万佳宏, 常佳伟, 魏彦琴, 马强, 王桂琴


宁夏大学农学院, 宁夏银川 750021
收稿日期:2020-06-26;修回日期:2020-10-20;网络出版日期:2021-01-28
基金项目:国家自然科学基金(31760748)
*通信作者:王桂琴, Tel: +86-951-2061811;Fax: +86-951-2061897;E-mail: nxwgq@126.com.
摘要:[目的] 了解宁夏地区奶牛乳腺炎金黄色葡萄球菌(Staphylococcus aureus,SA)代表菌株的基因组序列基本特征,进一步探究其耐药基因型、毒力及进化关系,为兽医临床防治提供理论依据。[方法] 采用纸片法对97株金黄色葡萄球菌临床分离株进行抗菌药物敏感性试验,同时进行葡萄球菌蛋白A(Staphylococcus aureus protein A,spa)分型、多位点序列分型(multilocus sequence typing,MLST),根据分型结果选取16株代表菌株进行全基因组测序,并对获得的测序序列进行处理分析。[结果] 药敏试验结果显示97株分离株对18种抗菌药物存在不同程度的耐药,其中9株耐甲氧西林金黄色葡萄球菌(methicillin-resistantStaphylococcus aureus,MRSA)对青霉素、氨苄西林、苯唑西林、头孢噻呋、磺胺异噁唑、红霉素、庆大霉素和克林霉素等8种抗菌药物完全耐药,甲氧西林敏感金黄色葡萄球菌(methicillin-sensitiveStaphylococcus aureus,MSSA)菌株对青霉素、氨苄西林、磺胺异噁唑耐药率较高。耐药基因数据库(antibiotic resistance genes database,ARDB)注释分析显示16株代表菌株共携带21种耐药基因,其中norA、tet38、bacA、mepA的携带率较高,与药敏试验结果具有一定的相关性。毒力基因数据库(virulence factors of pathogenic bacteria,VFDB)注释分析显示所有菌株携带多种与粘附、宿主免疫逃逸、分泌、胞外酶编码、铁摄取等疾病相关的毒力基因,MRSA菌株均携带较多毒力因子,MSSA菌株携带毒力因子数目不等。基因岛预测结果显示16株代表菌株存在不同数量的基因岛且MRSA菌株携带基因岛数目及毒力基因岛较多,但耐药基因岛数目与MSSA差异不明显。SNP分析结果显示部分分离株同源性较高,同源性较高的两株MRSA的全基因组基本序列特征差异较小,携带的耐药、毒力基因情况相似。[结论] 宁夏地区牛源SA分离株耐药性情况严重且具有较高的毒力水平,本研究为家畜相关MRSA(livestock-associated MRSA,LA-MRSA)与MSSA基因组序列信息的比较分析及宁夏地区SA感染的临床防控提供参考依据。
关键词:金黄色葡萄球菌全基因组测序耐药性毒力
Whole-genome analysis of bovine Staphylococcus aureus strains in Ningxia from 2016 to 2017
Jiahong Wan, Jiawei Chang, Yanqin Wei, Qiang Ma, Guiqin Wang


College of Agricultural, Ningxia University, Yinchuan 750021, Ningxia Hui Autonomous Region, China
Received: 26 June 2020; Revised: 20 October 2020; Published online: 28 January 2021
*Corresponding author: Guiqin Wang, Tel: +86-951-2061811; Fax: +86-951-2061897; E-mail: nxwgq@126.com.
