史喜绢, 张婷, 杨博, 申超超, 张大俊, 陈学辉, 崔卉梅, 袁兴国, 赵登率, 张克山

 , 郑海学
, 郑海学
 , 刘湘涛
, 刘湘涛 中国农业科学院兰州兽医研究所, 家畜疫病病原生物学国家重点实验室, 农业部畜禽病毒学重点开放实验室, 国家口蹄疫参考实验室, 甘肃 兰州 730046
收稿日期:2021-01-20;修回日期:2021-04-16;网络出版日期:2021-08-04
基金项目:国家自然科学基金(31972684);新发塞内卡病毒应急防控关键技术研究及产品开发(19ZDNA001);中国农业科学院重大科研任务(ZDRW202006)
*通信作者:张克山, Tel: +86-931-8342086, Fax: +86-931-8342052, E-mail: zks009@126.com;
郑海学, Tel: +86-931-8343307, Fax: +86-931-8342052, E-mail: zhenghaixue@caas.cn.
摘要:[目的] 本研究旨在探究猪源组织蛋白酶S (cathepsin S,CTSS)对塞内卡病毒(Seneca Valley virus,SVV)复制的影响。[方法] SVV感染IBRS-2细胞,采用RT-qPCR在转录水平探究SVV感染对内源性CTSS表达的调控; 采用ELISA测定SVV感染对CTSS酶活性的影响; 通过Western blotting (WB)和RT-qPCR检测过表达CTSS对SVV复制及SVV诱导的抗病毒细胞因子的调控作用; 合成针对CTSS的特异性siRNA,利用WB和RT-qPCR检测siRNA对CTSS的干扰效果以及CTSS被干扰后对SVV复制的影响。[结果] 结果表明SVV感染IBRS-2细胞能显著上调内源性CTSS表达并增强CTSS酶活性; 过表达CTSS能显著抑制SVV在IBRS-2细胞中的复制,同时促进宿主抗病毒细胞因子的表达; siRNA-2947下调内源性CTSS表达进而促进SVV复制。[结论] CTSS通过增强宿主抗病毒细胞因子上调表达而抑制SVV复制,本研究为进一步深入探究宿主CTSS在抗SVV免疫应答中的作用及机制提供参考依据。
关键词:组织蛋白酶S塞内卡病毒IBRS-2细胞抗病毒功能
The replication of Seneca Valley virus in IBRS-2 cells was inhibited by cathepsin S
Xijuan Shi, Ting Zhang, Bo Yang, Chaochao Shen, Dajun Zhang, Xuehui Chen, Huimei Cui, Xingguo Yuan, Dengshuai Zhao, Keshan Zhang

 , Haixue Zheng
, Haixue Zheng
 , Xiangtao Liu
, Xiangtao Liu State Key Laboratory of Veterinary Etiological Biology, Key Laboratory of Animal Virology of Ministry of Agriculture, National Foot-and-Mouth Disease Reference Laboratory, Lanzhou Veterinary Research Institute of Chinese Academy of Agriculture Science, Lanzhou 730046, Gansu Province, China
Received: 20 January 2021; Revised: 16 April 2021; Published online: 4 August 2021
*Corresponding author: Keshan Zhang, Tel: +86-931-8342086, Fax: +86-931-8342052, E-mail: zks009@126.com;
Haixue Zheng, Tel: +86-931-8343307, Fax: +86-931-8342052, E-mail: zhenghaixue@caas.cn.
Foundation item: Supported by the National Natural Science Foundation of China (31972684), by the Research and Product Development of Key Technologies for Emergency Prevention and Control of Newly Developed Seneca Virus (19ZDNA001) and by the Collaborative Innovation Project of Chinese Academy of Agricultural Sciences (ZDRW202006)
Abstract: [Objective] The purpose was to explore the effect of porcine cathepsin S (cathepsin S, CTSS) on SVV (Seneca Valley virus, SVV) replication. [Methods] IBRS-2 cells were infected with SVV, and the regulation of endogenous CTSS expression by SVV infection was investigated by RT-qPCR at transcriptional levels. The effect of SVV infection on CTSS enzyme activity was determined by ELISA. The regulatory effects of overexpressed CTSS on SVV replication and antiviral cytokines induced by SVV were detected by Western blotting (WB) and RT-qPCR. The specific siRNA for CTSS was synthesized and the interference effect of siRNA on CTSS and the effect of CTSS interference on SVV replication were detected by WB and RT-qPCR. [Results] The expression of endogenous CTSS and the activity of CTSS enzyme were markedly up-regulated in IBRS-2 cells infected with SVV. SVV replication in IBRS-2 cells was significantly inhibited and the expression of host antiviral cytokines host antiviral cytokines was up-regulated by CTSS overexpression. siRNA-2947 down-regulate the expression of endogenous CTSS to promote SVV replication. [Conclusion] CTSS inhibits SVV replication by enhancing the up-regulated expression of host antiviral cytokines. This study provided the reference basis for further exploring the role and mechanisms of host CTSS in anti-SVV innate immune response.
