

1. 北京化工大学 生命科学与技术学院,北京 100029;
2. 北京联合大学 生物化学工程学院,北京 100023
收稿日期:2020-06-28;接收日期:2020-08-19
基金项目:国家重点研发计划(No. 2018YFA0901800),河北省重点研发计划(Nos. 20322501D, 19226509D),北京市教育委员会科研计划-北京市自然科学基金项目(No. KZ201911417049) 资助
摘要:薯蓣皂素是一种天然甾体皂苷元,可作为数百种类固醇药物的前体,具有重要药用价值。目前工业生产薯蓣皂素主要依赖化学提取法,因此该法依赖植物材料和耕地且对环境有害。随着代谢工程和合成生物学的发展,生物合成法受到广泛关注。文中综述了生物合成薯蓣皂素的代谢途径和关键酶,并在酿酒酵母Saccharomyces cerevisiae中设计其异源合成途径,提出改造策略,以期为全生物合成薯蓣皂素提供有价值的参考。
关键词:薯蓣皂素生物合成途径关键酶异源表达
Pathway design and key enzyme analysis of diosgenin biosynthesis
Zhongyi Sun1, Peng Zhao1, Xizhen Ge2, Pingfang Tian1


1. College of Life Science and Technology, Beijing University of Chemical Technology, Beijing 100029, China;
2. College of Biochemical Engineering, Beijing Union University, Beijing 100023, China
Received: June 28, 2020; Accepted: August 19, 2020
Supported by: National Key Research and Development Program of China (No. 2018YFA0901800), Key Research and Development Program of Hebei Province, China (Nos. 20322501D, 19226509D), Program of Beijing Municipal Commission of Education-Natural Science Foundation of Beijing, China (No. KZ201911417049)
Corresponding author: Pingfang Tian. E-mail: tianpf@mail.buct.edu.cn.
Abstract: As a naturally occurring steroid sapogenin, diosgenin acts as the precursor of hundreds of steroid medicines, and thereby has important medicinal value. Currently, industrial production of diosgenin relies primarily on chemical extraction from plant materials. Clearly, this strategy shows drawbacks of excessive reliance on plant materials and farmland as well as environment pollution. Due to development of metabolic engineering and synthetic biology, bio-production of diosgenin has garnered plenty of attention. Although the biosynthetic pathways of diosgenin have not been completely identified, in this review, we outline the identified biosynthetic pathways and key enzymes. In particular, we suggest heterologous biosynthesis of diosgenin in Saccharomyces cerevisiae. Overall, this review aims to provide valuable insights for future complete biosynthesis of diosgenin.
Keywords: diosgeninbiosynthetic pathwaykey enzymesheterologous expression
薯蓣皂苷(Dioscin) 属于甾体皂苷,主要存在于盾叶薯蓣Dioscorea zingiberensis、葫芦巴Trigonella foenum-graecum等植物中[1-2]。薯蓣皂苷由薯蓣皂素(Diosgenin)、两个鼠李糖基和一个葡萄糖基构成(图 1)[3]。薯蓣皂素与甾体激素类药物具有类似结构,因而具有药用价值[4]。通过对薯蓣皂素进行结构修饰,目前已得到多种甾体激素类药物[5],这些药物具有抗肿瘤[6]、抗炎[7]、增强免疫[8]、保护肝脏[9]等功效。薯蓣皂素在植物体内含量甚微,无法满足药用需求。微生物异源合成可解决该问题。目前已异源合成多种植物次生代谢产物,例如香叶醇(Geraniol)[10]、青蒿素前体紫穗槐二烯(Amorphadiene)[11]、青蒿酸(Artemisinic acid)[12]、紫杉烯(Taxadiene)[13]、β-胡萝卜素(β-carotene)[14]等。据报道,薯蓣皂素的生物合成分为3个阶段:(1) 乙酰辅酶A经甲羟戊酸途径(MVA pathway) 或甲基-赤藓醇-4-磷酸途径(MEP pathway) 分别生成异戊烯焦磷酸(Isopentenyl diphosphate,IPP) 和二甲基烯丙基焦磷酸(Dimethylallyl diphosphate,DMAPP);(2) IPP和DMAPP经多步酶促反应,碳链延长与环化,形成胆固醇;(3) 胆固醇经多步氧化和成环反应,生成薯蓣皂素[15]。环阿屯醇合酶(Cycloartenol synthase,CAS)、细胞色素P450酶(Cytochrome P450 enzyme,CYP450s) 和尿苷二磷酸糖基转移酶(UDP-glucosyltransferase,UGTs) 在薯蓣皂素的合成中起关键作用[16-17]。
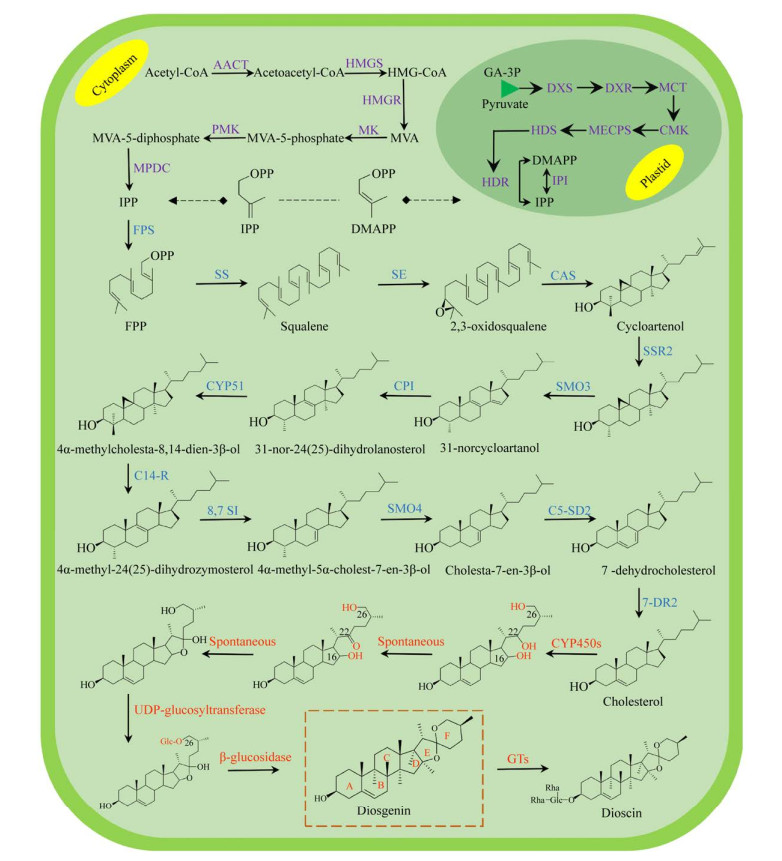 |
| 图 1 植物体内薯蓣皂素的合成途径 Fig. 1 Biosynthetic pathway of diosgenin in plants. Abbreviations: AACT: acetyl CoA acetyltransferase; HMGS: hydroxymethyl glutaryl CoA synthase; HMGR: 3-hydroxy-3-methylglutaryl-CoA reductase; MK: mevalonate kinase; PMK: phosphomevalonate kinase; MPDC: mevalonate diphosphosphate decarboxylase; DXS: 1-deoxy-D-xylulose-5- phosphate synthase; DXR: 1-deoxy-D-xylulose-5-phosphate reductoisomerase; MCT: 2-C-methyl-D-erythritol 4-phosphatecytidylyl transferase; CMK: 4-diphospho-cytidyl-2-C-methyl-D-erythritol kinase; MECPS: 2-C-methyl- D-erythritol-2, 4-cyclodiphosphate synthase; HDS: 4-hydroxy-3-methylbut-2-(E)-enyldiphosphate synthase; HDR: 4-hydroxy-3-methylbut-2-enyldiphosphate reductase; IPI: IPP diphosphate isomerase; SSR: sterol side chain reductase; SMO: C-4 sterol methyloxidase; CPI: cycloeucalenol cycloisomerase; CYP51: sterol C-14 demethylase; C14-R: sterol C-14 reductase; 8, 7-SI: sterol 8, 7-isomerase; C5-SD: sterol C-5 (6) desaturase; 7-DR: 7-dihydrocholesterol reductase. |