Foundation item: Supported by the National Natural Science Foundation of China (31760748)
Abstract: [Objective] To understand the basic characteristics of the genome sequence of the representative strain of Staphylococcus aureus from dairy cows with mastitis in Ningxia, and to further explore its drug-resistant genotype, virulence and evolutionary relationship, to serve as a theoretical basis for veterinary clinical prevention and treatment. [Methods] In total 97 clinical isolates of S. aureus were tested for antimicrobial susceptibility by the paper disk method. Staphylococcal protein A typing and multi-site sequence typing were done at the same time. According to the typing results, 16 representative strains were sequenced and the obtained sequences were analyzed by network database. [Results] Drug susceptibility test shows that 97 strains were resistant to 18 antibacterial drugs, of which 9 strains of methicillin-resistant Staphylococcus aureus (MRSA) were completely resistant to 8 antibacterial drugs, such as penicillin, ampicillin, oxacillin, ceftiofur, sulfisoxazole, erythromycin, gentamicin, and clindamycin. Methicillin-sensitive Staphylococcus aureus (MSSA) strains were highly resistant to penicillin, ampicillin, and sulfisoxazole. Antibiotic resistance genes database (ARDB) annotation analysis shows that 16 representative strains carried 21 drug resistance genes, among which the carrying rates of norA, tet38, bacA, and mepA were higher, correlating with to the results of drug sensitivity test. Virulence Factors Database (VFDB) annotation analysis shows that all strains carried a variety of virulence genes related to diseases such as adhesion, host immune escape, secretion, extracellular enzyme encoding, and iron uptake. MRSA strains all carried more virulence factors, MSSA strains carried different number of virulence factors. The results of gene island prediction show that 16 representative strains had different number of gene islands, and MRSA strains carried more gene islands and virulence gene islands, but the number of drug resistance gene islands was not significantly different from that of MSSA. The results of SNP analysis show that some isolates had higher homology, and the two MRSA with higher homology had little differences in the basic sequence characteristics of the whole genome, both carried similar resistance and virulence genes. [Conclusion] The drug resistance of S. aureus isolates from cattle in Ningxia is serious and highly virulent. Our findings provide a reference for the comparative analysis of genome sequence information of livestock-associated MRSA (LA-MRSA) and MSSA, and the clinical prevention and control of S. aureus infection in Ningxia.
