

中国科学院微生物研究所 中国科学院病原微生物与免疫学重点实验室,北京 100101
High-dose heat shock protein gp96 immunization prevents type 1 diabetes via inducing regulatory T cells
Mi Chen, Xinghui Li, Huaguo Zheng, Songdong Meng


CAS Key Laboratory of Pathogenic Microbiology and Immunology, Institute of Microbiology, Chinese Academy of Sciences, Beijing 100101, China
Received: February 20, 2016; Accepted: April 6, 2016
Supported by:National Natural Science Foundation of China (Nos. 31230026, 81321063, 81471960), Shenzhen Science and Technology Innovation Committee (Nos. JSGG20140516112337659, CYZZ20130826112642412)
Corresponding authors:Songdong Meng. Tel: +86-10-64807350; E-mail: mengsd@im.ac.cn
Abstract: Type 1 diabetes (T1D), the most prevalent human autoimmune disease, occurs in genetically susceptible individuals. Regulatory T cells (Tregs) are defective in T1D setting. Therefore, efforts to repair or restore Tregs in T1D may prevent or reverse this autoimmune disease. Here, we studied the potential role of rgp96 in preventing T1D, using non-obese diabetic (NOD) mice as an animal model. High-dose rgp96 immunization elicited efficient protection of mice against T1D, as evidenced by stable blood glucose, decreased disease incidence. Significantly increased CD4+CD25+Foxp3+ Tregs were observed in immunized mice. In vitro co-culture experiments demonstrated that rgp96 stimulation enhanced Treg proliferation and suppressive function by up-regulation of Foxp3 and IL-10. Our work shows that activation of Tregs by high-dose rgp96 immunization protects against T1D via inducing regulatory T cells and provides preventive and therapeutic potential for the development of an rgp96-based vaccine against T1D.
Key words: gp96 Tregs type 1 diabetes immunotherapy vaccine
随着现代生活水平的提高和生活方式、生活环境的改变,在全球范围内,1型糖尿病(Type 1 diabetes,T1D)的患病率正呈现快速上升的趋势。根据2011年国际糖尿病联盟(International diabetes federation,IDF)统计,在全球1.9亿0?15岁的儿童中,T1D患者约有490 100名,每年新诊断约77 800名,年增加率约为3.0%[1]。