| 图选项 |
1 薯蓣皂素的生物合成途径薯蓣皂素属于甾醇类物质,其合成前体为C5异戊二烯单位IPP和DMAPP。IPP和DMAPP在高等植物体内分别由MVA途径和MEP途径生成[18]。MVA途径存在于细胞质中,在薯蓣皂素骨架形成中起主要作用[19]。MVA途径起始于乙酰辅酶A (Acetyl-coenzyme A,Ac-CoA),Ac-CoA经6步酶促反应,生成薯蓣皂素的基本组成单元IPP[20]。质体中的MEP途径始于3-磷酸甘油醛(Glyceraldehyde 3-phosphate,G3P) 和丙酮酸,经7步酶促反应生成IPP和DMAPP (图 1)。IPP和DMAPP可相互转化[21],二者在法尼基焦磷酸合酶(Farnesyl diphosphate synthase,FPS) 催化下首尾相连,生成法尼基焦磷酸(Farnesyl pyrophosphate,FPP)。FPP经鲨烯合酶(Squalene synthase,SS) 和鲨烯环氧酶(Squalene epoxidase,SE) 连续催化,碳链延长并被氧化、环化,形成2, 3-氧化鲨烯(2, 3-oxidosqualene)[22]。2, 3-氧化鲨烯是合成甾醇和三萜的分支点。2, 3-氧化鲨烯经不同的氧化鲨烯环化酶(Oxidosqualene cyclase,OSCs) 催化,形成不同结构的次生代谢产物前体(图 2)。环阿屯醇和羊毛甾醇是甾醇类物质的合成前体,分别由CAS和羊毛甾醇合酶(Lanosterol synthase,LAS) 催化形成。Mohammadi等分析了葫芦巴T. foenum-graecum中薯蓣皂素生物合成的转录本和代谢物数据,未发现LAS[23],表明环阿屯醇为生物合成薯蓣皂素的中间体,又经同位素标记技术探明胆固醇为薯蓣皂素的合成前体[24],环阿屯醇合成胆固醇的9个酶促步骤也已在植物分子水平上表征[25]。迄今为止,胆固醇合成薯蓣皂素的相关基因尚未完全阐明。Zhou等用茉莉酸甲酯(Methyl jasmonate,MeJA) 诱导葫芦巴多产薯蓣皂素,分析诱导植株与未处理植株的差异基因,发现CYP450和UGT基因参与胆固醇后期修饰步骤[26]。Christ鉴定出葫芦巴中相关CYP450基因(TfCYP90B50和TfCYP82J17),并在酿酒酵母Saccharomyces cerevisiae中验证其功能。TfCYP90B50和TfCYP82J17可在胆固醇C-16和C-22位加氧修饰,但未得到C-26位氧化产物的结构信息[16]。胆固醇C-26位羟基需在UGTs催化下添加葡萄糖基,然后裂解葡萄糖基,形成薯蓣皂素F环(图 1)[27],但参与这步反应的相关基因还需挖掘。
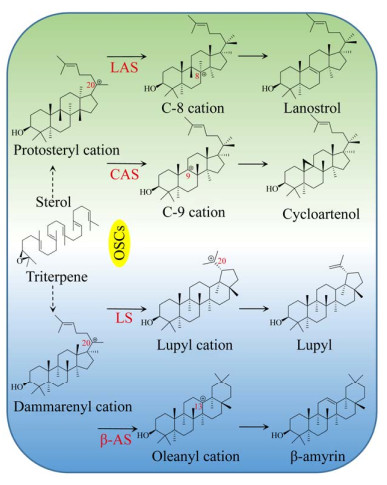 |
| 图 2 2, 3-氧化鲨烯代谢支路 Fig. 2 2, 3-oxidosqualene metabolic pathway. LS: lupyl synthase; β-AS: β-amyrin synthase. |
| 图选项 |
2 关键酶分析2.1 环阿屯醇合酶2, 3-氧化鲨烯在OSCs家族的催化下,可在不同位置脱质子,形成碳正离子。碳正离子经重排,2, 3-氧化鲨烯可形成多种不同结构的甾醇类和三萜类化合物前体(图 2)。CAS属于OSCs家族,可催化2, 3-氧化鲨烯在C-20位脱质子,形成原甾醇碳正离子(Protosteryl cation),之后C+-20重新排布到C-9位,并在此位发生成环反应,2, 3-氧化鲨烯形成环阿屯醇[22, 28]。迄今为止,CAS催化机理未明。在拟南芥Arabidopsis thaliana CAS研究中,通过定向进化与随机诱变,发现Tyr410、His477和Ile481是其催化功能的关键残基[29-31]。植物CAS序列高度保守,具有保守结构域Asp-Cys- Thr-Ala-Glu (DCTAE)[32]。DCTAE序列可影响CAS与底物的结合与环化[33-34]。Mashayekh等从葫芦巴中克隆出了编码756个氨基酸的CAS基因(GenBank登录号:APA19295.1)。TfCAS与已知CAS序列的相似性超过80%,且具有保守结构域DCTAE。目前植物OSCs家族的晶体结构尚未报道,仅有人类OSCs三维结构被解析(PDB ID: 1W6J (图 3B);1W6K (图 3C))。人类OSCs由两个环形相连的桶状α结构域和3个小的β结构域组成[34]。生物合成薯蓣皂素需异源表达CAS基因,同时降低LAS的表达可避免竞争性抑制,减少羊毛甾醇合成通量,使更多的2, 3-氧化鲨烯流向CAS通路[35]。此外,过表达宿主MVA途径中的HMGR、FPS、SS和SE,也可增加2, 3-氧化鲨烯的产量[36-37]。
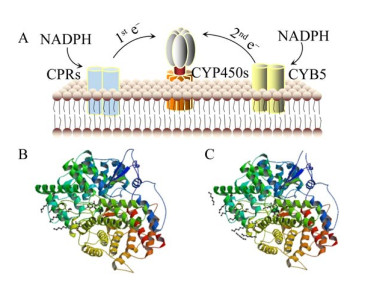 |
| 图 3 CYP450s电子传递体系(A) 和人类OSCs (B–C)的三维结构 Fig. 3 Structures of the electron transport system of CYP450s (A) and human OSCs (B–C). |
| 图选项 |
2.2 细胞色素P450酶CYP450是一个超基因家族,编码的单加氧酶可对底物特异性位点加氧修饰,参与多种次生代谢产物的后修饰。在薯蓣皂素生物合成中,CYP450s对胆固醇C-22、C-16和C-26位置氧化修饰[26]。目前已有多种植物CYP450s被分离鉴定,但参与生物合成薯蓣皂素CYP450s的报道很少。CYP450s是结合于内质网的氧化还原酶,需NADPH依赖型还原酶(NADPH-dependent reductase) 为其传递电子。异源表达CYP450s需解决两大问题:其一,保证CYP450s正确折叠;其二,保证CYP450s有效的电子传递效率。大肠杆菌Escherichia coli缺乏内质网,CYP450s无法正确锚定内膜系统,导致包涵体的形成。N-末端工程可解决此问题。Biggs等将CYP450s N-末端替换为来自牛(Bovine) 的亲水性八残基序列肽(8RP),实现了CYP450s在大肠杆菌E. coli中的正常表达[38]。8RP已成为N-末端工程的普选序列。以酿酒酵母为宿主,也可对CYP450s N-末端修饰,以保证CYP450s的高效折叠[39]。CYP450s在异源宿主内维持高催化效率,需为其设计有效的电子供体。还原力不足会降低CYP450s系统的催化效率;而还原当量过剩导致有毒中间体的产生。CYP450s与CYP450还原酶(Cytochrome P450 reductase,CPRs) 需优化配对。据报道,CYP450s表达量通常高于CPRs[40],且CYP450s与细胞色素b5 (Cytochrome b5,CYB5) 共表达可提高CYP450s系统反应效率[41] (图 3A)。