

中国农业科学院 北京畜牧兽医研究所,北京 100193
摘要:miRNA 是近年来发现的一类长约22 nt的内源性非编码RNA,在动物中主要通过抑制靶mRNA翻译,在转录后水平调控基因表达。大量研究表明脂肪组织中的miRNAs参与了脂肪细胞分化、脂代谢等多种生物过程调控,其自身也受到转录因子、脂肪细胞因子和环境因子等调控,这些复杂的相互作用关系构成了脂肪组织中miRNA的调控网络,循环miRNA的发现为这个网络加入了新元素。对肥胖等代谢疾病的研究,应该从这个复杂的动态网络中寻找答案。文中综述了脂肪组织中miRNA的最新研究进展,以期为利用miRNA进行肥胖等相关代谢失调疾病的治疗提供新思路。
关键词: miRNA脂肪分化脂代谢循环miRNA
Recent advances of miRNAs in adipose tissues
Yuntao Guo, Xiuxiu Zhang, Wanlong Huang, Xiangyang Miao


Institute of Animal Sciences, Chinese Academy of Agricultural Sciences, Beijing 100193, China
Received: April 9, 2015; Accepted: June 2, 2015
Supported by: The Major Science and Technology Project of New Variety Breeding of Genetically Modified Organisms (Nos.2009ZX08008-004B, 2008ZX08008-003), National High Technology Research Development Program of China (863 Program) (No. 2008AA10Z140), National Natural Science Foundation of China (No. 30571339), the Innovation Research Foundation of CAAS (No. 2004-CAAS-1), the Basic Research Fund for Central Public Research Institutes of CAAS (Nos. 2013ywf-yb-5, 2013ywf-zd-2), the Agricultural Science and Technology Innovation Program (No. ASTIP-IAS05).
Corresponding authors: Xiangyang Miao. Tel: +86-10-62895663; E-mail: mxy32@sohu.com
Abstract: microRNAs (miRNAs), a class of endogenous non-coding RNA about 22 nucleotide long, regulate gene expression at the post-transcription level by inhibiting the translation or inducing the degradation of their target mRNAs in organisms. A lot of studies reveal that miRNAs in adipose tissues are involved in adipocyte differentiation and lipid metabolism and modulated by multiple transcription factors, adipocytokines and environmental factors, which form a complex regulatory network maintaining the homeostasis of adipose tissues. The discovery of circulating miRNAs adds new elements to the regulatory network. To study the metabolic diseases such as obesity, we should keep a new insight into the complex dynamic network. In this review, we summarize the latest studies of miRNAs in adipose tissues, which might provide new strategies for the treatment of obesity and other related diseases.
Keywords: miRNAadiposedifferentiationlipid metabolismcirculating miRNA
哺乳动物体内的脂肪组织主要分布在皮下、肌肉、腹部和内脏等部位,由脂肪细胞、丰富的血管和神经组成,它可以将体内过剩的能量以甘油三酯的形式贮存下来,起着缓冲、维持体温和贮存能量等重要作用。然而,近年来随着人们生活水平的提高,高脂食物的过量摄入以及不良的生活习惯导致超重和肥胖人群的数目越来越大,究其原因是脂肪组织的过度积聚,其细胞水平的表现就是脂肪细胞体积增大和数目增加。肥胖引起的机体能量代谢失调导致了一系列相关慢性疾病如Ⅱ型糖尿病、高血压和心脑血管疾病等的发生,已成为威胁人类健康的重大隐患。microRNA (miRNA,微RNA) 是一类小分子非编码RNA,可以在转录后水平调控基因表达。已有研究表明[1],miRNA参与了脂代谢、脂肪细胞分化、能量稳态、葡萄糖刺激的胰岛素分泌和炎症反应等多种生物学过程,研究脂肪组织中的miRNA及其作用机制对于解决肥胖等代谢疾病有重要意义。本文综述了脂肪组织中有代表性的miRNA在脂肪细胞分化和脂代谢中功能的最新研究,并对循环miRNA和影响脂肪组织中miRNA功能行使的因素进行阐述,构建了脂肪组织中miRNA的调控网络,为利用miRNA进行肥胖等相关疾病的预防和治疗提供新思路。
1 miRNA简介 miRNA是在多种真核细胞和病毒中发现的一类长约21-22 nt的内源性非编码单链RNA,通过特异性碱基互补的方式与靶基因mRNA的3'-UTR结合,抑制靶mRNA翻译或诱导其降解,从而在转录后水平调控基因的表达。miRNA的产生包括以下几个过程:1) 编码miRNA的基因在RNA聚合酶Ⅱ的作用下转录形成初级转录本pri-miRNA;2) Pri-miRNA在Drosha/DGCR8复合体的切割下形成miRNA前体pre-miRNA;3) Pre-miRNA被Exportin5从细胞核转运到细胞质;4) Pre-miRNA被Dicer酶加工成双链成熟miRNA,随后双链解旋,形成单链成熟miRNA。miRNA的产生过程受到了许多转录因子调控,随后成熟单链miRNA形成沉默诱导复合体RISC,抑制或者降解靶mRNA。自从第一个miRNA lin-4在线虫中被发现以来,越来越多的miRNA被鉴定出来,截止到2014年6月,miRbase (http://www.mirbase.org/) 收录的miRNA条目已达28 645条,并且数目在逐年增长。miRNA参与了各种生命过程的调控,包括细胞的增殖、分化和物质代谢等,在生物体生长、发育和疾病发生等过程中扮演着重要角色[2]。