Keywords: cathepsin SSeneca Valley virusIBRS-2 cellantiviral function
塞内卡病毒(Seneca Valley virus, SVV)是新出现的一种引起猪水泡病的病原体, SVV是小RNA病毒科(Picornaviridae)塞内卡病毒属(Senecavirus)的唯一成员[1-2]。该病毒基因组长度约为7.3 kb, 包含一个开放阅读框(ORF), 编码一个大的多聚蛋白前体, 随后被加工成各种成熟的病毒蛋白, ORF分为L区、P1区、P2区和P3区, L区从新生病毒多聚蛋白前体中自我裂解为前导蛋白(Lpro);P1区产生4种病毒结构蛋白(VP1、VP2、VP3和VP4);P2和P3区编码病毒非结构蛋白(2A、2B、2C、3A、3B、3C和3D), 其中2C是非结构蛋白中最保守的蛋白[3]。
SVV于2002年首次在被污染的细胞中偶然发现, 被认为可能是通过胎牛血清或猪胰蛋白酶引入细胞培养物中的污染物, 由于其具有潜在的溶瘤活性, 最初被用于治疗人类癌症, 认为与特定病的病症无关[4-5]。2007年加拿大报道了SVV感染猪与特发性水泡病(PIVD)有关, 时隔两年多美国又报道SVV可引起猪的口部、蹄部等皮肤黏膜出现水泡或溃疡病变[6-7]。随后美国、巴西、泰国和中国等国家均报道猪群中出现了大量SVV感染[8-11]。这表明SVV在不同地区发生着持续感染, 意味着该病原存在暴发或大流行的潜在风险, 可能导致巨大的经济损失, 因此我们需要探究SVV的致病机制, 为开发免受SVV感染的候选疫苗或治疗药物提供理论支撑。
初乳在抵抗病原感染中具有重要作用[12], 猪的胎盘类型为上皮绒毛膜型, 故不能通过胎盘传递免疫球蛋白, 说明初乳是新生仔猪获得被动免疫的唯一途径, 这一特性决定了初乳在仔猪免疫中具有更为重要的作用。本团队前期应用iTRAQ技术研究发现母猪初乳中组织蛋白酶S (cathepsin S, CTSS)的含量显著高于常乳。CTSS是木瓜蛋白酶家族的半胱氨酸蛋白酶, 主要在树突状细胞、B细胞和巨噬细胞等抗原呈递细胞中表达[13], 在抗原(Ag)加工和呈递、基质降解以及促进新生血管形成和肿瘤细胞侵袭转移中起重要作用, 分泌的CTSS能够裂解膜结合的底物[14-16]。CTSS酶活性是其发挥功能的关键, 如棕榈酸酯抑制组织蛋白酶诱导内皮细胞侵袭从而抗血管生成, 部分是通过抑制CTSL和CTSS活性起作用的[17]。CTSS通过调节p38 MAPK和JNK1途径参与MP诱导的细胞凋亡和自噬;通过激活NF-κB和Caspase-3从而诱导肝癌细胞凋亡并增加其化学敏感性, 也可激活CD74调控趋化因子CCL2的表达, 从而对肿瘤微环境产生影响[18]。目前对宿主CTSS在病原感染中的作用研究较少, 而主要集中在自身免疫性疾病[19]、心血管疾病[20]及肿瘤相关疾病[21]的研究。
SVV与口蹄疫病毒(foot-and-mouth disease virus, FMDV)同属于小RNA病毒科, 感染猪表现为鼻部和蹄冠部出现水泡、溃烂等与FMDV相似的临床症状[22]。我们已经证明了CTSS通过促进FMDV诱导的干扰素及其他抗病毒细胞因子产生而抑制FMDV复制, 但宿主CTSS在SVV感染中的作用及其调控机制至今尚未阐明。为阐明宿主CTSS在SVV感染过程中发挥的作用, 发现宿主CTSS能够抑制SVV在IBRS-2细胞中复制, 而SVV复制增加了宿主CTSS的表达量及其酶活性, 进一步研究发现过表达CTSS促进SVV诱导的抗病毒细胞因子mRNA水平, 明确了宿主CTSS抑制SVV复制的初步原因。本研究结果为更深层次探究猪源CTSS在SVV触发的免疫应答中的作用机制奠定了基础。
1 材料和方法 1.1 材料 SVV株CH-FJ-2017和IBRS-2细胞由中国农业科学院兰州兽医研究所口蹄疫与新发病流行病学团队保存;SVV抗体由本实验室制备;兔多克隆抗体CTSS购于Abcam公司;鼠抗Flag单抗、鼠抗Myc单抗、鼠抗β-actin单抗、HRP标记山羊抗鼠IgG二抗和HRP标记山羊抗兔IgG二抗均购于Thermo Scientific公司。
RNA抽提试剂Trizol、5× Prime script RT Master Mix、SYBR Permix Ex Taq Ⅱ和蛋白预染Marker均购于宝生物工程大连有限公司;LipofectamineTM 2000转染试剂购于Invitrogen公司;Opti-MEM、100×青霉素-链霉素溶液、0.25% EDTA胰酶和胎牛血清(FBS)均购于Gibco公司;DMEM培养基和PBS溶液购于健顺公司;ECL显色剂购于Thermo Scientific公司;细胞裂解液和PMSF购于碧云天公司;组织蛋白酶S试剂盒(货号ab65306)购于艾博抗(上海)贸易有限公司;CTSS干扰序列由上海吉玛制药有限公司合成。
1.2 SVV对内源性CTSS mRNA水平的影响 将IBRS-2细胞按1×105接种于35 mm小皿中, 培养至80%-90%, 用MOI为0.5的SVV感染IBRS-2细胞, 接毒后0、4、8、12、16 h分别收取细胞样品。提取细胞总RNA, 利用RT-qPCR方法检测CTSS的mRNA表达(定量引物信息见表 1)。