T1D是一种自身免疫性疾病(Autoimmune disease,AID),由自身反应性T细胞持续活化并破坏胰岛β细胞,使胰岛素分泌功能受损而引发的代谢紊乱综合征[2]。T1D的确切病因迄今尚未阐明,但自身免疫异常是其最主要的致病因素[3]。CD4+CD25+Foxp3+调节性T细胞(Regulatory T cells,Tregs)是一类免疫抑制性T淋巴细胞,它在防止自身免疫性疾病及维持自身免疫耐受中发挥重要作用[4]。有研究发现,T1D患者体内的CD4+CD25+Foxp3+ Tregs出现数量减少或者调节能力减弱的现象[5];同样地,针对非肥胖型糖尿病(NOD)小鼠的研究也发现了患病小鼠体内该亚群T细胞水平的降低[6]。一些动物实验和临床研究结果显示,体外扩增的多克隆或抗原特异性Tregs的过继性转移疗法能够有效地预防或缓解T1D[7-10]。这些结果说明了Tregs在T1D的发病及疾病进程中发挥重要作用。
Gp96 (GRP96)是内质网热休克蛋白(Heat shock protein,HSP) 90家族的成员之一,与细胞质HSP90高度同源。我们前期的研究发现,低剂量的gp96免疫主要活化CD8+ T细胞应答,而高剂量的gp96能够通过Toll样受体2和Toll样受体4介导的NF-κB的激活而显著地增强Tregs的增殖能力和抑制性功能[11-13]。Chandawarkar等的研究也发现高剂量gp96免疫能够通过诱导具有免疫调节功能的CD4+T细胞的产生防止多种自体免疫疾病,包括T1D[14]。但这些研究所用的gp96均为天然提取,很难研发成疫苗或药物。我们利用酵母菌和昆虫细胞首次表达具有较高免疫活性的重组gp96 (Recombinant gp96,rgp96)蛋白,在此基础上,拟通过应用高剂量rgp96预先免疫NOD小鼠,了解其是否具有预防糖尿病发生的作用,并探讨其可能的作用机制。研究结果为开发基于rgp96的新型T1D预防或治疗性疫苗提供了依据。
1 材料与方法1.1 动物所有动物为NOD小鼠。NOD小鼠来源于北京中国科学院微生物研究所周旭宇研究员实验室。在北京大学医学院实验动物中心SPF级环境下饲养,繁殖出第一代,取雌性小鼠用于实验。
1.2 rgp96和MSA的制备及Western blotting鉴定利用昆虫细胞表达系统表达纯化重组热休克蛋白gp96 (Recombinant gp96,rgp96)和小鼠血清白蛋白(Mouse serum albumin,MSA),获得的目的蛋白通过10%的SDS-PAGE和Western blotting (Santa Cruz,CA,USA)进行鉴定。纯化和鉴定后的蛋白样品用液相内毒素清除剂(购自北京天恩泽公司)去除内毒素,然后用鲎试剂、工作品及工作用水等试剂(购自厦门市鲎试剂实验厂有限公司)检测蛋白样品中内毒素含量,结果显示蛋白样品中内毒素浓度低于10 EU/mg,可以用于免疫。
1.3 小鼠免疫和血糖测定按照每组10只小鼠随机分为3组:PBS组、MSA组和rgp96组(100 μg/只)。在小鼠6、7、9周龄时皮下各免疫1次,共3次,从最后一次免疫后的第1周开始监测小鼠血糖值,取小鼠尾静脉血1滴,用血糖仪(德国罗氏公司ACCU-CHEK? Performa)参照说明书测定血糖值,并统计患病率。以血糖值 > 13.3 mmol/L且持续2次以上诊断为T1D。
1.4 小鼠脾脏淋巴细胞的分离用颈脱臼法处死小鼠,取出小鼠脾脏,根据Liu等[11]方法分离获得小鼠脾脏淋巴细胞。
1.5 细胞内因子染色每只小鼠各取3×106的脾脏淋巴细胞,用PBS洗2次,用含5%BSA的PBS封闭30 min,加Percp-Cy5.5-anti-CD3、PE-anti-CD4、FITC-anti-CD25 (eBioscience),4 ℃避光孵育30 min,PBS清洗,每管加入250 μL固定/破膜剂(eBioscience),4 ℃孵育20 min后用清洗剂(eBioscience)清洗2次,在50 μL体系中加入APC-anti-Foxp3 (eBioscience),4 ℃避光孵育30 min,清洗剂清洗2次,用流式细胞仪(FACSCaliber,BectonDickinson,Mountain View,CA)检测Treg细胞。
1.6 体外Tregs增殖检测按CFSE细胞增殖试剂盒说明书标记小鼠脾脏淋巴细胞,将CFSE标记的脾脏淋巴细胞(3×105/孔)加入至96-孔圆底培养板中,培养基内分别加入1 μg/mL的anti-CD3和anti-CD28及50 IU/mL的IL-2,在5% CO2 37 ℃无菌条件下培养。培养24 h收集上清检测细胞因子;培养72 h收集细胞检测细胞体外增殖能力:按细胞内因子染色方法标记Treg细胞(不要用FITC通道的抗体,因为CFSE占用此通道);用流式细胞仪检测Treg细胞的CFSE荧光衰减情况,即为Tregs增殖情况。
