L, et al. The Saccharomyces cerevisiae YLL012/YEH1, YLR020/YEH2, and TGL1 genes encode a novel family of membrane-anchored lipases that are required for steryl ester hydrolysis. Mol Cell Biol, 2005, 25(5): 1655-1668. DOI:10.1128/MCB.25.5.1655-1668.2005 |
| [54] | Jandrositz A, Petschnigg J, Zimmermann R, et al. The lipid droplet enzyme Tgl1p hydrolyzes both steryl esters and triglycerides in the yeast, Saccharomyces cerevisiae. Biochim Biophys Acta Mol Cell Biol Lipids, 2005, 1735(1): 50-58. |
| [55] | Tiwari R, K?ffel R, Schneiter R. An acetylation/deacetylation cycle controls the export of sterols and steroids from S. cerevisiae. EMBO J, 2007, 26(24): 5109-5119. DOI:10.1038/sj.emboj.7601924 |
| [56] | Nagasawa N, Bogaki T, Iwamatsu A, et al. Cloning and nucleotide sequence of the alcohol acetyltransferase Ⅱ gene (ATF2) from Saccharomyces cerevisiae Kyokai No. 7. Biosci Biotechnol Biochem, 1998, 62(10): 1852-1857. DOI:10.1271/bbb.62.1852 |
| [57] | Rust S, Rosier M, Funke H, et al. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet, 1999, 22(4): 352-355. DOI:10.1038/11921 |
| [58] | Cauet G, Degryse E, Ledoux C, et al. Pregnenolone esterification in Saccharomyces cerevisiae. A potential detoxification mechanism. Eur J Biochem, 1999, 261(1): 317-324. DOI:10.1046/j.1432-1327.1999.00282.x |
| [59] | Gibbs GM, Roelants K, O'Bryan MK. The CAP superfamily: cysteine-rich secretory proteins, antigen 5, and pathogenesis-related 1 proteins-roles in reproduction, cancer, and immune defense. Endocr Rev, 2008, 29(7): 865-897. DOI:10.1210/er.2008-0032 |
| [60] | van Loon LC, van Strien EA. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol Mol Plant Pathol, 1999, 55(2): 85-97. DOI:10.1006/pmpp.1999.0213 |
| [61] | Choudhary V, Schneiter R. Pathogen-related yeast (PRY) proteins and members of the CAP superfamily are secreted sterol-binding proteins. Proc Natl Acad Sci USA, 2012, 109(42): 16882-16887. DOI:10.1073/pnas.1209086109 |
| [62] | Xie WP, Ye LD, Lv XM, et al. Sequential control of biosynthetic pathways for balanced utilization of metabolic intermediates in Saccharomyces cerevisiae. Metab Eng, 2015, 28: 8-18. DOI:10.1016/j.ymben.2014.11.007 |
| [63] | Ta MT, Kapterian TS, Fei WH, et al. Accumulation of squalene is associated with the clustering of lipid droplets. FEBS J, 2012, 279(22): 4231-4244. DOI:10.1111/febs.12015 |
| [64] | Valachovic M, Garaiova M, Holic R, et al. Squalene is lipotoxic to yeast cells defective in lipid droplet biogenesis. Biochem Bioph Res Commun, 2016, 469(4): 1123-1128. DOI:10.1016/j.bbrc.2015.12.050 |
| [65] | Peng HD, He LZ, Haritos VS. Metabolic engineering of lipid pathways in Saccharomyces cerevisiae and staged bioprocess for enhanced lipid production and cellular physiology. J Ind Microbiol Biotechnol, 2018, 45(8): 707-717. DOI:10.1007/s10295-018-2046-0 |
| [66] | Teixeira PG, David F, Siewers V, et al. Engineering lipid droplet assembly mechanisms for improved triacylglycerol accumulation in Saccharomyces cerevisiae. FEMS Yeast Res, 2018, 18(6). DOI:10.1093/femsyr/foy060 |
| [67] | Qian YD, Tan SY, Dong GR, et al. Increased campesterol synthesis by improving lipid content in engineered Yarrowia lipolytica. Appl Microbiol Biotechnol, 2020, 104(16): 7165-7175. DOI:10.1007/s00253-020-10743-4 |
| [68] | Fei WH, Shui GH, Gaeta B, et al. Fld1p, a functional homologue of human seipin, regulates the size of lipid droplets in yeast. J Cell Biol, 2008, 180(3): 473-482. DOI:10.1083/jcb.200711136 |
| [69] | Lee JJL, Chen LW, Cao B, et al. Engineering Rhodosporidium toruloides with a membrane transporter facilitates production and separation of carotenoids and lipids in a bi-phasic culture. Appl Microbiol Biotechnol, 2016, 100(2): 869-877. DOI:10.1007/s00253-015-7102-3 |
| [70] | Demessie Z, Woolfson KN, Yu F, et al. The ATP binding cassette transporter, VmTPT2/VmABCG1, is involved in export of the monoterpenoid indole alkaloid, vincamine in Vinca minor leaves. Phytochemistry, 2017, 140: 118-124. DOI:10.1016/j.phytochem.2017.04.019 |
| [71] | Chen J, Fan FY, Qu G, et al. Identification of Absidia orchidis steroid 11βa-hydroxylation system and its application in engineering Saccharomyces cerevisiae for one-step biotransformation to produce hydrocortisone. Metab Eng, 2020, 57: 31-42. DOI:10.1016/j.ymben.2019.10.006 |
| [72] | Levin DE. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: the cell wall integrity signaling pathway. Genetics, 2011, 189(4): 1145-1175. DOI:10.1534/genetics.111.128264 |
| [73] | Nunez LR, Jesch SA, Gaspar ML, et al. Cell wall integrity MAPK pathway is essential for lipid homeostasis. J Biol Chem, 2008, 283(49): 34204-34217. DOI:10.1074/jbc.M806391200 |
| [74] | Kim JE, Jang IS, Son SH, et al. Tailoring the Saccharomyces cerevisiae endoplasmic reticulum for functional assembly of terpene synthesis pathway. Metab Eng, 2019, 56: 50-59. DOI:10.1016/j.ymben.2019.08.013 |
| [75] | Prasad R, Goffeau A. Yeast ATP-binding cassette transporters conferring multidrug resistance. Annu Rev Microbiol, 2012, 66: 39-63. DOI:10.1146/annurev-micro-092611-150111 |
| [76] | Mahé Y, Lemoine Y, Kuchler K. The ATP binding cassette transporters Pdr5 and Snq2 of Saccharomyces cerevisiae can mediate transport of steroids in vivo. J Biol Chem, 1996, 271(41): 25167-25172. DOI:10.1074/jbc.271.41.25167 |
| [77] | Bu X, Lin JY, Cheng J, et al. Engineering endogenous ABC transporter with improving ATP supply and membrane flexibility enhances the secretion of β-carotene in Saccharomyces cerevisiae. Biotechnol Biofuels, 2020, 13: 168. DOI:10.1186/s13068-020-01809-6 |
| [78] | Zhou YJ, Hu YT, Zhu ZW, et al. Engineering 1-alkene biosynthesis and secretion by dynamic regulation in yeast. ACS Synth Biol, 2018, 7(2): 584-590. DOI:10.1021/acssynbio.7b00338 |
| [79] | Darwiche R, Kelleher A, Hudspeth EM, et al. Structural and functional characterization of the CAP domain of pathogen-related yeast 1 (Pry1) protein. Sci Rep, 2016, 6: 28838. DOI:10.1038/srep28838 |