表 1. 引物序列信息 Table 1. Informations of primer sequence
| Genes | Primers (5′→3′) |
| P-CTSS | Forward: ATGAGTTGCGTGAGAGTTCC |
| Reverse: ACAAGAACCACAAGAACCCTG | |
| P-GAPDH | Forward: ACATGGCCTCCAAGGAGTAAGA |
| Reverse: GATCGAGTTGGGGCTGTGACT | |
| P-IFN-β | Forward: GGCTGGAATGAAACCGTCAT |
| Reverse: TCCAGGATTGTCTCCAGGTCA | |
| P-IFN-γ | Forward: CATTCAAGTGCTGTCTGACATG |
| Reverse: GATCGGTGTGCCTGCCTTC | |
| P-IL-6 | Forward: GGCATCACCTTTGGCATCTT |
| Reverse: AGTTTTCCTGCTTTCTGCAGCT | |
| NF-κB(p65) | Forward: TTCTTTCAAACAAAGGACCAGA |
| SVV | Reverse: GCAACCCAAGTAACCCTTAAAG |
| Forward: GAATTTGGAAGCCATGCTC | |
| Reverse: AGCCAACATAGAR(A)ACCAGATTGC | |
| 3D: TTCAAACCAGGAACACTACTCCGAGA-BHQ1 |
表选项
1.3 CTSS酶活性测定 将IBRS-2细胞按1×104接种于48孔板中, 培养至70%-80%, 用MOI为0.5的SVV感染IBRS-2细胞, 接毒后0、4、8、12、16 h分别收取细胞样品。收集1×106-5×106个细胞, 取CTSS试剂盒(货号ab65306, Abcam)中的100 μL裂解液裂解细胞, 在微量离心机中以最高速度离心5 min, 取50 μL上清于96孔板, 加等量反应缓冲液和10 mmol/L Ac-VVR-AFC (CTSS底物), 根据试剂盒说明书进行处理。使用SpectraMax M5荧光计在400 nm激发波长和505 nm发射波长下测量荧光。
1.4 过表达CTSS对SVV复制影响的检测 IBRS-2细胞消化后接种于细胞培养板中, 待细胞长至70%-80%时, 将CTSS真核表达质粒与Lip2000试剂(DNA: Lip2000=1 μg: 2 μL)分别加至Opti-MEM中, 混合后静置15 min, 将Opti-MEM混合物直接加至细胞中, 培养24 h后感染SVV。用无血清的DMEM清洗细胞, 将SVV稀释至MOI为0.5时感染IBRS-2细胞, 并设不感染对照组, 置于37 ℃、5% CO2培养箱孵育1 h之后, 弃去病毒液, 用含2% FBS的维持液继续培养。12 h后收取两份细胞样品, 一份提取总RNA用于RT-qPCR检测SVV拷贝数的变化, 并以猪源GAPDH作为内参检测CTSS转录水平变化;一份用含β-巯基乙醇的5×SDS Loading Buffer处理, 用于Western blotting (WB)检测CTSS和SVV蛋白水平的变化, 并以β-actin为内参。
1.5 siRNA干扰效果及其对SVV复制影响的检测 将状态良好的IBRS-2细胞按1×104接种于12孔板, 培养至70%左右, 转染50 μmol、150 μmol siRNA-2947以及siRNA-NC, 36 h后用MOI 0.5的SVV感染细胞, 12 h后收取细胞样品, 一部分提取总RNA, RT-qPCR检测SVV拷贝数的变化, 另一部分用于WB检测CTSS和SVV蛋白水平的变化。
1.6 抗病毒细胞因子mRNA水平检测 按1.4制备样品, 提取RNA, 采用RT-qPCR检测猪源IFN-γ、IL-6、NF-κB转录水平的变化。
1.7 数据分析 所有实验至少重复3次, 应用GraphPad Prism 7软件进行分析并作图, 使用单因素方差分析进行统计学分析(*:P < 0.05表示数据具有统计学意义, **:P < 0.01表示数据具有显著性差异, ***:P < 0.001表示数据间具有极显著性差异)。
2 结果和分析 2.1 SVV上调内源性CTSS的mRNA水平 将IBRS-2细胞接种于35 mm小皿中, 待细胞长至80%-90%, 用0.5 MOI的SVV感染IBRS-2细胞, 在0、4、8、12、16 h后收取细胞样品, 利用RT-qPCR方法检测内源性CTSS的变化。结果表明SVV感染IBRS-2细胞后内源性CTSS的mRNA水平升高(图 1), 提示SVV感染促进宿主细胞内源性CTSS的表达。
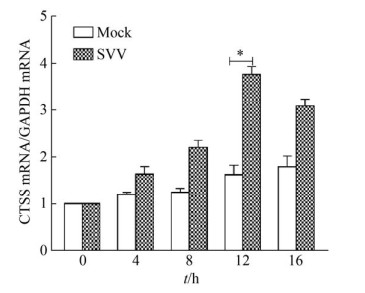 |
| 图 1 SVV感染上调内源性CTSS的mRNA水平 Figure 1 The mRNA levels of endogenous CTSS was up-regulated by SVV infection. |
| 图选项 |