2 miRNA调控脂肪细胞分化 脂肪细胞由间充质干细胞 (Mesenchymal stem cells,MSCs) 分化而来。MSCs首先经历细胞系定型,确定向脂肪细胞分化,随后形成的前体脂肪细胞经历克隆增殖、生长停滞和终末分化形成成熟的脂肪细胞,在这一过程中pRB-E2F、MAPK、SMAD/TGFβ和WNT等关键信号通路以及CCAAT/增强子结合蛋白 (CCAAT/enhancer-binding proteins,C/EBPs) 和过氧化物酶体增殖物激活受体 (Peroxisome proliferator-activated receptors,PPARs) 等核心转录因子发挥了重要的调控作用。2004年,Esau等[3]发现miR-143可以通过其靶基因蛋白激酶5 (Mitogen-activated protein kinase 5,MAP2K5) 促进脂肪细胞分化,自此第一个影响脂肪细胞分化的miRNA被鉴定出来。随后,越来越多的参与脂肪细胞分化miRNA如let-7、miR-17-92等被陆续发现,这些研究结果表明,miRNA对脂肪细胞分化调节的实质是靶向作用于细胞分化过程中相关通路 (如pRB-E2F、MAPK、SMAD/TGFβ和WNT等) 的分子组件进而影响下游转录因子转录活动,或者是直接作用于关键转录因子 (如C/EBPs和PPARs等),从而转录调控脂肪酸结合蛋白4 (Fatty acid binding protein 4,FABP4)、脂肪酸合成酶 (Fatty acid synthase,FASN)、硬脂酰辅酶A去饱和酶 (Stearoyl-CoA desaturase,SCD) 和葡萄糖转运蛋白4 (Glucose transporter 4,GLUT4) 等脂肪相关基因的表达,影响脂肪细胞分化。
miR-143是第一个发现的促进脂肪细胞分化的miRNA。Esau等[3]运用芯片技术分析了miRNA在人脂肪细胞中的表达情况,鉴定出miR-143能促进脂肪细胞的分化,进一步分析表明,miR-143对脂肪细胞分化的促进作用是通过其靶基因MAP2K5实现的。Yi等[4]也发现miR-143可以通过抑制其靶基因多效生长因子 (Pleiotrophin,PTN) 的表达促进脂肪细胞分化。Takanabe等[5]用高能量饲料喂养构建的肥胖小鼠模型中miR-143出现上调表达,导致小鼠体重和脂肪的增加,这些现象表明miR-143不仅在细胞水平参与了脂肪细胞的分化,在成体时也同样参与了脂质的积累调控。Chen等[6]对人脂肪基质细胞 (Human adipose-derived mesenehymal stem cells,hADSCs) 不同分化时序中miR-143的作用进行研究时发现,在不同的分化阶段将miR-143转染进hADSCs细胞会发挥不同的作用。在有丝分裂克隆增殖阶段转染会抑制脂肪细胞分化,而在生长停滞或终末分化阶段转染时却会促进分化,说明miR-143对脂肪细胞分化的调控作用因分化阶段不同而异。总之,miR-143可以通过MAPK信号通路中的分子组件调节脂肪细胞分化。
Wang等[7]研究发现,在小鼠3T3L1前体脂肪细胞分化前的克隆扩增阶段,miR-17-92簇上调表达。在激素的诱导下,miR-17-92过表达加速了3T3L1前体脂肪细胞分化,增加了甘油三脂积聚。后续的荧光素酶报告实验表明肿瘤抑制因子成视网膜细胞瘤样蛋白2 (Retinoblastoma-like protein 2,Rb2/P130) 是miR-17-92的直接靶基因,在有丝分裂克隆增殖阶段下调表达。siRNA介导的Rb2/P130抑制重现了miR-17-92过表达的表型。针对这些现象推测:在激素诱导后3T3L1前体脂肪细胞中,miR-17-92表达量升高,抑制了其靶基因Rb2/P130表达,致使没有足够的Rb2/P130与转录因子E2F (E2F transcription factor,E2F) 组成二聚体来抑制E2F,活化状态的E2F4和E2F5增加,激活了pRB-E2F信号通路,启动细胞进入下一个周期。在另外一项研究中,Chen等[8]用芯片技术研究大鼠ADSCs向成熟脂肪细胞分化时miRNA的表达谱时发现:miR-363通过转录后抑制其靶基因E2F3的翻译水平,激活了成视网膜瘤蛋白信号通路pRB-E2F,进而抑制了周期蛋白E (Cyclin E,CYCE) 的表达并阻止细胞从G1向S期的转变,从而抑制了脂肪细胞分化中的克隆增殖过程,同时也引起基因C/EBPα表达下调,抑制了细胞终末分化。miR-17-92和miR-363都通过pRB-E2F信号通路调控了脂肪细胞分化,二者的区别是前者促进分化,后者抑制分化。
Kim等[9]在研究hADSCs向脂肪细胞分化的机制时发现miR-21出现了上调表达,过表达miR-21后hADSCs细胞的成脂分化被加强,抑制miR-21得到了相反的结果。进一步的研究证明,在诱导成脂分化时miR-21的上调表达抑制了其靶基因转化生长因子β受体2 (Transforming growth factor beta receptor 2,TGFβR2),进而降低了TGFβ/SMAD信号通路下游信号分子母系抗皮肤生长因子3 (Mothers against decapentaplegic homolog 3,SMAD3) 的磷酸化,减弱了TGFβ/SMAD信号通路对脂肪分化的抑制作用,随后的RNAi干扰SMAD3基因表达实验验证了这一点。miR-21通过抑制其靶基因TGFβR2,调控了TGFβ/SMAD信号通路,是白色脂肪细胞分化的一个正调控因子。有****利用MDI诱导和LiCl处理3T3L1前体脂肪细胞,分化成熟后,分别得到了WNT/β抑制和激活两个细胞模型,然后利用芯片研究两个细胞中差异表达miRNA,筛选出的miR-210可以通过抑制其靶基因转录因子TCF7L2 (Transcription factor 7-like 2,TCF7L2) 调控WNT信号通路,进而调控脂肪生成。
miR-143、miR-17-92、miR-363、miR-21和miR-210分别通过对MAPK、pRB-E2F、TGFβ/SMAD和WNT信号通路中的靶标直接或间接作用调控了脂肪细胞分化,此外miR-27、miR-130、miR-146b和miR-31等通过直接靶向作用C/EBPα和PPARγ等脂肪细胞分化关键转录因子调控了这一过程,关于调控脂肪细胞分化的miRNAs的研究详见表1。
表1 调控脂肪细胞分化的miRNAsTable 1 miRNAs in the regulation of white adipocytes differentiation
| miRNAs | Target gene | Style | Cell model of pre-adipocytes | Species | References |
| miR-17-5p | BMPR2, BMP2 | + | ADSCs | H | [10] |
| miR-17-92 | RB2/p130 | + | 3T3L1 | M | [7] |
| miR-21 | TGFBR2 | + | C3H10T1/2 | H | [9] |
| miR-22 | HDAC6 | - | ADSCs | H | [11] |
| miR-33b | EBF1 | - | PSPA | P | [12] |
| miR-103 | MEF2D | + | 3T3L1 | M | [13] |
| miR-106a | BMP2 | + | ADSCs | H | [10] |
| miR-124 | DLX5 | + | 3T3L1 | M | [14] |
| miR-128 | ABCA1, ABCG1 | + | HEK293T, HepG2, MCF7 | H | [15] |
| miR-135a-5p | APC | - | 3T3L1 | M | [16] |
| miR-137 | CDC42 | - | ADSCs | H | [17] |