基于上述研究,Paddon等在酿酒酵母中异源表达黄花蒿Artemisia annua的CYP71AV1、CPR1和CYB5基因,以启动子强弱来控制CYP71AV1、CPR1和CYB5的表达量,研究其最佳配比,保证CYP450s系统的催化效率[12]。不同菌株及载体也会影响CYP450s系统的催化效率,Hausjell等进行了探索[42],但难点仍是CYP450s系统的电子通量。Nielsen等通过异源酶亚细胞区域化策略(Subcellular compartmentalization),将高粱Sorghum bicolor的CYP79A1、CYP71E1和UGT85B1表达于烟草Nicotiana benthamiana的叶绿体,利用烟草光合作用产生的还原力,启动CYP450s系统[43]。植物光合作用以铁氧还原蛋白(Ferredoxins) 为电子载体。铁氧还原蛋白处于光合还原力中心,与异源CYP450s系统存在还原当量竞争。Mellor等比较了3种植物铁氧还原蛋白和一种类黄素工程蛋白电子载体(Flavodoxin-like engineered protein) 对CYP450s系统的催化效率,发现以类黄素工程蛋白为电子载体,可减小还原力内源竞争,为异源CYP450s系统提供最佳的氧化还原电位[44]。
2.3 尿苷二磷酸糖基转移酶糖基转移酶(Glucosyltransferase,GTs) 是一个蛋白质超家族,催化糖基转移到甾醇、三萜等特定受体分子。根据氨基酸序列相似性、催化机制和保守序列,GTs可分为110个家族http://journals.im.ac.cn/html/cjbcn/2021/4/(http://www.cazy.org/GlycosylTransferases.html)。UGTs属于GTs家族1,它以尿苷二磷酸(Uridine diphosphate UDP) 活化的糖分子为供体,将葡萄糖、鼠李糖、半乳糖、阿拉伯糖、木糖和葡萄糖醛酸等添加至受体分子的特定位置[22]。植物中存在大量UGT基因,例如,已在拟南芥A. thaliana基因组中鉴定出107个UGT基因[45],其中UGT73C6、UGT78D1、UGT74F2和UGT75C1的功能已明确[46-48],但参与薯蓣皂素合成的UGT基因尚未报道[26]。已报道的UGTs序列同源性相对较低,蛋白结构高度相似。目前已有10个植物UGTs晶体结构被解析(表 1),这些植物UGTs晶体结构显示其均含有GT-B型折叠(GTs家族两种普遍折叠之一)。
表 1 已解析晶体结构的尿苷二磷酸糖基转移酶Table 1 The UGTs of resolved crystal structures
| Protein | Organism | GenBank | Uniprot | PDB |
| UGT72B1 | Arabidopsis thaliana | CAB8091.6 | Q04622 | 2VCE |
| UGT74F2 | Arabidopsis thaliana | AAB64024.1 | O22822 | 5U6M |
| UGT89C1 | Arabidopsis thaliana | AAF80123.1 | Q8LGD9 | 6IJ7 |
| UGT78K6 | Clitoria ternatea | BAF49297.1 | A4F1R4 | 3WC4 |
| UGT71G1 | Medicago truncatula | AAW56092.1 | Q5IFH7 | 2ACV |
| UGT85H2 | Medicago truncatula | ABE87250.1 | A6XNC5 | 2PQ6 |
| UGT78G1 | Medicago truncatula | ABI94025.1 | A6XNC6 | 3HBF |
| UGT74AC1 | Siraitia grosvenorii | AEM42999.1 | – | 6L8W |
| UGT76G1 | Stevia rebaudiana | AAR06912.1 | Q6VAB4 | 6INF |
| VvGT1 | Vitis vinifera | AAB81683.1 | O22304 | 2C1X |
表选项
这种GT-B型折叠包含与Rossmann折叠结构类似的两个C-末端和两个N-末端结构域。每个结构域包含一个携带双侧α螺旋的β折叠。UGTs的N-和C-末端结构域都形成可容纳底物结合的凹陷。核苷酸糖供体与UGTs C-末端结构域结合,受体分子与N-末端结构域结合。不同UGTs C-末端结构域高度相似,包含保守序列PSPG (Plant secondary product glycosyltransferase)。PSPG序列含44个氨基酸残基,核苷酸糖供体主要与此基序中的残基作用[49]。UDP-葡萄糖(UDP-glucose,UDP-glu) 主要与位于PSPG最后一位的谷氨酰胺互作[50]。PSPG关键残基对特异性供体的识别至关重要。Han等将UGT78D3的PSPG基序进行单点突变(H380Q),UGT78D3由识别UDP-阿拉伯糖转为识别UDP-glu和UDP-木糖[51]。不同UGTs N-末端结构域差异较大,可结合类型多样的糖基受体。结构修饰N-末端可影响UGTs催化效率和糖基化位点。以山奈酚(Kaempferol) 为受体,单点突变(I305T) 使UGT85H2催化效率提高37倍[52]。通过域交换策略(Domain-swapping) 可设计酶嵌合体,改变酶特性。Cartwright等设计了UGT74F1与UGT74F2嵌合体,改变了针对槲皮素(Quercetin) 的糖基化位点[53]。基于结构酶工程策略,设计突变体与嵌合体,可改变UGTs的催化效率、底物特异性和区域选择性。
3 异源合成薯蓣皂素的途径设计与改造策略异源合成薯蓣皂素需首先选择合适宿主。拟南芥和烟草遗传转化体系成熟,是理想表达宿主。Schnee等在拟南芥中异源表达玉米萜烯合酶(TPS10),成功合成了(E)-β-金合欢烯((E)-beta- farnesene)[54]。在烟草中也成功构建萜类[55]、黄酮类[56]等植物次生代谢产物合成途径。模式植物虽可为植物次生代谢产物的异源合成提供相似细胞环境,但生长缓慢、代谢复杂。藻类也是理想的表达宿主,可通过光合作用固定CO2并为CYP450s代谢通路提供还原力,且同时存在MVA和MEP途径(其他异养微生物体内只存在其中一条),但光合固碳的效率低,严重制约次级产物的合成能力[57-59]。因此,可选用生长快且易培养的大肠杆菌和酿酒酵母。目前大肠杆菌已用于生产青蒿素前体紫穗槐二烯[11]、紫杉烯[13]、β-胡萝卜素[14]等高值药物。与大肠杆菌相比,酿酒酵母可为CYP450s提供内质网等内膜系统,以便参与次生代谢产物的后修饰步骤。同时,作为模式真核生物,酿酒酵母异源表达植物源基因有更好的适配性。Paddon等利用酿酒酵母生产青蒿酸,产量达25 g/L[12]。Galanie等在酿酒酵母中成功合成二甲基吗啡(Thebaine) 和氢可酮(Hydrocodone)[60]。科学家虽已深入探讨薯蓣皂素的上游合成途径,但胆固醇的下游修饰步骤仍缺乏详细研究。根据植物基因组和转录组数据库,利用诸如plantiSMASH[61]和1KP Project[62]计算方法分析不同植株表达差异、挖掘关键基因使得在酿酒酵母中构建薯蓣皂素全生物合成途径成为可能[16, 63] (图 4)。目前已构建多个平台菌。Meadows等构建了高产FPP酿酒酵母(130 g/L)[64],Souza等构建了产胆固醇的酿酒酵母菌株RH6829[63],二者均可作为薯蓣皂素的生产宿主。
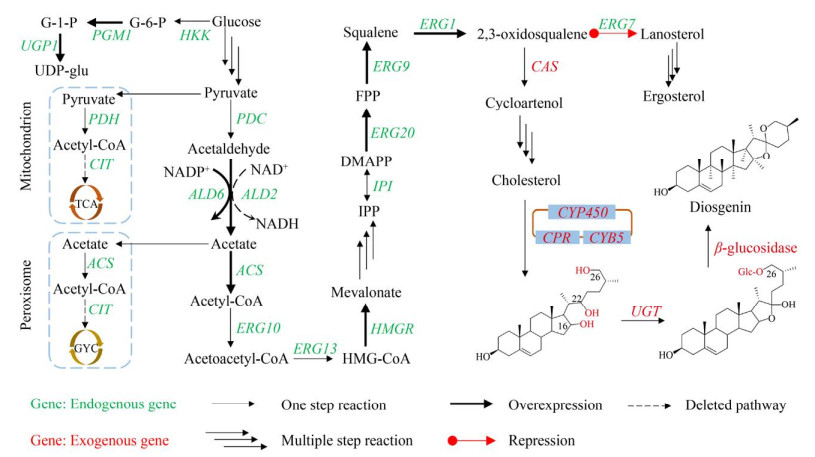 |
| 图 4 酿酒酵母中构建薯蓣皂素合成途径 Fig. 4 Protocol for engineering biosynthetic pathway of diosgenin in S. cerevisiae. G6P: glucose 6-phosphate; G1P: glucose 1-phosphate; PDH: pyruvate dehydrogenase; PDC: pyruvate decaboxylase. |