2.2 SVV感染能显著增强CTSS酶活性 转染0.25 μg CTSS重组质粒至IBRS-2细胞, 20 h后用SVV (MOI=0.5)感染细胞, 同时设不用病毒刺激的对照组, 收取0、4、8、12、16 h细胞样品, 裂解细胞用Fluorometric Method检测CTSS酶活性, 结果表明SVV感染能显著增强CTSS酶活性, 且随着SVV感染时间的增加, CTSS的活性也随之增强(图 2)。
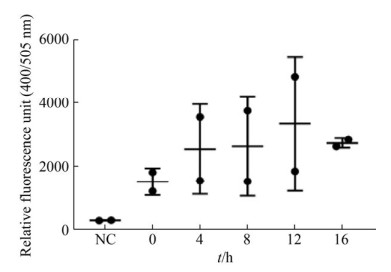 |
| 图 2 SVV感染上调CTSS酶活性 Figure 2 The activity of CTSS was up-regulated by SVV infection. |
| 图选项 |
2.3 过表达CTSS抑制SVV复制 转染0、1、3、5 μg pcDNA3.1-CTSS-Myc至IBRS-2细胞, 24 h后用SVV (MOI=0.5)感染细胞, 并设Mock为对照, 12 h后收取细胞样品检测其对SVV复制的调控作用, 发现随着CTSS表达量增加(图 3-A, C), SVV复制水平呈现剂量依赖性降低(图 3-B, C)。结果表明, 过表达CTSS抑制SVV在IBRS-2细胞中复制。
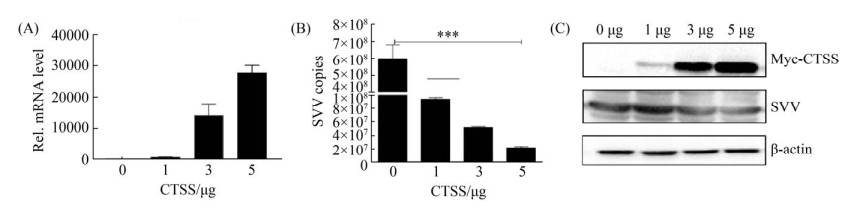 |
| 图 3 过表达CTSS抑制SVV的复制 Figure 3 SVV replication was inhibited by overexpression CTSS. A: the mRNA level of CTSS was detected by qPCR; B: copies number of SVV was detected by RT-qPCR; C: the Protein level of CTSS and SVV was detected. |
| 图选项 |
2.4 siRNA敲低CTSS促进SVV复制 为进一步确定宿主CTSS对SVV复制的影响, 将50、150 μmol siRNA-2947转染至IBRS-2细胞, 以siRNA-NC为对照, 在36 h后用等量SVV (MOI=0.5)感染细胞, 用RT-qPCR和WB检测siRNA-2947对CTSS表达及SVV复制的影响, 结果表明siRNA-2947能下调宿主CTSS的表达(图 4-A, 4-C)而促进SVV在IBRS-2细胞中复制(图 4-B, 4-C)。
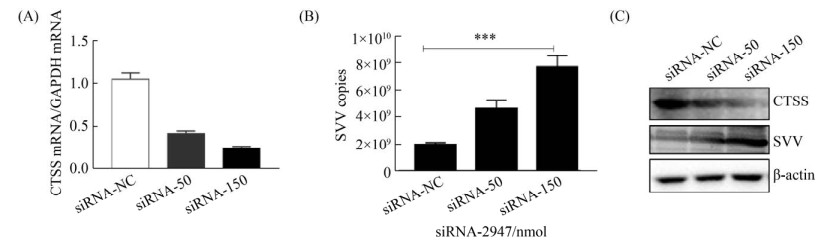 |
| 图 4 siRNA敲低CTSS促进SVV复制 Figure 4 SiRNA knocked down CTSS to promote SVV replication. A: the mRNA level of CTSS was detected by RT-qPCR; B: copies number of SVV was detected by RT-qPCR; C: WB to detect the changes of CTSS and SVV protein levels. |
| 图选项 |
2.5 过表达CTSS促进SVV诱导的宿主抗病毒细胞因子的转录 为明确CTSS抑制SVV复制的原因, 检测宿主CTSS是否影响SVV诱导的抗病毒细胞因子产生。在IBRS-2细胞中转染pcDNA3.1和pcDNA3.1-CTSS-Myc, 24 h后感染SVV (MOI=0.5), 并设不接毒刺激的对照组, 12 h后收集细胞处理样品, RT-qPCR结果显示, 在IBRS-2细胞中能检测到IFN-γ、IL-6、NF-κB, CTSS可促进SVV诱导的IFN-γ、IL-6、NF-κB的上调表达实验结果表明宿主CTSS能激活SVV诱导的宿主抗病毒细胞因子的转录水平(图 5)。
 |
| 图 5 过表达CTSS促进SVV诱导的抗病毒细胞因子的上调表达 Figure 5 Overexpression of CTSS promoted up-regulation of antiviral cytokines induced by SVV. A: the mRNA level of IFN-γ was detected by RT-qPCR; B: the mRNA level of IL-6 was detected by RT-qPCR; C: the mRNA level of NF-κ B was detected by RT-qPCR. |
| 图选项 |
3 讨论 CTSS是一种溶酶体蛋白酶, 主要在抗原呈递细胞中表达, 其活性调节对于MHC-Ⅱ信号传导及CD4+ T细胞介导的免疫反应激活非常重要。研究报道CTSS活性可以由肠道菌群调节, 共生体触发生理性CTSS活性, 而病原体引起病理性CTSS活性增加, 病理性CTSS活性增加导致T细胞活化和增殖, 进而加速宿主免疫反应[23]。本研究用SVV感染IBRS-2, WB和RT-qPCR检测发现SVV感染上调内源性CTSS的表达, SVV感染也能使CTSS活性增加, 其具体机制还有待进一步探究。