| miR-139-5p | NOTCH1, IRS1 | - | 3T3L1 | M | [18] |
| miR-143 | ERK5, MAP2K5, PTN, ORP8 | + | 3T3L1, ADSCs | H, M | [3, 4, 6, 19] |
| miR-146b | SIRT1 | + | 3T3L1 | M | [20] |
| miR-204-5p | DVL3 | + | ADSCs | H | [21] |
| miR-221 | CDKN1 | + | 3T3L1 | M, H | [22] |
| miR-222 | ERα | + | 3T3L1 | M, H | [23] |
| miR-224 | EGR2, ACSL4 | - | 3T3L1 | M | [24] |
| miR-302 | CDKN1A | + | ASDCs | H | [25] |
| miR-335 | MEST | + | 3T3L1, MSCs | M | [26] |
| miR-363 | E2F3 | - | ADSCs | R | [8] |
| miR-378a-3p | MAPK1 | + | 3T3L1 | M | [27] |
| miR-486-5p | SIRT1 | - | ADSCs | H | [28] |
| miR-540 | PPARγ | - | ADSCs | H | [29] |
| miR-548d-5p | PPARγ | + | hBMSCs | H | [30] |
| miR-561 | HSD11B1 | - | A549, HepG2 | H | [31] |
| miR-579 | HSD11B1 | - | A549, HepG2 | H | [31] |
| “+” means promote; “-” means suppress; “H” means human; “M” mean mouse; “R” means rat; “P” means pig. | |||||
表选项
3 miRNA调控脂代谢 2003 年,Xu等[32]在研究果蝇时发现miR-14的缺失会导致三酰基甘油和二酰基甘油水平的增加,自此miRNA对脂代谢调控的作用得到肯定。脂代谢包括脂肪酸合成和分解、磷脂和胆固醇代谢等。与miRNA调控脂肪细胞分化的机制不同,miRNA对脂代谢的调控,是通过对脂代谢过程中相关的酶、转录因子或者对影响脂代谢的激素的调控作用实现的。脂代谢过程中的关键酶类有乙酰辅酶A羧化酶α (Acetyl-CoA carboxylase alpha,ACACA)、乙酰辅酶A羧化酶β (Acetyl-CoA carboxylase beta,ACACB)、还原型烟酰胺腺嘌呤二核苷酸磷酸 (Triphosphopyridine nucleotide,NADPH)、ATP-柠檬酸裂解酶 (ATP-citrate lyase,ACLY)、FASN、肝脏甘油三酯激酶 (Hepatic triglyceride lipase,LIPC) 和SCD等,一些miRNA如miR-122可直接或间接调控这些酶类的合成,进而影响脂代谢活动。固醇调节元件结合蛋白 (Sterol-regulatory element binding proteins,SREBPs) 是脂代谢中一个重要的转录因子,在人类中由SREBF1和SREBF2基因编码,对胆固醇稳态有重要的调节作用。胰岛素是机体内唯一降低血糖的激素,同时促进糖原、脂肪、蛋白质合成,对脂类合成代谢有促进作用,一些miRNA如miR-375可通过胰岛素信号通路调节胰岛素释放,进而影响脂代谢。其他的一些激素如胰高血糖素、肾上腺素、甲状腺素等也在脂代谢中发挥调控功能。
作为肝脏中表达丰度最高的miRNA,miR-122在肝脏中的的功能也是第一个被确定的,它能够参与肝脏发育、肝脏脂肪代谢等多个生命过程。Esau等[33]研究表明miR-122缺失会导致ACACA、FASN等多种脂代谢相关基因的下调表达。Krutzfeldt等[34]发现利用antagomir-122处理小鼠后其血浆中的胆固醇水平也出现下降现象,而过表达miR-122会引起肝脏内多种胆固醇生物合成相关基因表达上调,增强机体内胆固醇的合成。miR-122可能对肝脏中胆固醇合成有促进作用,是脂代谢的一个重要的调控元件。
多项研究表明,miR-33是维持细胞内胆固醇动态平衡的关键转录后调节因子,通过对脂代谢的多个途径产生影响,是最具代表性的调控脂代谢的miRNA。人类的miR-33可以分为miR-33a和miR-33b,二者仅差2个核苷酸,非常相似。miR-33a和miR-33b分别位于17号染色体SREBF2基因和22号染色体上SREBF1基因的内含子区域,SREBF1基因编码的SREBP1转录因子可以激活参与脂肪酸、磷脂和甘油三酯合成的基因FASN、SCD、ACC的表达,而SREBF2编码的SREBP2转录因子调控了参与胆固醇合成和摄取的基因,如3-羟基-3-甲基戊二酰-辅酶A合成酶 (3-hydroxy-3-methylglutaryl- CoA synthase,HMGCR)、低密度脂蛋白受体 (Low density lipoprotein receptor,LDLR) 和3-羟基-3-甲基戊二酰-辅酶A还原酶 (3-hydroxy-3- methylglutaryl-CoA reductase,HMGCS) 的表达。miR-33a和miR-33b可以通过对靶基因过氧化物酶体肉碱O型辛基转移酶 (Carnitine O-octanoyltransferase,CROT)、三功能蛋白酶β亚基 (Trifunctional protein,beta subunit,HADHB) 和肉碱棕榈酰基转移酶1A (Carnitine palmitoyltransferase 1A,CPT1A) 的转录后抑制参与脂肪酸β氧化,通过靶基因三磷酸腺苷结合盒转运因子A1 (The ATP-binding cassette transporter A1,ABCA1)[35, 36]调节胆固醇流出,也可以对SREBP蛋白的负调控因子如胰岛素受体底物蛋白2 (Insulin receptor substrate 2,IRS2)、AMP活化蛋白激酶α1亚基 (AMP-activated protein kinase alpha 1 subunit,AMPKA1) 或AMP活化蛋白激酶α1催化亚基 (AMP-activated protein kinase alpha 1 catalytic subunit,PRKAA1) 和去乙酰化酶6 (Sirtuin 6,SIRT6)[37]产生抑制作用。SREBP1/2和miR-33a/b四者相互协同,调控了脂肪酸、甘油三酯和胆固醇的稳态[38, 39, 40, 41]。
ABCA1蛋白是控制巨噬细胞胆固醇流出的重要调控者,可以防止胆固醇在细胞内的过量积累。Ramirez等[42]在研究高脂饮食的小鼠腹膜巨噬细胞胆固醇流出时,发现miR-758出现了下调表达,肝脏中也出现了相同的现象。在小鼠和人类的体外培养细胞中,miR-758 能够抑制ABCA1基因,降低其表达水平。使用anti-miR-758处理细胞则发现这一miRNA的抑制会导致ABCA1表达的增加,随后的荧光素酶报告试验证实ABCA1是miR-758的直接靶基因。在小鼠细胞中,miR-758能够减少细胞内胆固醇流向载脂蛋白A1 (Apolipoprotein A1,APOA1),而anti-miR-758 的应用则会促进胆固醇的流出。由此可知,miR-758 具有调节细胞内胆固醇水平的能力。