| 图选项 |
薯蓣皂素的异源合成途经长,需保证多步骤间整体催化效率,而实现高催化效率需充足的底物供应。Ac-CoA是合成薯蓣皂素的前体物,在酵母菌中过表达合成Ac-CoA的关键基因可增加其产量。Zhao等过表达NADP依赖型醛脱氢酶基因ALD6,并引入肠道沙门氏菌Salmonella enterica的Ac-CoA合成酶基因ACSseL641P(Acetyl-CoA synthase),提高了Ac-CoA产量[65]。酵母菌细胞质产生的Ac-CoA可进入三羧酸循环(Tricarboxylic acid cycle,TCA) 和过氧化物酶体乙醛酸循环(Glyoxylate cycle,GYC)。敲除柠檬酸合酶基因CIT (Citrate synthase) 可抑制Ac-CoA进入TCA和GYC循环,促使Ac-CoA流向MVA途径[66]。UDP-glu是合成薯蓣皂素的关键底物。葡萄糖由己糖激酶(Hexokinase,HXK1)、葡萄糖磷酸变位酶(Phosphoglucomutase,PGM1) 和葡萄糖-1-磷酸尿苷转移酶(Glucose-1 phosphate uridylyltransferase,UGP1) 催化,产生UDP-glu。过表达PGM1和UGP1可提高UDP-glu产量[67]。维持高催化效率还需要充足的辅因子。NADPH是生物合成薯蓣皂素的主要辅因子。乙醛脱氢酶(Aldehyde dehydrogenases) 催化乙醛转化为乙酸,由ALD6或ALD2编码。ALD6以NADP+为辅因子,ALD2以NAD+为辅因子[68]。Kim等在酿酒酵母中过表达ALD6并敲除ALD2,提高了细胞质中NADPH的浓度,目标产物原人参二醇(Protopanaxadiol) 产量随之提高[69]。维持高催化效率还需抑制有毒中间体的产生。设计敏感调控元件,响应细胞内外有毒中间体的浓度变化,可动态调控代谢流。Dahl等分析鉴定出对有毒中间体FPP敏感的启动子,并将其整合到紫穗槐二烯异源表达途径中。筛选的启动子可对FPP上下游途径实施反馈调节,使得FPP积累减少,紫穗槐二烯产量提高2倍[70]。
4 总结与展望薯蓣皂素作为数百种类固醇药物的前体,具有重要药用价值。由于化学提取法作为现今其主要生产手段,存在产率较低且污染环境等问题,所以生物合成法日益受到关注。目前,薯蓣皂素的基因工程全合成鲜有报道。植物宿主生长缓慢,代谢网络复杂,改造难度大;酵母作为宿主虽有成功案例,但各步骤间适配性差,产量低,无法工业化生产[16]。因此,需利用代谢工程和合成生物学最新技术手段对薯蓣皂素的生物合成进行系统研究。迄今面临的困难主要有以下几点:(1) 关键酶的催化效率低;(2) 产物合成途径长;(3) 异源途径与底盘细胞的适配性差;(4) 目的产物或中间代谢物积累造成胁迫,限制产量的提高。
为构建稳定高效的微生物细胞工厂,需从酶、途径、代谢网络、细胞等不同维度研究其适配与平衡的关系。因此提出以下研究方向:(1) 利用结构生物学和计算生物学手段,筛选并建立关键酶库(如CYP450s和UGTs)。通过比较不同酶的催化差异,揭示关键酶催化效率和专一性的分子机制,进而建立理性设计酶分子的策略。(2) 针对多步催化的长合成途径,建立并完善基因组整合机制。利用CRISPR和Red重组等技术对宿主基因组进行编辑,构建整合位点库,为长途径的分散高效组装奠定基础[71-72]。(3) 探究异源途径在底盘细胞中的最优表达策略,利用N-末端工程、信号肽工程实现胞内关键酶的空间定位[73];建立以RNA聚合酶与核糖体为枢纽的正交表达系统,将外源途径与底盘细胞的自身代谢网络分割开来,实现细胞资源在不同途径间的合理分配[74]。(4) 针对某些中间代谢物或终产物的细胞毒性大且造成反馈抑制等问题,开发代谢物传质及转运技术,可利用蛋白支架将多步酶捆绑,强化中间物的转化效率;同时设计转运蛋白,以减少胞内代谢物积累,解除胁迫效应。随着代谢工程及合成生物学策略的运用,薯蓣皂素的大规模发酵生产必将实现。
参考文献
| [1] | Ge L, Guo LC, Yang KD, et al. Solubility of diosgenin in several imidazolium-based ionic liquids. J Chem Eng Data, 2015, 60(1): 11-15. DOI:10.1021/je5004324 |
| [2] | Zhang XX, Wang XB, Khurm M, et al. Alterations of brain quantitative proteomics profiling revealed the molecular mechanisms of diosgenin against cerebral ischemia reperfusion effects. J Proteome Res, 2020, 19(3): 1154-1168. DOI:10.1021/acs.jproteome.9b00667 |
| [3] | Cai BR, Zhang Y, Wang ZT, et al. Therapeutic potential of diosgenin and its major derivatives against neurological diseases: recent advances. Oxidat Med Cell Longev, 2020, 2020: 3153082. |
| [4] | Shaikh S, Shriram V, Khare T, et al. Biotic elicitors enhance diosgenin production in Helicteres isora L. suspension cultures via up-regulation of CAS and HMGR genes. Physiol Mol Biol Plants, 2020, 26(3): 593-604. DOI:10.1007/s12298-020-00774-6 |
| [5] | Sautour M, Mitaine-Offer AC, Lacaille-Dubois MA. The dioscorea genus: a review of bioactive steroid saponins. J Nat Med, 2007, 61: 91-101. DOI:10.1007/s11418-006-0126-3 |
| [6] | Dong MX, Meng ZF, Kuerban K, et al. Diosgenin promotes antitumor immunity and PD-1 antibody efficacy against melanoma by regulating intestinal microbiota. Cell Death Dis, 2018, 9(10): 1039. DOI:10.1038/s41419-018-1099-3 |
| [7] | Zhang R, Wen L, Shen Y, et al. One compound of saponins from Disocorea zingiberensis protected against experimental acute pancreatitis by preventing mitochondria-mediated necrosis. Sci Rep, 2016, 6: 35965. DOI:10.1038/srep35965 |
| [8] | Huang CH, Liu DZ, Jan TR. Diosgenin, a plant-derived sapogenin, enhances regulatory T-cell immunity in the intestine of mice with food allergy. J Nat Prod, 2010, 73(6): 1033-1037. DOI:10.1021/np900690z |