病原微生物通过入侵宿主后破坏宿主防御系统, 从而达到成功感染宿主的目的, 当然其生命周期也受不同宿主因素影响。有研究报道SVV 3Cpro可以通过切割或降解天然免疫接头蛋白以逃避宿主抗病毒天然免疫反应[24];SVV 3Cpro也可以通过去泛素化关键信号分子RIG-Ⅰ、TBK1和TRAF3负调节Ⅰ型IFN从而逃避先天免疫反应[25]。SVV 3Dpro是一种RNA依赖的RNA聚合酶, 对于病毒复制是必不可少的[26], E2泛素结合酶UBE2L6通过泛素化SVV 3D从而促进SVV感染[27]。本研究发现过表达CTSS抑制SVV在IBRS-2细胞中复制, 而下调内源性CTSS能促进SVV复制。作者又探究了宿主CTSS对SVV诱导的抗病毒细胞因子产生的影响, 有报道称CTSS通过加强结合膜CX3CL1脱落并生成可溶性CX3CL1, 使CX3CL1与CX3CR1相互作用, 将免疫细胞募集到炎症部位, 从而增加CX3CL1脱落进入间质, 改变自身免疫性泪腺炎和泪腺分泌[28]。SVV感染还会诱导明显的T细胞反应, 研究表明感染后第10天观察到与IFN-γ产生相关的CD8+和双阳性CD4+ CD8+ T细胞增加[29]。干燥综合征患者泪液中CTSS活性升高可诱导促炎细胞因子上调表达[30], 这提示CTSS可能与炎症有关。因此我们随后检测了CTSS对促炎细胞因子产生的影响, RT-qPCR结果显示CTSS可促进SVV诱导的IL-6、IFN-γ、NF-κB的mRNA表达。研究表明CTSS在体外能够切割IL-6R, 释放的sIL-6R具有生物活性, 并能诱导IL-6反式信号转导, 这说明CTSS在炎症方面有相当重要的作用[31]。天然杀伤细胞(NKT)可调节多种免疫反应, NKT细胞分泌Th1细胞因子(IFN-γ)和Th2细胞因子(包括IL-4), 它们募集并激活其他先天免疫细胞以加剧肝脏中的炎症反应, CTSB和CTSS抑制剂可降低LPS诱导的炎症过程中NKT细胞的活化[32]。先天免疫是宿主抵抗病原体感染的第一道防线, 据报道RIG-Ⅰ对SVV发挥重要的抗病毒作用, 它主要负责SVV感染过程中Ⅰ型干扰素信号通路的激活。这表明CTSS促进SVV诱导的抗病毒细胞因子的表达, 可能是宿主CTSS抑制SVV复制的原因之一, 具体详细机制还需要进一步研究。
本研究首次证明了CTSS在SVV感染过程中发挥抗病毒作用的新功能, 为宿主CTSS拮抗SVV感染提供了依据, 为下一步探究宿主CTSS在SVV触发的免疫应答中的作用积累了素材;同时也提示CTSS可能作为抑制SVV复制的潜在靶点发挥作用。
4 结论 CTSS可调节多种生理和病理过程, 本研究通过WB和RT-qPCR检测发现SVV感染能上调内源性CTSS的表达, 利用过表达和特异性siRNA实验证明了CTSS能抑制SVV在IBRS-2细胞中复制, SVV感染细胞过程中宿主CTSS能上调SVV诱导的抗病毒细胞因子表达, 从而抑制SVV复制, 这说明SVV感染与宿主CTSS之间存在相互调节作用。
References
| [1] | Zhang JQ, Pi?eyro P, Chen Q, Zheng Y, Li GW, Rademacher C, Derscheid R, Guo BQ, Yoon KJ, Madson D, Gauger P, Schwartz K, Harmon K, Linhares D, Main R. Full-length genome sequences of Senecavirus A from recent idiopathic vesicular disease outbreaks in US swine. Genome Announcements, 2015, 3(6): e01270-15. |
| [2] | Hales LM, Knowles NJ, Reddy PS, Xu L, Hay C, Hallenbeck PL. Complete genome sequence analysis of Seneca Valley virus-001, a novel oncolytic picornavirus. The Journal of General Virology, 2008, 89(Pt 5): 1265-1275. |
| [3] | Venkataraman S, Reddy SP, Loo J, Idamakanti N, Hallenbeck PL, Reddy VS. Structure of Seneca valley virus-001:an oncolytic picornavirus representing a new genus. Structure, 2008, 16(10): 1555-1561. DOI:10.1016/j.str.2008.07.013 |
| [4] | Coffin RS. From virotherapy to oncolytic immunotherapy: where are we now?. Current Opinion in Virology, 2015, 13: 93-100. DOI:10.1016/j.coviro.2015.06.005 |