miR-370 通过上调miR-122 的表达间接促进脂类合成,还可以通过抑制其靶基因CPT1A的表达,降低脂肪酸β-氧化的速率[43]。此外,miR-27[44, 45, 46]、miR-30c[47]、miR-168a[48]、miR-223[49]、miR-302a[50]和miR-378[51]也可以对脂代谢产生调控作用。
4 脂肪组织中的循环miRNA 循环miRNA (Circulating miRNA) 存在于微囊泡、高密度脂蛋白复合体 (High-density lipoproteins,HDLs) 和RNA诱导的沉默复合体中,可以在同一组织不同细胞间和不同组织的细胞间充当相互交流的媒介。脂肪细胞能够分泌含有循环miRNA和细胞特异性蛋白标志物的微囊泡,约7 000种脂肪组织特异性mRNA和140种miRNA存在于小鼠脂肪细胞系分泌的微囊泡中[52]。研究发现,脂肪组织细胞间传递的循环miRNA参与了脂类合成和细胞生长过程[53],亦可调节脂肪细胞的脂质积累和细胞大小[54]。
在研究miRNA在脂肪组织中的功能时,发现一些miRNA如miR-130b[55]可以充当脂肪细胞和肌肉细胞之间交流的媒介。在这项关于肥胖的研究中,患者的TGFβ基因表达升高,刺激前体和成熟脂肪细胞分泌miR-130b,随后miR-130b进入血液后被运输到肌肉组织,靶向抑制肌肉细胞激活物受体γ共活化剂1α (Peroxisome proliferator-activated receptor gamma coactivator 1-alpha,PGC1α) 基因表达,而PGC1α在肌肉中起到脂质氧化作用,脂肪细胞通过分泌miR-130b抑制了肌肉中的脂质氧化。Vickers等[56]发现,巨噬细胞等外围组织分泌的内源性的miRNA如miR-223、miR-135a*和miR-375等能够被组装进HDLs中,这个过程受到神经酰胺生物合成的限速酶中性鞘磷脂酶2 (Neutral sphingomyelinase 2,nSMase2/SMPD3) 和ABCA1的调控。随后,组装好的HDLs经由经典的胆固醇反向转运通路 (Reverse cholesterol transport,RCT) 进入到肝细胞中,清道夫B族受体成员1 (Scavenger receptor class B member 1,SRB1) 参与了这一过程。HDLs进入到肝细胞后,其中的循环miRNA通过对细胞中靶基因的抑制发挥调控作用。
循环miRNA一般以内分泌或者旁分泌的形式发挥作用,只是对靶细胞转录本产生非特异性修饰作用,而一般的内分泌激素如胰岛素等是对靶标的特异性的有力调控,故利用循环miRNA作为肥胖等代谢疾病治疗靶点的作用有限,但是可以利用它作为病理状态的标志物。目前,多种循环miRNA已经被用到病理状态的诊断上,如miR-122和脂肪肝[57]、miR-223和动脉粥样硬化[56]、miR-101/miR-375/miR-802和Ⅱ型糖尿病[58]以及let-7e和高血压[59]等。循环miRNA具有作为脂肪代谢相关疾病标志物的潜能,这种新的miRNA的存在和调控方式的发现为脂肪组织中相关的病理研究指明了方向。
5 脂肪组织中的miRNA受到多种因素调控 脂肪组织可以分泌肿瘤坏死因子α (Tumor necrosis factor α,TNFα)、白细胞介素6 (Interleukin-6,IL-6)、抵抗素 (Resistin,RSTN)、瘦素 (Leptin,LEP) 和脂联素 (Adiponectin,ADPN) 等脂肪细胞因子,这些细胞因子介导了肥胖引起的胰岛素抵抗以及炎症反应。脂肪组织中的miRNAs在生成时候除了受到转录因子调控[60]之外,还受到脂肪细胞因子调控。TNFα处理前体脂肪细胞,引起了miR-143和miR-103的下调[61],二者都是重要的脂肪生成调控者,miR-143的表达也受到脂肪组织中游离脂肪酸和脂肪细胞因子的调控[62]。此外miR-99、miR-146b、miR-155、miR-221、miR-325、miR-335和miR-378都受到了脂肪细胞因子的调控[22, 26, 63, 64, 65, 66],它们在成脂分化、脂质代谢和胰岛素抵抗中发挥着重要的调节作用。除此之外,脂肪组织中的miRNA还受到一些调控蛋白如KH型剪接调控蛋白 (KH-type splicing regulatory protein,KSRP)、骨形态发生蛋白2 (Bone morphogenetic protein 2,BMP2) 的影响。KSRP是一种RNA结合蛋白,通过促进miR-145形成抑制了脂质分解[67],BMP2通过上调MSCs中的miR-24-1和miR-31的表达调控了前体脂肪细胞增殖[68]。另外内环境葡萄糖浓度以及饮食[19]也影响了miRNA表达,相关的机制暂不清楚。总之,脂肪组织中的miRNA的表达水平受到了转录因子、脂肪细胞因子、调控蛋白和一些理化因素的调控,维持在一个相对稳定的状态。
6 展望 近年来,由于肥胖及其并发症等发病率的飙升,miRNA在脂肪组织中功能的研究也越来越多,通过对这些研究进行归纳,所采用的思路大致都是:首先利用高通量测序或者芯片技术筛选影响脂肪细胞分化过程中或者脂代谢过程中的miRNA,然后利用生物信息学手段预测其靶基因并进行双荧光素酶报告实验证实靶基因,继而在3T3L1或者MSC细胞中对目标miRNA进行干扰或过表达,观察细胞分化状态变化和脂质积聚情况,最后在动物水平观察抑制或过表达模型。在实验第一步手段的选择上,高通量测序愈来愈受到青睐,归其原因主要是在测序成本的逐年降低和测序技术本身的优势如准确定量和新miRNA的鉴定等,利用二代测序技术对miRNA进行研究已经成为一个主要的方法。本实验室前期对不同脂肪沉积能力的两个牛种皮下脂肪组织进行了Illumina测序,获得了17个差异表达miRNA,通过对这些差异表达miRNA的预测靶基因进行功能和信号通路富集分析表明这些miRNA可能通过PPARα等靶基因,参与了脂肪酸代谢等生物学过程和PPAR等信号通路,调控了脂肪细胞分化和脂代谢,进而影响了牛脂肪沉积。牛脂肪沉积机制的研究有望鉴定出影响脂肪细胞分化和脂代谢过程的调控分子,对肥胖等人类疾病研究也有理论意义。
在生物体内,一个miRNA可能作用于多个靶基因,也可能是多个miRNA调控一个靶基因,这种作用构成一个调控网络,在某些信号的刺激下,从整体上调控有机体的生命活动,因此miRNA的作用是通过其复杂的靶基因协同调节实现的。miRNA通过对其靶基因的抑制作用调控了脂肪细胞分化和脂代谢相关的复合体和信号通路 (图1),同时它受到许多上游转录因子以及细胞因子等调节。转录因子、环境因素、miRNA和下游靶基因及其所在的信号通路,形成了一个复杂的调控网络,调控了机体前体脂肪细胞分化和脂代谢[69],miR-130b等循环miRNA的发现又增加了这个调控网络的复杂性 (图2)。
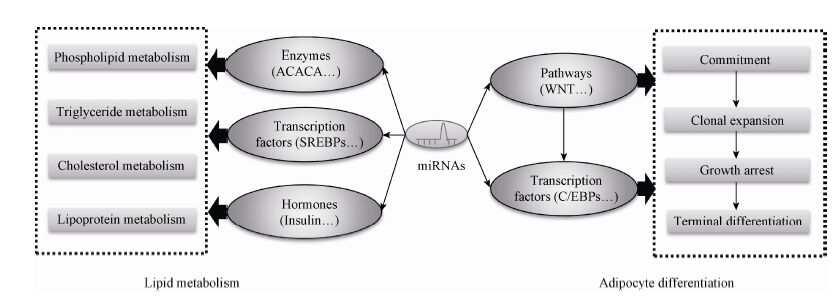 |
| 图1 miRNA调控脂肪细胞分化和脂代谢的机制 Fig.1 The regulation mechanism of miRNAs in adipocyte differentiation and lipid metabolism. |
| 图选项 |
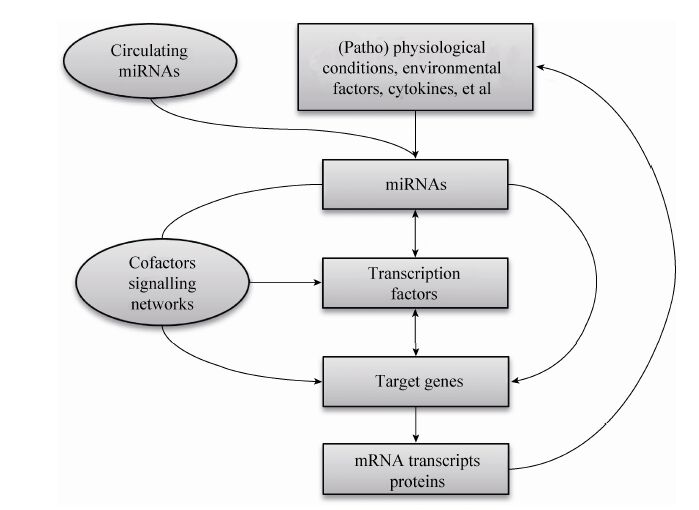 |
| 图2 脂肪组织中的miRNAs调控网络 Fig.2 The regulating network of miRNAs in adipose tissues. |
| 图选项 |