| [9] | Zhang XL, Han X, Yin LH, et al. Potent effects of dioscin against liver fibrosis. Sci Rep, 2015, 5: 9713. DOI:10.1038/srep09713 |
| [10] | Jiang GZ, Yao MD, Wang Y, et al. Manipulation of GES and ERG20 for geraniol overproduction in Saccharomyces cerevisiae. Metab Eng, 2017, 41: 57-66. DOI:10.1016/j.ymben.2017.03.005 |
| [11] | Tsuruta H, Paddon CJ, Eng D, et al. High-level production of amorpha-4, 11-diene, a precursor of the antimalarial agent artemisinin, in Escherichia coli. PLoS ONE, 2009, 4(2): e4489. DOI:10.1371/journal.pone.0004489 |
| [12] | Paddon CJ, Westfall PJ, Pitera DJ, et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature, 2013, 496(7446): 528-532. DOI:10.1038/nature12051 |
| [13] | Ajikumar PK, Xiao WH, Tyo KEJ, et al. Isoprenoid pathway optimization for taxol precursor overproduction in Escherichia coli. Science, 2010, 330(6000): 70-74. DOI:10.1126/science.1191652 |
| [14] | Yang JM, Guo LZ. Biosynthesis of β-carotene in engineered E. coli using the MEP and MVA pathways. Microb Cell Fact, 2014, 13: 160. DOI:10.1186/s12934-014-0160-x |
| [15] | Li J, Liang Q, Li CF, et al. Comparative transcriptome analysis identifies putative genes involved in dioscin biosynthesis in Dioscorea zingiberensis. Molecules, 2018, 23(2): 454. DOI:10.3390/molecules23020454 |
| [16] | Christ B, Xu CC, Xu ML, et al. Repeated evolution of cytochrome P450-mediated spiroketal steroid biosynthesis in plants. Nat Commun, 2019, 10: 3206. DOI:10.1038/s41467-019-11286-7 |
| [17] | Miettinen K, Pollier J, Buyst D, et al. The ancient CYP716 family is a major contributor to the diversification of eudicot triterpenoid biosynthesis. Nat Commun, 2017, 8: 14153. DOI:10.1038/ncomms14153 |
| [18] | Schaller H. New aspects of sterol biosynthesis in growth and development of higher plants. Plant Physiol Biochem, 2004, 42(6): 465-476. DOI:10.1016/j.plaphy.2004.05.012 |
| [19] | Wang X, Chen DJ, Wang YQ, et al. De novo transcriptome assembly and the putative biosynthetic pathway of steroidal sapogenins of Dioscorea composita. PLoS ONE, 2015, 10(4): e0124560. DOI:10.1371/journal.pone.0124560 |
| [20] | Vranová E, Coman D, Gruissem W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu Rev Plant Biol, 2013, 64: 665-700. DOI:10.1146/annurev-arplant-050312-120116 |
| [21] | Jung JD, Park HW, Hahn Y, et al. Discovery of genes for ginsenoside biosynthesis by analysis of ginseng expressed sequence tags. Plant Cell Rep, 2003, 22(3): 224-230. DOI:10.1007/s00299-003-0678-6 |
| [22] | Thimmappa R, Geisler K, Louveau T, et al. Triterpene biosynthesis in plants. Annual Rev Plant Biol, 2014, 65: 225-257. DOI:10.1146/annurev-arplant-050312-120229 |
| [23] | Mohammadi M, Mashayekh T, Rashidi-Monfared S, et al. New insights into diosgenin biosynthesis pathway and its regulation in Trigonella foenum-graecum L. Phytochem Anal, 2020, 31(2): 229-241. DOI:10.1002/pca.2887 |
| [24] | Yin Y, Gao LH, Zhang XA, et al. A cytochrome P450 monooxygenase responsible for the C-22 hydroxylation step in the Paris polyphylla steroidal saponin biosynthesis pathway. Phytochemistry, 2018, 156: 116-123. DOI:10.1016/j.phytochem.2018.09.005 |
| [25] | Sonawane PD, Pollier J, Panda S, et al. Plant cholesterol biosynthetic pathway overlaps with phytosterol metabolism. Nat Plants, 2016, 3: 16205. |
| [26] | Zhou C, Li XH, Zhou ZL, et al. Comparative transcriptome analysis identifies genes involved in diosgenin biosynthesis in Trigonella foenum-graecum L. Molecules, 2019, 24(1): 140. DOI:10.3390/molecules24010140 |