1.7 体外Tregs抑制功能实验用磁珠分选试剂盒(Miltenyi Biotec,Auburn,CA)分离得到Tregs和效应T细胞(CD4+CD25- T细胞)。按CFSE细胞增殖试剂盒说明书标记CD4+CD25- T细胞,将CFSE标记的CD4+CD25- T细胞(3×105 /孔)加入至96-孔圆底培养板中,将其单独培养或加入1/3效应细胞数目的Tregs,培养基内分别加入1 μg/mL的anti-CD3和anti-CD28及50 IU/mL的IL-2,在5% CO2、37 ℃无菌条件下培养3 d。收集上述细胞,按细胞内因子染色方法标记效应T细胞(不要用FITC通道的抗体,因为CFSE占用此通道);用流式细胞仪检测效应T细胞的CFSE荧光衰减情况,计算Tregs对其增殖的抑制,抑制率=[CFSElow (Teff without Tregs)-CFSElow(Teff with Tregs)]/ CFSElow (Teff without Tregs)×100%。
1.8 细胞因子的ELISA测定高剂量rgp96刺激24 h后收集Tregs培养上清液,用ELISA试剂盒(eBioscience,San Diego,CA)按照说明书检测IL-10含量。
1.9 蛋白质免疫印迹蛋白免疫印记测定Tregs中Foxp3的水平。高剂量rgp96刺激24 h后收集细胞提取蛋白,10% SDS-PAGE电泳分离,转印于PVDF膜,5%的脱脂牛奶封闭1 h,一抗(1:1 000) 4 ℃孵育过夜,以PBST洗3次,每次10 min,辣根过氧化物酶标记的二抗(1:2 000)室温孵育1 h,同样步骤用PBST洗去二抗后加显色液曝光显影。
2 结果与分析2.1 SDS-PAGE和Western blotting鉴定热休克蛋白gp96和小鼠血清白蛋白MSA本实验所用的热休克蛋白gp96和小鼠血清白蛋白MSA均为从昆虫细胞表达系统表达纯化的重组蛋白。纯化的蛋白通过10%的SDS-PAGE胶分离,经考马斯亮蓝染色鉴定,对染色条带进行扫描分析gp96纯度在95%以上,采用Western blotting鉴定为目的蛋白gp96和MSA (图 1)。
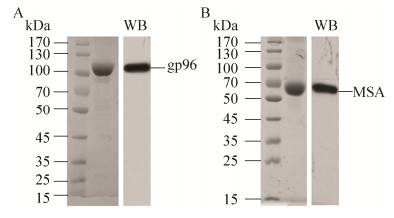 |
| 图 1 人工表达重组gp96和MSA的纯化与鉴定 Figure 1 Purification and identification of recombinant gp96 and MSA. Purified recombinant gp96 (A) and MSA (B) expressed in the Bac-to-Bac? baculovirus expression system was analyzed on a 10% SDS-PAGE gel and visualized with coomassie staining (left lane) or immunoblotted with an anti-gp96 or anti-MSA antibody (right lane). |
| 图选项 |
2.2 高剂量rgp96免疫显著降低NOD小鼠1型糖尿病发生首先比较不同rgp96蛋白免疫剂量对NOD小鼠Tregs的诱导情况,分别在6、7和9周龄时免疫雌性NOD小鼠(图 2A),结果显示采用100 μg/次可诱导高比例的Tregs (图 2B),推测疫苗的EC50应该在60 μg/次左右,这与我们前期的试验结果相吻合[11]。
 |
| 图 2 高剂量rgp96免疫显著降低小鼠1型糖尿病患病率 Figure 2 Immunization with high-dose rgp96 prevents the onset of T1D in NOD mice. Schematic representation of the immunization schedule (A). Female NOD mice were immunized with the indicated amounts of rgp96 (B), or PBS, MSA or rgp96 (100μg/time) (C) for three times at 6, 7 and 9 weeks of age, respectively. FACS analysis of the frequency of CD4+CD25+Foxp3+ Tregs in the spleen of mice at week 10 (B). Blood glucose were measured weekly for eleven weeks after the last immunization (C). Diabetes incidence is shown (D). Mice were determined diabetic with two consecutive readings of blood glucose > 13.3 mmol/L. Data show the x±s of ten mice. Student's t-test was used to determine P-values. **P < 0.01 compared to PBS-or MSA-immunized mice. |
| 图选项 |