目前对肥胖和相关代谢失调疾病治疗的研究聚焦在一些易受到药物影响的酶类上面,如降胆固醇药物可以通过HMGCR降低血液胆固醇含量。对脂肪组织中miRNA的研究表明一些miRNA可以对脂肪细胞分化和脂代谢产生重要影响,有成为治疗靶点的潜能。然而,鉴于miRNA与其靶基因的作用以及生物过程时序的复杂性等,这个庞大的调控网络远远没有研究透彻,将miRNA应用于临床治疗尚有诸多难点。最近成人体内功能性棕色脂肪的发现为肥胖等代谢疾病的研究带来了契机,miR-133[70]等参与棕色脂肪细胞分化调节的miRNA被鉴定出来,利用miRNA治疗肥胖等疾病有望在棕色脂肪细胞分化方面取得突破。另外,针对肥胖以及肥胖相关并发症的治疗,不应仅局限于miRNA,其上游的转录因子、下游的靶基因、调控蛋白和环境理化因素亦具有成为治疗靶标的潜能。对脂肪细胞分化乃至肥胖等的研究应该在这个动态的网络中寻找答案,对脂肪组织中miRNA的功能研究,也可借鉴Calura等[71]在通路中研究miRNA作用机制的方法,结合上游转录因子、细胞因子、表型相关的靶基因所在通路,甚至代谢组、蛋白质组等,绘制miRNA参与的调控网络,在网络中发现关键的基因或代谢途径。随着调控脂肪细胞分化研究的深入,人们对这个复杂的调控网络的了解越来越透彻,相信在不久的将来一定能够研究出更加有效的治疗肥胖的药物或方法。
参考文献
| [1] | Ambros V. MicroRNA pathways in flies and worms. Cell, 2003, 113(6): 673-676. |
| [2] | Hyun S, Lee JH, Jin H, et al. Conserved microRNA miR-8/miR-200 and its target USH/FOG2 control growth by regulating PI3K. Cell, 2009, 139(6): 1096-1108. |
| [3] | Esau C, Kang XL, Peralta E, et al. MicroRNA-143 regulates adipocyte differentiation. J Biol Chem, 2004, 279(50): 52361-52365. |
| [4] | Yi C, Xie WD, Li F, et al. MiR-143 enhances adipogenic differentiation of 3T3-L1 cells through targeting the coding region of mouse pleiotrophin. FEBS Lett, 2011, 585(20): 3303-3309. |
| [5] | Takanabe R, Ono K, Abe Y, et al. Up-regulated expression of microRNA-143 in association with obesity in adipose tissue of mice fed high-fat diet. Biochem Biophys Res Commun, 2008, 376(4): 728-732. |
| [6] | Chen L, Hou J, Ye LF, et al. MicroRNA-143 regulates adipogenesis by modulating the MAP2K5-ERK5 signaling. Sci Rep, 2014, 4: 3819. |
| [7] | Wang Q, Li YC, Wang JH, et al. miR-17-92 cluster accelerates adipocyte differentiation by negatively regulating tumor-suppressor Rb2/p130. Proc Natl Acad Sci USA, 2008, 105(8): 2889-2894. |
| [8] | Chen L, Cui JH, Hou J, et al. A novel negative regulator of adipogenesis: microRNA-363. Stem Cells, 2014, 32(2): 510-520. |
| [9] | Kim YJ, Hwang SJ, Bae YC, et al. MiR-21 regulates adipogenic differentiation through the modulation of TGF-beta signaling in mesenchymal stem cells derived from human adipose tissue. Stem Cells, 2009, 27(12): 3093-3102. |
| [10] | Li HL, Li TP, Wang SH, et al. miR-17-5p and miR-106a are involved in the balance between osteogenic and adipogenic differentiation of adipose-derived mesenchymal stem cells. Stem Cell Res, 2013, 10(3): 313-324. |
| [11] | Huang S, Wang SH, Bian CJ, et al. Upregulation of miR-22 promotes osteogenic differentiation and inhibits adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells by repressing HDAC6 protein expression. Stem cells Dev, 2012, 21(13): 2531-2540. |
| [12] | Taniguchi M, Nakajima I, Chikuni K, et al. MicroRNA-33b downregulates the differentiation and development of porcine preadipocytes. Mol Biol Rep, 2014, 41(2): 1081-1090. |
| [13] | Li MH, Liu ZJ, Zhang ZZ, et al. miR-103 promotes 3T3-L1 cell adipogenesis through AKT/mTOR signal pathway with its target being MEF2D. Biol Chem, 2015, 396(3): 235-244. |
| [14] | Qadir AS, Woo KM, Ryoo HM, et al. Insulin suppresses distal-less homeobox 5 expression through the up-regulation of microRNA-124 in 3T3-L1 cells. Exp Cell Res, 2013, 319(14): 2125-2134. |
| [15] | Adlakha YK, Khanna S, Singh R, et al. Pro-apoptotic miRNA-128-2 modulates ABCA1, ABCG1 and RXRα expression and cholesterol homeostasis. Cell Death Dis, 2013, 4: e780. |
| [16] | Chen C, Peng YD, Peng YL, et al. miR-135a-5p inhibits 3T3-L1 adipogenesis through activation of canonical Wnt/β-catenin signaling. J Mol Endocrinol, 2014, 52(3): 311-320. |