| [27] | Mehrafarin A, Qaderi A, Rezazadeh S, et al. Bioengineering of important secondary metabolites and metabolic pathways in fenugreek (Trigonella foenum-graecum L.). J Med Plants, 2010, 9(35): 1-18. |
| [28] | Segura MJR, Lodeiro S, Meyer MM, et al. Directed evolution experiments reveal mutations at cycloartenol synthase residue His477 that dramatically alter catalysis. Org Lett, 2002, 4(25): 4459-4462. DOI:10.1021/ol0269897 |
| [29] | Wu TK, Griffin JH. Conversion of a plant oxidosqualene-cycloartenol synthase to an oxidosqualene-lanosterol cyclase by random mutagenesis. Biochemistry, 2002, 41(26): 8238-8244. DOI:10.1021/bi0200920 |
| [30] | Lodeiro S, Schulz-Gasch T, Matsuda SP. Enzyme redesign: two mutations cooperate to convert cycloartenol synthase into an accurate lanosterol synthase. J Am Chem Soc, 2005, 127(41): 14132-14133. DOI:10.1021/ja053791j |
| [31] | Kolesnikova MD, Xiong QB, Lodeiro S, et al. Lanosterol biosynthesis in plants. Arch Biochem Biophys, 2006, 447(1): 87-95. DOI:10.1016/j.abb.2005.12.010 |
| [32] | Souza-Moreira TM, Alves TB, Pinheiro KA, et al. Friedelin synthase from Maytenus ilicifolia: leucine 482 plays an essential role in the production of the most rearranged pentacyclic triterpene. Sci Rep, 2016, 6: 36858. DOI:10.1038/srep36858 |
| [33] | Ito R, Masukawa Y, Hoshino T. Purification, kinetics, inhibitors and CD for recombinant β-amyrin synthase from Euphorbia tirucalli L. and functional analysis of the DCTA motif, which is highly conserved among oxidosqualene cyclases. FEBS J. FEBS J, 2013, 280(5): 1267-1280. DOI:10.1111/febs.12119 |
| [34] | Thoma R, Schulz-Gasch T, D'Arcy B, et al. Insight into steroid scaffold formation from the structure of human oxidosqualene cyclase. Nature, 2004, 432(7013): 118-122. DOI:10.1038/nature02993 |
| [35] | Baker MAB, Brown AJ. A detour to sterol synthesis. Nat Microbiol, 2019, 4(2): 214-215. DOI:10.1038/s41564-018-0347-8 |
| [36] | Kim YK, Kim YB, Uddin MR, et al. Enhanced triterpene accumulation in Panax ginseng hairy roots overexpressing mevalonate-5-pyrophosphate decarboxylase and farnesyl pyrophosphate synthase. ACS Synth Biol, 2014, 3(10): 773-779. DOI:10.1021/sb400194g |
| [37] | Dai ZB, Liu Y, Zhang XA, et al. Metabolic engineering of Saccharomyces cerevisiae for production of ginsenosides. Metab Eng, 2013, 20: 146-156. DOI:10.1016/j.ymben.2013.10.004 |
| [38] | Biggs BW, Lim CG, Sagliani K, et al. Overcoming heterologous protein interdependency to optimize P450-mediated taxol precursor synthesis in Escherichia coli. Proc Natl Acad Sci USA, 2016, 113(12): 3209-3214. DOI:10.1073/pnas.1515826113 |
| [39] | Li SJ, Li YR, Smolke CD. Strategies for microbial synthesis of high-value phytochemicals. Nat Chem, 2018, 10(4): 395-404. DOI:10.1038/s41557-018-0013-z |
| [40] | Peterson JA, Ebel RE, O'Keeffe DH, et al. Temperature dependence of cytochrome P-450 reduction. A model for NADPH-cytochrome P-450 reductase: cytochrome P-450 interaction. J Biol Chem, 1976, 251(13): 4010-4016. DOI:10.1016/S0021-9258(17)33349-5 |
| [41] | Zhang HM, Im SC, Waskell L. Cytochrome b5 increases the rate of product formation by cytochrome P450 2B4 and competes with cytochrome P450 reductase for a binding site on cytochrome P450 2B4. J Biol Chem, 2007, 282(41): 29766-29776. DOI:10.1074/jbc.M703845200 |