Keywords: Staphylococcus aureuswhole genome sequencingdrug resistancevirulence
奶牛乳腺炎是奶牛乳腺受到创伤、化学刺激或微生物感染引起乳腺发生炎症的过程[1],而研究表明大多数病例是由细菌侵袭乳腺所引起[2-3]。奶牛养殖业是宁夏的重要经济产业,每年由SA感染引起的乳腺炎给奶牛养殖业造成了严重的经济损失[4]。SA作为人类皮肤感染中的常见病原体,是医院感染的主要原因,也是全世界奶牛乳腺炎的重要病原体之一[5],其耐药菌株MRSA通常通过携带多种耐药基因、毒力基因或形成生物被膜等方式快速适应生存环境而产生耐药性[6-7],给公共卫生造成严重的压力。SA的致病性由多种毒力因子决定,因此不同MSSA、MRSA感染的乳房炎严重程度也存在差异,主要与SA毒力因子的缺失、存在及组合等有关[8]。由于SA含有多种可移动的遗传元件,其携带的毒力因子也具有一定差异,并且可通过其水平基因的转移而发生进化[9],调控各种耐药和毒力因子的表达。与医院获得性MRSA (healthcare-associated MRSA,HA-MRSA)和社区获得性MRSA (community associated MRSA,CA-MRSA)相比,LA-MRSA的毒力相关研究数据仍有限,并且不同克隆群体的SA通常具有不同的遗传特征,某些特定毒力因子的携带存在一定差异,其致病能力存在显著差异。因此探究不同克隆群体的MSSA或LA-MRSA的毒力因子分布规律,对于SA感染性疾病的防控具有重要意义。
本研究对宁夏地区乳腺炎患牛牛乳中分离出的97株SA进行抗菌药物敏感性测试及spa分型、MLST及MRSA SCCmec分型,根据分型结果选取16株代表菌株进行全基因组测序分析,重点分析其耐药情况、毒力因子及基因岛,并对其遗传进化关系进行了分析比较,旨在发现宁夏地区牛源MRSA、MSSA的毒力特征,为研究宁夏地区LA-MRSA与MSSA之间的毒力差异以及SA感染的防控提供理论依据。
1 材料和方法 1.1 样品来源 质控菌株ATCC 29213由中国农业大学国家兽药安全评价中心惠赠;97株试验菌株是本实验室在2016–2017年宁夏银川、吴忠、永宁、平罗、贺兰5个地区共10个规模化奶牛养殖场临床型和隐形型乳腺炎500份乳样中分离鉴定的(表 1)。
表 1. 97株菌株的来源及分离情况 Table 1. Sources and isolation of 97 strains
| Sampling areas | Farm ID | Number of samples | Separation rate | Sampling date |
| Yinchuan | 1 | 64 | 20.3% (13/64) | 201607 |
| 2 | 56 | 17.9% (10/56) | ||
| Wuzhong | 3 | 42 | 21.4% (9/42) | 201607 |
| 4 | 56 | 19.6% (11/56) | ||
| Yongning | 5 | 45 | 22.2% (10/45) | 201609 |
| 6 | 40 | 15.0% (6/40) | ||
| Pingluo | 7 | 67 | 20.1% (14/67) | 201709 |
| Helan | 8 | 31 | 12.9% (4/31) | 201706 |
| 9 | 46 | 19.5% (9/46) | ||
| 10 | 53 | 20.8% (11/53) |
表选项
1.2 主要试剂和仪器 BHI肉汤、BHI琼脂培养基,北京陆桥技术有限公司;细菌基因组提取试剂盒、溶菌酶、基因引物spa-f、spa-r、arcC、aroE、glpF、gmk、pta、tpi、ypil、mec、ccr,北京博迈德基因技术有限公司;Qubit dsDNA HS Assay Kit (荧光定量试剂盒),美国Life Tech公司;micro TUBE AFA Fiber Screw Cap (超声破碎管),美国Covaris公司;KAPA HyperPlus DNA Library Prep Kit for Illumina (建库试剂盒),Kapa Biosystems公司;Agencourt AMPure XP beads (筛选片段所需磁珠),美国Beckman Coulter公司;DNA chips (定量文库所需芯片),美国Agilent公司;药敏纸片,中国兽医药品监察所。
1.3 抗菌药物敏感性测定 根据美国临床实验室标准委员会(Clinical and Laboratory Standards,CLSI)推荐的纸片法测定18种抗菌药物对试验菌株的敏感值,ATCC 29213为质控菌,结果判定参照CLSI推荐标准。
1.4 菌株基因组的提取 根据细菌基因组DNA提取试剂盒说明书进行提取。
1.5 spa、MLST和MRSA的SCCmec分型 对spa分型的2个基因、MLST分型的金黄色葡萄球菌7个看家基因与SCCmec分型的mec、ccr基因复合群进行PCR扩增、电泳、纯化,将纯化产物送北京博迈德生物技术有限公司进行测序,测序序列提交至数据库,并进行分析处理。所有的基因引物根据参考文献[10]合成。
1.6 文库质检、测序及结果处理 DNA文库送于北京安诺优达基因科技有限公司运用IlluminaHiseq 2500进行高通量测序,以NCTC 8325 (NC-007795.1)为参考基因组,运用分析软件CLC genomic workbench 9.0对测序结果进行拼接组装,得到所测菌株的全基因组序列。
1.7 数据分析 使用GeneMarkets程序检索相关的编码基因;使用RepeatMasker (http://www.repeatmasker.org/)预测散布的重复序列;使用IslandPath-DIOMB程序用于预测基因组岛;使用VFDB、ARDB进行毒力及耐药性分析;使用TreeBeST、PhyML构建系统发育树。