| [17] | Shin KK, Kim YS, Kim JY, et al. miR-137 controls proliferation and differentiation of human adipose tissue stromal cells. Cell Physiol Biochem, 2014, 33(3): 758-768. |
| [18] | Mi L, Chen YS, Zheng XL, et al. MicroRNA-139-5p suppresses 3T3-L1 preadipocytedifferentiation through Notch and IRS1/PI3K/Akt insulin signaling pathways. J Cell Biochem, 2014, 116(7): 1195-1204. |
| [19] | Jordan SD, Krüger M, Willmes DM, et al. Obesity-induced overexpression of miRNA-143 inhibits insulin-stimulated AKT activation and impairs glucose metabolism. Nat Cell Biol, 2011, 13(4): 434-446. |
| [20] | Ahn J, Lee H, Jung CH, et al. MicroRNA-146b promotes adipogenesis by suppressing the SIRT1-FOXO1 cascade. EMBO Mol Med, 2013, 5(10): 1602-1612. |
| [21] | He HH, Chen K, Wang F, et al. MiR-204-5p promotes the adipogenic differentiation of human adipose-derived mesenchymal stem cells by modulating DVL3 expression and suppressing Wnt/β-catenin signaling. Int J Mol Med, 2015, 35(6): 1587-1595. |
| [22] | Meerson A, Traurig M, Ossowski V, et al. Human adipose microRNA-221 is upregulated in obesity and affects fat metabolism downstream of leptin and TNF-α. Diabetologia, 2013, 56(9): 1971-1979. |
| [23] | Shi ZH, Zhao C, Guo XR, et al. Differential expression of microRNAs in omental adipose tissue from gestational diabetes mellitus subjects reveals miR-222 as a regulator of ERα expression in estrogen-induced insulin resistance. Endocrinology, 2014, 155(5): 1982-1990. |
| [24] | Peng YD, Xiang H, Chen C, et al. MiR-224 impairs adipocyte early differentiation and regulates fatty acid metabolism. Int J Biochem Cell Biol, 2013, 45(8): 1585-1593. |
| [25] | Kim JY, Shin KK, Lee AL, et al. MicroRNA-302 induces proliferation and inhibits oxidant-induced cell death in human adipose tissue-derived mesenchymal stem cells. Cell Death Dis, 2014, 5: e1385. |
| [26] | Zhu L, Chen L, Shi CM, et al. MiR-335, an adipogenesis-related microRNA, is involved in adipose tissue inflammation. Cell Biochem Biophys, 2014, 68(2): 283-290. |
| [27] | Huang NN, Wang J, Xie WD, et al. MiR-378a-3p enhances adipogenesis by targeting mitogen-activated protein kinase 1. Biochem Biophys Res Commun, 2015, 457(1): 37-42. |
| [28] | Kim YJ, Hwang SH, Lee SY, et al. miR-486-5p induces replicative senescence of human adipose tissue-derived mesenchymal stem cells and its expression is controlled by high glucose. Stem cells Dev, 2012, 21(10): 1749-1760. |
| [29] | Chen L, Chen YW, Zhang S, et al. MiR-540 as a novel adipogenic inhibitor impairs adipogenesis via suppression of PPARγ. J Cell Biochem, 2015, 116(6): 969-976. |
| [30] | Sun JK, Wang YS, Li YB, et al. Downregulation of PPARγ by miR-548d-5p suppresses the adipogenic differentiation of human bone marrow mesenchymal stem cells and enhances their osteogenic potential. J Transl Med, 2014, 12: 168. |
| [31] | Han YY, Staab-Weijnitz CA, Xiong GM, et al. Identification of microRNAs as a potential novel regulatory mechanism in HSD11B1 expression. J Steroid Biochem Mol Biol, 2013, 133: 129-139. |
| [32] | Xu PZ, Vernooy SY, Guo M, et al. The Drosophila microRNA mir-14 suppresses cell death and is required for normal fat metabolism. Curr Biol, 2003, 13(9): 790-795. |
| [33] | Esau C, Davis S, Murray SF, et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab, 2006, 3(2): 87-98. |