| [42] | Hausjell J, Schendl D, Weissensteiner J, et al. Recombinant production of a hard-to-express membrane-bound cytochrome P450 in different yeasts—Comparison of physiology and productivity. Yeast, 2020, 37(2): 217-226. DOI:10.1002/yea.3441 |
| [43] | Nielsen AZ, Ziersen B, Jensen K, et al. Redirecting photosynthetic reducing power toward bioactive natural product synthesis. ACS Synth Biol, 2013, 2(6): 308-315. DOI:10.1021/sb300128r |
| [44] | Mellor SB, Vinde MH, Nielsen AZ, et al. Defining optimal electron transfer partners for light-driven cytochrome P450 reactions. Metab Eng, 2019, 55: 33-43. DOI:10.1016/j.ymben.2019.05.003 |
| [45] | Li Y, Baldauf S, Lim EK, et al. Phylogenetic analysis of the UDP-glycosyltransferase multigene family of Arabidopsis thaliana. J Biol Chem, 2001, 276(6): 4338-4343. DOI:10.1074/jbc.M007447200 |
| [46] | Jones P, Messner B, Nakajima JI, et al. UGT73C6 and UGT78D1, Glycosyltransferases involved in flavonol glycoside biosynthesis in Arabidopsis thaliana. J Biol Chem, 2003, 278(45): 43910-43918. DOI:10.1074/jbc.M303523200 |
| [47] | Quiel JA, Bender J. Glucose conjugation of anthranilate by the Arabidopsis UGT74F2 glucosyltransferase is required for tryptophan mutant blue fluorescence. J Biol Chem, 2003, 278(8): 6275-6281. DOI:10.1074/jbc.M211822200 |
| [48] | Tohge T, Nishiyama Y, Hirai MY, et al. Functional genomics by integrated analysis of metabolome and transcriptome of Arabidopsis plants over-expressing an MYB transcription factor. Plant J, 2005, 42(2): 218-235. DOI:10.1111/j.1365-313X.2005.02371.x |
| [49] | Wang XQ. Structure, mechanism and engineering of plant natural product glycosyltransferases. FEBS Lett, 2009, 583(20): 3303-3309. DOI:10.1016/j.febslet.2009.09.042 |
| [50] | Caputi L, Malnoy M, Goremykin V, et al. A genome-wide phylogenetic reconstruction of family 1 UDP-glycosyltransferases revealed the expansion of the family during the adaptation of plants to life on land. Plant J, 2012, 69(6): 1030-1042. DOI:10.1111/j.1365-313X.2011.04853.x |
| [51] | Han SH, Kim BG, Yoon JA, et al. Synthesis of flavonoid O-pentosides by Escherichia coli through engineering of nucleotide sugar pathways and glycosyltransferase. Appl Environ Microbiol, 2014, 80(9): 2754-2762. DOI:10.1128/AEM.03797-13 |
| [52] | Modolo LV, Escamilla-Trevi?o LL, Dixon RA, et al. Single amino acid mutations of medicago glycosyltransferase UGT85H2 enhance activity and impart reversibility. FEBS Lett, 2009, 583(12): 2131-2135. DOI:10.1016/j.febslet.2009.05.046 |
| [53] | Cartwright AM, Lim EK, Kleanthous C, et al. A kinetic analysis of regiospecific glucosylation by two glycosyltransferases of Arabidopsis thaliana: domain swapping to introduce new activities. J Bio Chem, 2008, 283(23): 15724-15731. DOI:10.1074/jbc.M801983200 |
| [54] | Schnee C, K?llner TG, Held M, et al. The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc Natl Acad Sci USA, 2006, 103(4): 1129-1134. DOI:10.1073/pnas.0508027103 |
| [55] | Brückner K, Tissier A. High-level diterpene production by transient expression in Nicotiana benthamiana. Plant Methods, 2013, 9: 46. DOI:10.1186/1746-4811-9-46 |
| [56] | Zhang HB, Wang L, Hunter D, et al. A Narcissus mosaic viral vector system for protein expression and flavonoid production. Plant Methods, 2013, 9: 28. DOI:10.1186/1746-4811-9-28 |
| [57] | Jaramillo-Madrid AC, Ashworth J, Fabris M, et al. Phytosterol biosynthesis and production by diatoms (Bacillariophyceae). Phytochemistry, 2019, 163: 46-57. DOI:10.1016/j.phytochem.2019.03.018 |