我们用高剂量的rgp96 (100 μg/次)免疫,从免疫后第1周(小鼠10周龄)开始,用血糖仪测量小鼠的血糖值,每周1次,连续测量11周,结果显示,用高剂量rgp96免疫的小鼠的血糖值基本保持不变且维持在正常范围内,而对照组(注射PBS或MSA)小鼠的血糖值显著升高,在13周龄时约为免疫组小鼠血糖值的3倍(P < 0.01) (图 2C)。此外,我们统计了小鼠的患病率(血糖值大于13.3 mmol/L),结果显示免疫组小鼠的患病率明显低于对照组患病率,在14周龄时对照组小鼠全部发病,而免疫组只有40%的小鼠发病,该发病率一直延续到本试验观察期结束(20周龄)。免疫组小鼠的T1D发病时间约在12周龄,与对照组小鼠(约10周龄)相比明显延缓(图 2D)。
2.3 高剂量rgp96免疫诱导Tregs产生我们之前的研究发现高剂量天然提取的gp96免疫可以诱导Tregs产生,因此推测高剂量人工表达重组gp96免疫通过诱导Tregs抑制小鼠自身免疫应答,从而预防1型糖尿病。
NOD小鼠分别在第6、7、9周接受rgp96免疫,在第10周处死小鼠,通过流式细胞术检测小鼠脾脏中Treg细胞的变化,结果显示,用高剂量rgp96免疫的小鼠脾脏中Treg细胞的频率明显高于对照组小鼠(PBS组对比免疫组,4.487±0.461 9 vs 8.760±0.390 9;MSA组对比免疫组,4.643±0.327 3 vs 8.760±0.390 9) (P < 0.01) (图 3)。
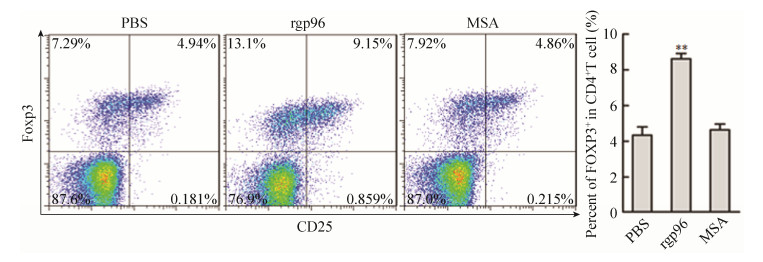 |
| 图 3 高剂量rgp96免疫诱导Tregs产生 Figure 3 High-dose rgp96 immunization induces Tregs in mice. Female NOD mice were immunized with PBS, MSA or rgp96 (100 μg/time) for three times. Mice were sacrificed at age week 10. Flow cytometric analysis was performed to determine the frequency of CD4+CD25+Foxp3+ Tregs in the spleen of mice. Data show the x±s of ten mice. Student's t-test was used to determine P-values. **P < 0.01. Data are representative of two independent experiments. |
| 图选项 |
2.4 高剂量rgp96增强Tregs的增殖和抑制功能,上调Foxp3表达和IL-10分泌进一步查明rgp96对Tregs的增殖和功能的影响。分离NOD小鼠脾脏淋巴细胞,用CFSE染色方法研究高浓度rgp96刺激对Tregs增殖能力的影响,结果显示,相比PBS处理,rgp96孵育显著提高Tregs的增殖能力(P < 0.05) (图 4A)。采用磁珠分选CD4+CD25+ Tregs和CD4+CD25- T细胞,用100 μg/mL rgp96体外刺激Tregs 24 h,用CFSE染色的CD4+CD25-T细胞作为效应细胞(Teff)与预刺激的Tregs以3:1的比例混合,在anti-CD3、anti-CD28和IL-2存在的条件下培养72 h,通过流式细胞术检测Teff的增殖情况(图 4B)。可以看到,高浓度rgp96刺激的Tregs对Teff增殖的抑制能力明显高于未刺激的Tregs (P < 0.01) (图 4C)。同时,高浓度rgp96刺激显著提高Tregs分泌IL-10的水平(图 4D)及Foxp3的表达(图 4E)。
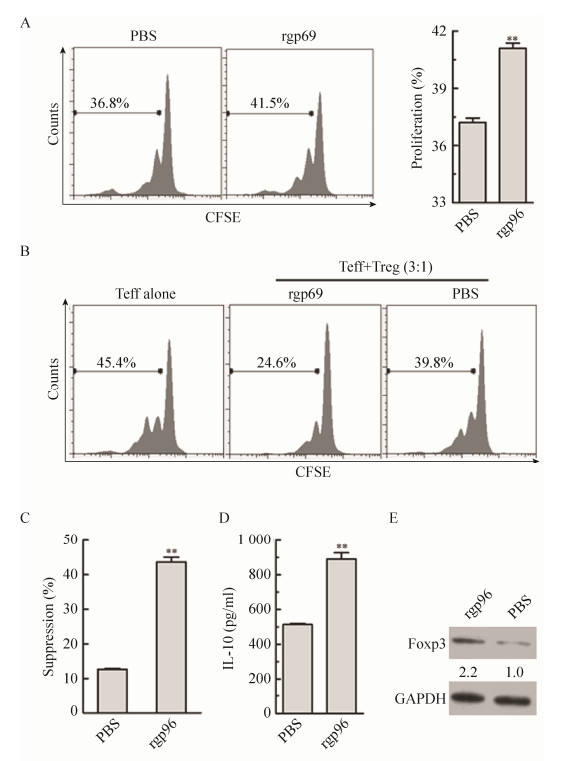 |
| 图 4 高剂量rgp96增强Tregs的增殖和抑制功能,上调Foxp3表达和IL-10分泌 Figure 4 High-dose rgp96 promotes Tregs inhibitory function and proliferation, and up-regulates Foxp3 expression and IL-10 secretion. Mice spleenocytes (A-C) or Treg cells (D-E) were incubated with 100 μg/mL rgp96 or PBS in vitro in the present of 1 μg/mL anti-CD3, anti-CD28 Abs and 50 IU/mL IL-2. (A) Proliferation of Tregs was determined by CSFE staining 3 d later. (B) A total of 3×105 CSFE-labeled CD4+CD25- Teff cells were