| [34] | Krützfeldt J, Rajewsky N, Braich R, et al. Silencing of microRNAs in vivo with 'antagomirs'. Nature, 2005, 438(7068): 685-689. |
| [35] | Horie T, Ono K, Horiguchi M, et al. MicroRNA-33 encoded by an intron of sterol regulatory element-binding protein 2 (Srebp2) regulates HDL in vivo. Proc Natl Acad Sci USA, 2010, 107(40): 17321-17326. |
| [36] | Rayner KJ, Suárez Y, Dávalos A, et al. MiR-33 contributes to the regulation of cholesterol homeostasis. Science, 2010, 328(5985): 1570-1573. |
| [37] | Dávalos A, Goedeke L, Smibert P, et al. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc Natl Acad Sci USA, 2011, 108(22): 9232-9237. |
| [38] | Marquart TJ, Allen RM, Ory DS, et al. miR-33 links SREBP-2 induction to repression of sterol transporters. Proc Natl Acad Sci USA, 2010, 107(27): 12228-12232. |
| [39] | Najafi-Shoushtari SH, Kristo F, Li YX, et al. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science, 2010, 328(5985): 1566-1569. |
| [40] | Rottiers V, Näär AM. MicroRNAs in metabolism and metabolic disorders. Nat Rev Mol Cell Biol, 2012, 13(4): 239-250. |
| [41] | Ono K, Horie T, Nishino T, et al. MicroRNA-33a/b in lipid metabolism - novel "thrifty" models. Circ J, 2015, 79(2): 278-284. |
| [42] | Ramirez CM, Dávalos A, Goedeke L, et al. MicroRNA-758 regulates cholesterol efflux through posttranscriptional repression of ATP-binding cassette transporter A1. Arterioscler Thromb Vasc Biol, 2011, 31(11): 2707-2714. |
| [43] | Iliopoulos D, Drosatos K, Hiyama Y, et al. MicroRNA-370 controls the expression of microRNA-122 and Cpt1α and affects lipid metabolism. J Lipid Res, 2010, 51(6): 1513-1523. |
| [44] | Kang T, Lu W, Xu W, et al. MicroRNA-27 (miR-27) targets prohibitin and impairs adipocyte differentiation and mitochondrial function in human adipose-derived stem cells. J Biol Chem, 2013, 288(48): 34394-34402. |
| [45] | Kim SY, Kim AY, Lee HW, et al. miR-27a is a negative regulator of adipocyte differentiation via suppressing PPARgamma expression. Biochem Biophys Res Commun, 2010, 392(3): 323-328. |
| [46] | Karbiener M, Fischer C, Nowitsch S, et al. microRNA miR-27b impairs human adipocyte differentiation and targets PPARγ. Biochem Biophys Res Commun, 2009, 390(2): 247-251. |
| [47] | Irani S, Hussain MM. Role of microRNA-30c in lipid metabolism, adipogenesis, cardiac remodeling and cancer. Curr Opin Lipidol, 2015, 26(2): 139-146. |
| [48] | Zhang L, Hou DX, Chen X, et al. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of cross-kingdom regulation by microRNA. Cell Res, 2012, 22(1): 107-126. |
| [49] | Vickers KC, Landstreet SR, Levin MG, et al. MicroRNA-223 coordinates cholesterol homeostasis. Proc Natl Acad Sci USA, 2014, 111(40): 14518-14523. |
| [50] | Meiler S, Baumer Y, Toulmin E, et al. MicroRNA 302a is a novel modulator of cholesterol homeostasis and atherosclerosis. Arterioscler Thromb Vasc Biol, 2015, 35(2): 323-331. |
| [51] | Gerin I, Bommer GT, McCoin CS, et al. Roles for miRNA-378/378* in adipocyte gene expression and lipogenesis. Am J Physiol Endocrinol Metab, 2010, 299(2): e198-e206. |
| [52] | Ogawa R, Tanaka C, Sato M, et al. Adipocyte-derived microvesicles contain RNA that is transported into macrophages and might be secreted into blood circulation. Biochem Biophys Res Commun, 2010, 398(4): 723-729. |