| [5] | Reddy PS, Burroughs KD, Hales LM, Ganesh S, Jones BH, Idamakanti N, Hay C, Li SS, Skele KL, Vasko AJ, Yang JP, Watkins DN, Rudin CM, Hallenbeck PL. Seneca valley virus, a systemically deliverable oncolytic picornavirus, and the treatment of neuroendocrine cancers. JNCI: Journal of the National Cancer Institute, 2007, 99(21): 1623-1633. DOI:10.1093/jnci/djm198 |
| [6] | Pasma T, Davidson S, Shaw SL. Idiopathic vesicular disease in swine in Manitoba. Can Vet J, 2008, 49(1): 84-85. |
| [7] | Corner S SK. Seneca valley virus and vesicular lesions in a pig with idiopathic vesicular disease. Journal of Veterinary Science & Technology, 2012, 3(6). DOI:10.4172/2157-7579.1000123 |
| [8] | Leme R, Alfieri A, Alfieri A. Update on Senecavirus infection in pigs. Viruses, 2017, 9(7): 170. DOI:10.3390/v9070170 |
| [9] | Zhao X, Wu Q, Bai Y, Chen G, Zhou L, Wu Z, Li Y, Zhou W, Yang H, Ma J. Phylogenetic and genome analysis of seven Senecavirus A isolates in China. Transboundary and Emerging Diseases, 2017, 64(6): 2075-2082. DOI:10.1111/tbed.12619 |
| [10] | Leme RA, Oliveira TES, Alcantara BK, Headley SA, Alfieri AF, Yang M, Alfieri AA. Clinical manifestations of Senecavirus A infection in neonatal pigs, Brazil, 2015. Emerging Infectious Diseases, 2016, 22(7): 1238-1241. DOI:10.3201/eid2207.151583 |
| [11] | Saeng-Chuto K, Rodtian P, Temeeyasen G, Wegner M, Nilubol D. The first detection of Senecavirus A in pigs in Thailand, 2016. Transboundary and Emerging Diseases, 2018, 65(1): 285-288. DOI:10.1111/tbed.12654 |
| [12] | Imus JK, Lehmkuhl HD, Woods LW. Resistance of colostrum-deprived domestic lambs to infection with deer adenovirus. Journal of Veterinary Diagnostic Investigation, 2019, 31(1): 78-82. DOI:10.1177/1040638718817508 |
| [13] | Wiener JJM, Wickboldt AT Jr, Wiener DK Jr, Lee-Dutra A Jr, Edwards JP Jr, Karlsson L Jr, Nguyen S Jr, Sun SQ Jr, Jones TK Jr, Grice CA Jr. Discovery and SAR of novel pyrazole-based thioethers as cathepsin S inhibitors. Part 2:Modification of P3, P4, and P5 regions. Bioorganic & Medicinal Chemistry Letters, 2010, 20(7): 2375-2378. |
| [14] | Fonovi? UP, Jevnikar Z, Kos J. Cathepsin S generates soluble CX3CL1(fractalkine) in vascular smooth muscle cells. Biological Chemistry, 2013, 394(10): 1349-1352. DOI:10.1515/hsz-2013-0189 |
| [15] | Thanei S, Theron M, Silva AP, Reis B, Branco L, Schirmbeck L, Kolb FA, Haap W, Schindler T, Trendelenburg M. Cathepsin S inhibition suppresses autoimmune-triggered inflammatory responses in macrophages. Biochemical Pharmacology, 2017, 146: 151-164. DOI:10.1016/j.bcp.2017.10.001 |