co-cultured with Tregs at a ratio of 3:1 for 3 d. Teff cells cultured without Tregs served as a negative control. The CFSE-labelled Teff cell division cycle was measured by FACS. (C) The suppression rate for Teff proliferation was calculated. (D) The IL-10 levels in the supernatants of Tregs were measured by ELISA. (E) The Foxp3 expression of Tregs was detected by Western blotting. Student's t-test was used to determine P-values. **P < 0.01. Results are representative of three independent experiments, and FACS analyses were performed for at least five times in each experiment. |
| 图选项 |
3 讨论热休克蛋白gp96诱导天然性免疫和获得性免疫的功能已经在多种动物模型中得到验证,其作为免疫佐剂治疗肿瘤和感染性疾病已应用于临床试验。其作用机理包括与APC的相互作用、TLR的转录后调控、抗原交叉呈递以及激活CTL[15]。然而关于gp96用于自体免疫疾病预防或治疗的研究却很少被报道。本研究检测了高剂量rgp96免疫对T1D的预防作用,发现在NOD小鼠模型中,高剂量rgp96免疫能够有效地预防或者延缓NOD小鼠T1D的发生,高剂量rgp96显著提高抑制性T细胞Tregs数目和抑制功能,上调其标志性转录因子Foxp3的水平并增强主要抑制性细胞因子IL-10的表达,提示高剂量的rgp96通过上调Tregs数量并增强其抑制功能,从而抑制导致小鼠糖尿病的自身免疫过度活化。
尽管一些报道表明Tregs与T1D的疾病进程相关[16],对于Tregs在T1D相关的病理学中的功能研究才刚刚开始。Tregs的频率降低或功能受损会导致T1D的发生[17-18],抗胸腺细胞球蛋白(Antithymocyte globulin,ATG)通过诱导Tregs产生能够有效预防或缓解T1D[19-20]。这些研究均表明Tregs对于预防和治疗T1D的潜在价值。
NOD小鼠是模拟人类的自发出现由T淋巴细胞介导的胰岛β细胞破坏而发生T1D的理想动物模型[21],被广泛用于T1D相关的研究之中。本研究中,我们在NOD小鼠模型中检测rgp96免疫对T1D的预防作用。结果显示,高剂量rgp96可有效预防或延缓T1D的发生,鉴于gp96在临床上已经用于肿瘤等疾病的治疗,因此采用rgp96诱导Tregs对于预防和治疗T1D是一种安全有效的方法;同时,相对于体外大量培养Tregs治疗糖尿病需要高成本[10],采用高剂量rgp96免疫的策略成本相对低廉。
本研究发现采用100 μg的单次免疫剂量可在小鼠体内诱导高水平的Tregs,由于gp96自体肿瘤治疗性疫苗在小鼠和肿瘤患者的合适免疫剂量分别为10?20 μg/次和25 μg/次[22-23],我们推测将来临床试验中150?300 μg/次的免疫剂量就可能在人体中诱导出高比例的Tregs,这需要进一步试验验证。
我们之前的研究发现,天然提取gp96可直接与Tregs表面TLR2和TLR4相互作用,进而激活NF-κB信号通路,促进Foxp3、IL-10及TGF-β1的表达,从而增强Tregs的增殖能力和抑制性功能[11-12]。本研究采用昆虫细胞表达的rgp96,高剂量免疫同样能活化Tregs,证明人工表达的重组gp96与天然蛋白具有同样的免疫学功能,更重要的是通过NOD小鼠试验发现高剂量重组gp96免疫对1型糖尿病有显著的预防效果,为开发基于重组gp96的新型糖尿病预防性和治疗性疫苗提供了依据。
参考文献
| [1] | Chinese Diabetes Society. China guidelines for diagnosis and treatment of type 1 diabetes.Clin Diabetes, 2013, 7(3): 6–27.(in Chinese). 中华医学会糖尿病学分会. 中国1型糖尿病诊治指南.糖尿病临床, 2013, 7(3): 6-27. |
| [2] | McDevitt HO, Unanue ER. Autoimmune diabetes mellitus-much progress, but many challenges.Adv Immunol, 2008, 100: 1–12.DOI: 10.1016/S0065-2776(08)00801-8 |