2 结果和分析 2.1 药敏试验结果 质控菌株ATCC 29213 MIC值在质控范围内,且阳性对照呈浑浊状态,阴性对照呈清亮状态,表明试验结果可靠。药敏试验结果显示,97株SA对18种抗菌药物存在不同程度的耐药(图 1),其中9株MRSA菌株对青霉素、氨苄西林、苯唑西林、头孢噻呋、磺胺异噁唑、红霉素、庆大霉素和克林霉素等8种抗菌药物全部耐药,对环丙沙星(22.2%)、左氧氟沙星(22.2%)、四环素(88.9%)、多西环素(77.8%)、氟苯尼考(44.4%)具有不同程度耐药;88株MSSA分离株对青霉素(100%)、氨苄西林(86.4%)、磺胺异噁唑(88.6%)耐药率较高,其次是红霉素(46.4%)、克林霉素(21.6%)和庆大霉素(26.1%)。无论是MRSA还是MSSA对替米考星、阿米卡星、万古霉素、达托霉素和利奈唑胺均100%敏感。
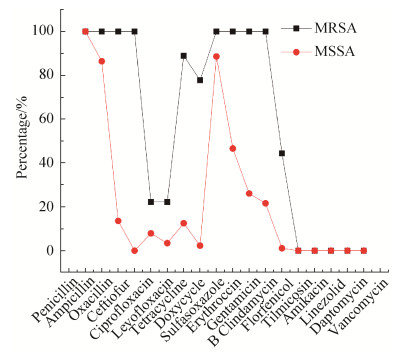 |
| 图 1 97株金黄色葡萄球菌对18种抗生素耐药情况 Figure 1 Resistance of 97 strains of Staphylococcus aureus to 18 antibiotics. |
| 图选项 |
2.2 spa、MLST、SCCmec分型结果与基因组基本特征 运用spa分型对97株SA进行鉴定,一共鉴定出16种spa型,其中t 224 (24,27.3%)、t 518 (22,25.0%)、t 359 (8,9.1%)是88株MSSA的常见spa型,而t 037 (7,77.8%)是9株MRSA菌株的主要spa型。MLST分型一共鉴定出11种ST型,其中ST 50 (31,35.2%)、ST 4053 (29,32.9%)、ST 97 (16,18.2%)是88株MSSA的主要ST型,ST 239为MRSA主要ST型。对9株MRSA进行SCCmec分型,结果显示7株MRSA为SCCmec Ⅲ型,2株MRSA为SCCmec Ⅳ型。
选取16株SA进行全基因组测序,其中包含16种spa型、11种MLST,WLD 11、WLD 19、XWBJ 6为MRSA菌株。16株分离株的基因组序列总长度为2533140–2908428 bp,基因数量为1892–2760个,基因序列中GC含量为33.48%– 34.91% (表 2)。COG功能分类显示,16株菌株所有注释蛋白分布于24个条目中,其中有较多氨基酸转运和代谢相关基因,其次是翻译、核糖体的结构及生物发生、一般功能预测基因、无机离子转运和代谢、功能未知的基因、碳水化合物的运输和代谢等维持菌体基本功能的相关基因占优势。
表 2. 16株分离株分型结果与全基因组序列基本特征 Table 2. Genotyping results and whole genome sequence basic characteristics of 16 isolates
| Bacterial strains | spa | MLST | Total length of gene sequence/bp | Number of genes | GC content/% |
| JY 5 | t 359 | ST 97 | 2533140 | 1892 | 34.91 |
| LN 2 | t 114 | ST 1 | 2775400 | 2644 | 33.61 |
| LN 3 | t 521 | ST 97 | 2743563 | 2592 | 33.52 |
| LN 25 | t 224 | ST 4053 | 2857273 | 2758 | 33.52 |
| LNBJ 26 | t 021 | ST 243 | 2775027 | 2632 | 33.56 |
| M 4 | t 899 | ST 9 | 2797158 | 2658 | 33.55 |
| NM 50 | t 519 | ST 50 | 2668597 | 2480 | 33.60 |
| NM 86 | t 1939 | ST 9 | 2768142 | 2604 | 33.52 |
| NM 87 | t 002 | ST 50 | 2669851 | 2480 | 33.60 |
| NM 90 | t 518 | ST 50 | 2686041 | 2508 | 33.59 |
| RG 1 | t 034 | ST 398 | 2750820 | 2580 | 33.65 |
| ZD 2 | t 3696 | ST 4053 | 2854734 | 2739 | 33.53 |
| WLD 8 | t 5353 | ST 4066 | 2753300 | 2594 | 33.54 |
| WLD 11 | t 037 | ST 239 | 2903457 | 2760 | 33.48 |
| WLD 19 | t 932 | ST 239 | 2908428 | 2756 | 33.48 |
| XWB J6 | t 441 | ST 59 | 2828276 | 2671 | 33.58 |
表选项
2.3 ARDB数据库注释 对16株SA的基因组进行ARDB注释分析,结果显示16株分离株携带21种耐药基因,有氨基糖苷类耐药基因aadD、str、aac-aphD、aph(3’)、ant;β-内酰胺类耐药基因blaZ、mecA;氟喹诺酮类耐药基因norA;四环素类耐药基因tet(K)、tet(M)、tet(L)、tet 38、mepA,大环内酯类耐药基因erm(B)、erm(C)、lnu(B);氯霉素类耐药基因catA、cml,磷霉素耐药基因fosB,博来霉素耐药基因ble;杆菌肽耐药基因bacA。16株代表菌株中norA、tet 38、bacA、mepA的携带率较高,分别为100%、100%、94%、87.5% (图 2-B),将16株分离株携带的耐药基因进行比较,3株MRSA分离株较MSSA菌株携带耐药基因数目多,且MRSA ST 239 (WLD 11、WLD 19)耐药基因数目高达14个(图 2-A),与药敏试验结果具有一定相关性。
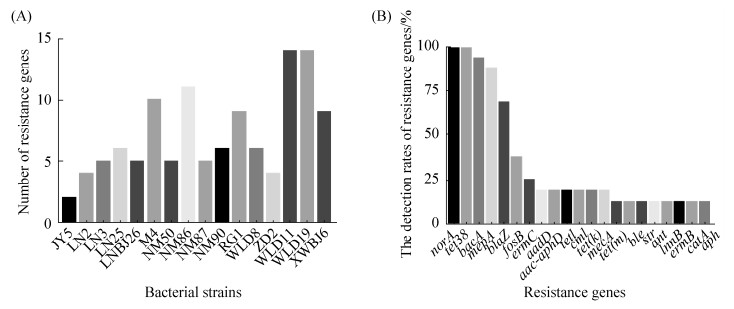 |