| [16] | Olson OC, Joyce JA. Cysteine cathepsin proteases: regulators of cancer progression and therapeutic response. Nature Reviews Cancer, 2015, 15(12): 712-729. DOI:10.1038/nrc4027 |
| [17] | Zhang J, Shan Y, Li Y, Luo XP, Shi HM. Palmitate impairs angiogenesis via suppression of cathepsin activity. Molecular Medicine Reports, 2017, 15(6): 3644-3650. DOI:10.3892/mmr.2017.6463 |
| [18] | Riese RJ, Wolf PR, Br?mme D, Natkin LR, Villadangos JA, Ploegh HL, Chapman HA. Essential role for cathepsin S in MHC class Ⅱ-associated invariant chain processing and peptide loading. Immunity, 1996, 4(4): 357-366. DOI:10.1016/S1074-7613(00)80249-6 |
| [19] | Kim SJ, Sch?tzle S, Sohail Ahmed S, Haap W, Jang SH, Gregersen PK, Georgiou G, Diamond B. Increased cathepsin S in Prdm1?/? dendritic cells alters the T FH cell repertoire and contributes to lupus. Nature Immunology, 2017, 18(9): 1016-1024. DOI:10.1038/ni.3793 |
| [20] | Figueiredo JL, Aikawa M, Zheng CY, Aaron J, Lax L, Libby P, de Lima Filho JL, Gruener S, Fingerle J, Haap W, Hartmann G, Aikawa E. Selective cathepsin S inhibition attenuates atherosclerosis in apolipoprotein E-deficient mice with chronic renal disease. The American Journal of Pathology, 2015, 185(4): 1156-1166. DOI:10.1016/j.ajpath.2014.11.026 |
| [21] | Kim S, Jin HE, Seo HR, Lee HJ, Lee YS. Regulating BRCA1 protein stability by cathepsin S-mediated ubiquitin degradation. Cell Death & Differentiation, 2019, 26(5): 812-825. |
| [22] | Leme RA, Zotti E, Alcantara BK, Oliveira MV, Freitas LA, Alfieri AF, Alfieri AA. Senecavirus A: an emerging vesicular infection in Brazilian pig herds. Transboundary and Emerging Diseases, 2015, 62(6): 603-611. DOI:10.1111/tbed.12430 |
| [23] | Steimle A, Gronbach K, Beifuss B, Sch?fer A, Harmening R, Bender A, Maerz JK, Lange AN, Michaelis L, Maurer A, Menz S, McCoy K, Autenrieth IB, Kalbacher H, Frick JS. Symbiotic gut commensal bacteria act as host cathepsin S activity regulators. Journal of Autoimmunity, 2016, 75: 82-95. DOI:10.1016/j.jaut.2016.07.009 |