| [3] | Bach JF, Chatenoud L. A historical view from thirty eventful years of immunotherapy in autoimmune diabetes.Semin Immunol, 2011, 23(3): 174–181.DOI: 10.1016/j.smim.2011.07.009 |
| [4] | Peterson RA. Regulatory T-cells: diverse phenotypes integral to immune homeostasis and suppression.Toxicol Pathol, 2012, 40(2): 186–204.DOI: 10.1177/0192623311430693 |
| [5] | Lindley S, Dayan CM, Bishop A, et al. Defective suppressor function in CD4+ CD25+ T-cells from patients with type 1 diabetes.Diabetes, 2005, 54(1): 92–99.DOI: 10.2337/diabetes.54.1.92 |
| [6] | Kukreja A, Cost G, Marker J, et al. Multiple immuno-regulatory defects in type-1 diabetes.J Clin Investig, 2002, 109(1): 131–140.DOI: 10.1172/JCI0213605 |
| [7] | Bluestone JA, Tang QZ. Therapeutic vaccination using CD4+ CD25+ antigen-specific regulatory T cells.Proc Natl Acad Sci USA, 2004, 101(Suppl 2): 14622–14626. |
| [8] | Bluestone JA, Trotta E, Xu DQ. The therapeutic potential of regulatory T cells for the treatment of autoimmune disease.Expert Opin Ther Targets, 2015, 19(8): 1091–1103.DOI: 10.1517/14728222.2015.1037282 |
| [9] | Tang QZ, Henriksen KJ, Bi MY, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes.J Exp Med, 2004, 199(11): 1455–1465.DOI: 10.1084/jem.20040139 |
| [10] | Bluestone JA, Buckner JH, Fitch M, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells.Sci Transl Med, 2015, 7(315): 315r. |
| [11] | Liu Z, Li XH, Qiu LP, et al. Treg suppress CTL responses upon immunization with HSP gp96.Eur J Immunol, 2009, 39(11): 3110–3120.DOI: 10.1002/eji.v39:11 |
| [12] | Li XH, Liu Z, Yan XL, et al. Induction of regulatory T cells by high-dose gp96 suppresses murine liver immune hyperactivation.PLoS ONE, 2013, 8(7): e68997.DOI: 10.1371/journal.pone.0068997 |
| [13] | Yan XL, Zhang XJ, Wang YZ, et al. Regulatory T cell depletion synergizes with gp96 mediated cellular responses and antitumor activity.Cancer Immunol Immunother, 2011, 60(12): 1763–1774.DOI: 10.1007/s00262-011-1076-5 |
| [14] | Chandawarkar RY, Wagh MS, Kovalchin JT, et al. Immune modulation with high-dose heat shock protein gp96: therapy of murine autoimmune diabetes and encephalomyelitis.Int Immunol, 2004, 16(4): 615–624.DOI: 10.1093/intimm/dxh063 |