| [53] | Müller G, Schneider M, Biemer-Daub G, et al. Microvesicles released from rat adipocytes and harboring glycosylphosphatidylinositol-anchored proteins transfer RNA stimulating lipid synthesis. Cell Signal, 2011, 23(7): 1207-1223. |
| [54] | Karbiener M, Pisani DF, Frontini A, et al. MicroRNA-26 family is required for human adipogenesis and drives characteristics of brown adipocytes. Stem Cells, 2014, 32(6): 1578-1590. |
| [55] | Wang YC, Li YY, Wang XY, et al. Circulating miR-130b mediates metabolic crosstalk between fat and muscle in overweight/obesity. Diabetologia, 2013, 56(10): 2275-2285. |
| [56] | Vickers KC, Palmisano BT, Shoucri BM, et al. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol, 2011, 13(4): 423-433. |
| [57] | Cermelli S, Ruggieri A, Marrero JA, et al. Circulating microRNAs in patients with chronic hepatitis C and non-alcoholic fatty liver disease. PLoS ONE, 2011, 6(8): e23937. |
| [58] | Higuchi C, Nakatsuka A, Eguchi J, et al. Identification of circulating miR-101, miR-375 and miR-802 as biomarkers for type 2 diabetes. Metabolism, 2015, 64(4): 489-497. |
| [59] | Li SQ, Zhu JG, Zhang WL, et al. Signature microRNA expression profile of essential hypertension and its novel link to human cytomegalovirus infection. Circulation, 2011, 124(2): 175-184. |
| [60] | Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol, 2014, 15(8): 509-524. |
| [61] | Xie HM, Lim B, Lodish HF. MicroRNAs induced during adipogenesis that accelerate fat cell development are downregulated in obesity. Diabetes, 2009, 58(5): 1050-1057. |
| [62] | Zhu LL, Shi CM, Ji CB, et al. FFAs and adipokine-mediated regulation of hsa-miR-143 expression in human adipocytes. Mol Biol Rep, 2013, 40(10): 5669-5675. |
| [63] | Shi CM, Zhu LJ, Chen XH, et al. IL-6 and TNF-α induced obesity-related inflammatory response through transcriptional regulation of miR-146b. J Interferon Cytokine Res, 2014, 34(5): 342-348. |
| [64] | Subedi A, Park PH. Autocrine and paracrine modulation of microRNA-155 expression by globular adiponectin in RAW 264.7 macrophages: involvement of MAPK/NF-κB pathway. Cytokine, 2013, 64(3): 638-641. |
| [65] | Chou WW, Wang YT, Liao YC, et al. Decreased microRNA-221 is associated with high levels of TNF-α in human adipose tissue-derived mesenchymal stem cells from obese woman. Cell Physiol Biochem, 2013, 32(1): 127-137. |
| [66] | Xu LL, Shi CM, Xu GF, et al. TNF-α, IL-6, and leptin increase the expression of miR-378, an adipogenesis-related microRNA in human adipocytes. Cell Biochem Biophys, 2014, 70(2): 771-776. |
| [67] | Lin YY, Chou CF, Giovarelli M, et al. KSRP and microRNA 145 are negative regulators of lipolysis in white adipose tissue. Mol Cell Biol, 2014, 34(12): 2339-2349. |
| [68] | Sun FY, Wang JY, Pan QH, et al. Characterization of function and regulation of miR-24-1 and miR-31. Biochem Biophys Res Commun, 2009, 380(3): 660-665. |
| [69] | Arner P, Kulyté A. MicroRNA regulatory networks in human adipose tissue and obesity. Nat Rev Endocrinol, 2015, 11(5): 276-288. |
| [70] | Trajkovski M, Ahmed K, Esau CC, et al. MyomiR-133 regulates brown fat differentiation through Prdm16. Nat Cell Biol, 2012, 14(12): 1330-1335. |
| [71] | Calura E, Martini P, Sales G, et al. Wiring miRNAs to pathways: a topological approach to integrate miRNA and mRNA expression profiles. Nucleic Acids Res, 2014, 42(11): e96. |