| [24] | Qian SH, Fan WC, Liu TT, Wu MG, Zhang HW, Cui XF, Zhou Y, Hu JJ, Wei SZ, Chen HC, Li XM, Qian P. Seneca valley virus suppresses host type Ⅰ interferon production by targeting adaptor proteins MAVS, TRIF, and TANK for cleavage. Journal of Virology, 2017, 91(16): e00823-17. |
| [25] | Xue Q, Liu HS, Zhu ZX, Yang F, Xue QH, Cai XP, Liu XT, Zheng HX. Seneca Valley Virus 3C protease negatively regulates the type Ⅰ interferon pathway by acting as a viral deubiquitinase. Antiviral Research, 2018, 160: 183-189. DOI:10.1016/j.antiviral.2018.10.028 |
| [26] | Kempf BJ, Barton DJ. Picornavirus RNA polyadenylation by 3Dpol, the viral RNA-dependent RNA polymerase. Virus Research, 2015, 206: 3-11. DOI:10.1016/j.virusres.2014.12.030 |
| [27] | Li L, Bai J, Fan H, Yan JF, Li SH, Jiang P. E2 ubiquitin-conjugating enzyme UBE2L6 promotes Senecavirus A proliferation by stabilizing the viral RNA polymerase. PLoS Pathogens, 2020, 16(10): e1008970. DOI:10.1371/journal.ppat.1008970 |
| [28] | Fu RZ, Guo H, Janga S, Choi M, Klinngam W, Edman MC, Hamm-Alvarez SF. Cathepsin S activation contributes to elevated CX3CL1(fractalkine) levels in tears of a Sj?gren's syndrome murine model. Scientific Reports, 2020, 10: 1455. DOI:10.1038/s41598-020-58337-4 |
| [29] | Maggioli MF, Lawson S, de Lima M, Joshi LR, Faccin TC, Bauermann FV, Diel DG. Adaptive immune responses following Senecavirus A infection in pigs. Journal of Virology, 2018, 92(3): e01717-17. |
| [30] | Klinngam W, Fu RZ, Janga S, Edman M, Hamm-Alvarez S. Cathepsin S alters the expression of pro-inflammatory cytokines and MMP-9, partially through protease-activated receptor-2, in human corneal epithelial cells. International Journal of Molecular Sciences, 2018, 19(11): 3530. DOI:10.3390/ijms19113530 |
| [31] | Flynn CM, Garbers Y, Düsterh?ft S, Wichert R, Lokau J, Lehmann CHK, Dudziak D, Schr?der B, Becker-Pauly C, Rose-John S, Aparicio-Siegmund S, Garbers C. Cathepsin S provokes interleukin-6(IL-6) trans-signaling through cleavage of the IL-6 receptor in vitro. Scientific Reports, 2020, 10: 21612. DOI:10.1038/s41598-020-77884-4 |
| [32] | De Mingo Pulido á, de Gregorio E, Chandra S, Colell A, Morales A, Kronenberg M, Marí M. Differential role of cathepsins S and B in hepatic APC-mediated NKT cell activation and cytokine secretion. Frontiers in Immunology, 2018, 9: 391. DOI:10.3389/fimmu.2018.00391 |