| [15] | Randazzo M, Terness P, Opelz G, et al. Active-specific immunotherapy of human cancers with the heat shock protein Gp96-revisited.Int J Cancer, 2012, 130(10): 2219–2231.DOI: 10.1002/ijc.v130.10 |
| [16] | Zóka A, Barna G, Somogyi A, et al. Extension of the CD4+Foxp3+CD25?/low regulatory T-cell subpopulation in type 1 diabetes mellitus.Autoimmunity, 2014, 48(5): 289–297. |
| [17] | Passerini L, Di Nunzio S, Gregori S, et al. Functional type 1 regulatory T cells develop regardless of FoxP3 mutations in patients with IPEX syndrome.Eur J Immunol, 2011, 41(4): 1120–1131.DOI: 10.1002/eji.201040909 |
| [18] | Salomon B, Lenschow DJ, Rhee L, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes.Immunity, 2000, 12(4): 431–440.DOI: 10.1016/S1074-7613(00)80195-8 |
| [19] | Ogawa N, Minamimura K, Kodaka T, et al. Short administration of polyclonal anti-T cell antibody (ALS) in NOD mice with extensive insulitis prevents subsequent development of autoimmune diabetes.J Autoimmun, 2006, 26(4): 225–231.DOI: 10.1016/j.jaut.2006.03.001 |
| [20] | Simon G, Parker M, Ramiya V, et al. Murine antithymocyte globulin therapy alters disease progression in NOD mice by a time dependent induction of immunoregulation.Diabetes, 2008, 57(2): 405–414.DOI: 10.2337/db06-1384 |
| [21] | Kikutani H, Makino S. The murine autoimmune diabetes model: NOD and related strains.Adv Immunol, 1992, 51: 285–322.DOI: 10.1016/S0065-2776(08)60490-3 |
| [22] | Testori A, Richards J, Whitman E, et al. Phase III comparison of vitespen, an autologous tumor-derived heat shock protein gp96 peptide complex vaccine, with physician's choice of treatment for stage IV melanoma: the C-100-21 study group.J Clin Oncol, 2008, 26(6): 955–962.DOI: 10.1200/JCO.2007.11.9941 |
| [23] | Crane CA, Han SJ, Ahn B, et al. Individual patient-specific immunity against high-grade glioma after vaccination with autologous tumor derived peptides bound to the 96 kDa chaperone protein.Clin Cancer Res, 2013, 19(1): 205–214.DOI: 10.1158/1078-0432.CCR-11-3358 |