| [58] | Fabris M, George J, Kuzhiumparambil U, et al. Extrachromosomal genetic engineering of the marine diatom Phaeodactylum tricornutum enables the heterologous production of monoterpenoids. ACS Synth Biol, 2020, 9(3): 598-612. DOI:10.1021/acssynbio.9b00455 |
| [59] | Gallo C, Landi S, D'Ippolito G, et al. Diatoms synthesize sterols by inclusion of animal and fungal genes in the plant pathway. Sci Rep, 2020, 10: 4204. DOI:10.1038/s41598-020-60993-5 |
| [60] | Galanie S, Thodey K, Trenchard IJ, et al. Complete biosynthesis of opioids in yeast. Science, 2015, 349(6252): 1095-1100. DOI:10.1126/science.aac9373 |
| [61] | Kautsar SA, Duran HGS, Blin K, et al. PlantiSMASH: automated identification, annotation and expression analysis of plant biosynthetic gene clusters. Nucleic Acids Res, 2017, 45(W1): W55-W63. DOI:10.1093/nar/gkx305 |
| [62] | Matasci N, Hung LH, Yan ZX, et al. Data access for the 1 000 Plants (1KP) project. Gigascience, 2014, 3: 17. DOI:10.1186/2047-217X-3-17 |
| [63] | Souza CM, Schwabe TME, Pichler H, et al. A stable yeast strain efficiently producing cholesterol instead of ergosterol is functional for tryptophan uptake, but not weak organic acid resistance. Metab Eng, 2011, 13(5): 555-569. DOI:10.1016/j.ymben.2011.06.006 |
| [64] | Meadows AL, Hawkins KM, Tsegaye Y, et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production. Nature, 2016, 537(7622): 694-697. DOI:10.1038/nature19769 |
| [65] | Zhao FL, Bai P, Nan WH, et al. A modular engineering strategy for high-level production of protopanaxadiol from ethanol by Saccharomyces cerevisiae. AIChE J, 2019, 65(3): 866-874. DOI:10.1002/aic.16502 |
| [66] | Chen Y, Bao JC, Kim IK, et al. Coupled incremental precursor and co-factor supply improves 3-hydroxypropionic acid production in Saccharomyces cerevisiae. Metab Eng, 2014, 22: 104-109. DOI:10.1016/j.ymben.2014.01.005 |
| [67] | Zhuang Y, Yang GY, Chen XH, et al. Biosynthesis of Plant-derived ginsenoside Rh2 in yeast via repurposing a key promiscuous microbial enzyme. Metab Eng, 2017, 42: 25-32. DOI:10.1016/j.ymben.2017.04.009 |
| [68] | Minard KI, McAlister-Henn L. Sources of NADPH in yeast vary with carbon source. J Bio Chem, 2005, 280(48): 39890-39896. DOI:10.1074/jbc.M509461200 |
| [69] | Kim JE, Jang IS, Sung BH, et al. Rerouting of NADPH synthetic pathways for increased protopanaxadiol production in Saccharomyces cerevisiae. Sci Rep, 2018, 8: 15820. DOI:10.1038/s41598-018-34210-3 |
| [70] | Dahl RH, Zhang FZ, Alonso-Gutierrez J, et al. Engineering dynamic pathway regulation using stress-response promoters. Nat Biotechnol, 2013, 31(11): 1039-1046. DOI:10.1038/nbt.2689 |
| [71] | Wu XL, Li BZ, Zhang WZ, et al. Genome-wide landscape of position effects on heterogeneous gene expression in Saccharomyces cerevisiae. Biotechnol Biofuels, 2017, 10: 189. DOI:10.1186/s13068-017-0872-3 |
| [72] | Li SW, Ding WT, Zhang XL, et al. Development of a modularized two-step (M2S) chromosome integration technique for integration of multiple transcription units in Saccharomyces cerevisiae. Biotechnol Biofuels, 2016, 9: 232. DOI:10.1186/s13068-016-0645-4 |
| [73] | Liu GS, Li T, Zhou W, et al. The yeast peroxisome: a dynamic storage depot and subcellular factory for squalene overproduction. Metab Eng, 2019, 57: 151-161. |
| [74] | Zhao P, Ren MR, Ge XZ, et al. Development of orthogonal T7 expression system in Klebsiella pneumoniae. Biotechnol Bioeng, 2020, 117(8): 2446-2459. DOI:10.1002/bit.27434 |