| 图 2 16株分离株携带耐药基因情况 Figure 2 Test results of resistance genes in 16 isolates. A: Number of resistance genes carried by a 16 isolates; B: Detection rate of 21 resistance genes. |
| 图选项 |
2.4 VFDB数据库注释及与重要毒力因子比较 对16株试验菌株的基因组进行VFDB注释分析,结果显示16株菌株中含有大量与疾病相关的毒力因子,其中与细菌粘附功能相关的毒力因子有18个,与胞外酶相关的毒力因子有15个,与铁摄取相关的毒力因子有7个,与宿主免疫逃逸相关的毒力基因有39个,与分泌系统相关的毒力基因有8个,编码毒素基因有36个(表 3)。
表 3. 16株分离株的毒力因子检测结果 Table 3. Test results of virulence factors of 16 isolate strains
| Virulence factors | Related genes | Function |
| Autolysin | atl | Adherence |
| Binding protein | ebh | |
| Elastin binding protein | ebp | |
| Extracellular adherence protein/MHC Analogous protein | eap/map | |
| Fibronectin binding proteins | fbpA, fnbA, fnbB | |
| Clumping factor | clfA, clfB | |
| Intercellular adhesin | icaA, icaB, icaC, icaR | |
| Collagen adhesin | can | |
| Staphylococcal protein A | spa | |
| Ser-Asp rich fibrinogen-binding proteins | sdrC, sdrD, sdrE | |
| Serine protease | SplA, SplB, SplC, SplD, SplE, SplF | Exoenzyme |
| Staphylokinase | sak | |
| Hyaluronate lyase | hysA | |
| Thermonuclease | nuc | |
| Serine V8 protease | sspA | |
| Cysteine protease | sspB, sspC, SplD | |
| Lipase | Geh, lip | |
| Capsule | capA, capB, capC, capD, capE, capF, capG, capH, capI, capJ, capK, capL, capM, capN, capO, capP, cap5A, cap5B, cap5E, cap5F, cap5G, cap5H, cap5I, cap5K, cap5M, cap5N, cap8B, cap8C, cap8D, cap8E, cap8G, cap8H, cap8I, cap8J, cap8K, cap8L, cap8M, cap8O, cap8P | Host immune escape |
| Type Ⅶ secretion system | esaA, esaB, esaC, essA, essB, essC, esxA, esxB | Secretion system |
| Exfoliative toxin type A | eta | Toxins |
| Hemolysin | hly/hla, hld, hlb | |
| Enterotoxin-like | sea, seb, sec, seg, seh, sei, sej, selk, selm, seln, selo, selq, selu | |
| Exotoxin | Set 1, set 2, set 3, set 4, set 5, set 6, set 7, set 8, set 9, set 11, set 12, set 13, set 15, set 16, set 22, set 23, set 25, set 26, set 26, set 30, set 31, set 33, set 34, set 35, set 36, set 37, set 39, set 40 | |
| Gamma hemolysin | hlgA, hlgB, hlgC | |
| Leukotoxin E | lukE | |
| Leukotoxin D | lukD | |
| Leukocidin | lukF, lukM, lukS | |
| Tsst-1 toxic shock syndrome toxin-1 | TSST-1 | |
| Heme transporter-related proteins | isdA, isdB, isdC, isdD, isdE, isdF, isdG | Iron uptake |
表选项
将16株分离株的重要毒力因子进行比较,发现所有菌株均携带atl、ebh、ebp、eap/map、spa、icaA、icaB、icaC、icaR等与细菌粘附功能相关的基因;nuc、geh、lip、sak、hysA、sspB、sspC、spl等与编码胞外酶相关基因;isdA、isdG等与铁摄取相关基因;esaB、essA、essB、essC、esxA、esxB等与分泌系统相关基因;多种与宿主免疫逃逸相关毒力基因;eta、多种肠毒素、类毒素、溶血素等毒素基因,而cna、efb、fbpA、fnbA、fnbB、TSST-1、LukS-PV、LukM-PV、LukF-PV、lukD、lukE等毒力因子携带情况存在一定差异(图 3)。LA-MRSA菌株XWBJ 6、WLD 19、WLD 11均携带较多毒力因子,且XWBJ 6携带两种杀白细胞素基因LukS-PV、LukM-PV,WLD 19、WLD 11同时携带lukD和lukE。MSSA菌株携带毒力因子数目不等,WLD 8携带TSST-1,JY 5和LNBJ 26携带LukS-PV,LN 2携带LukF-PV等杀白细胞素基因。
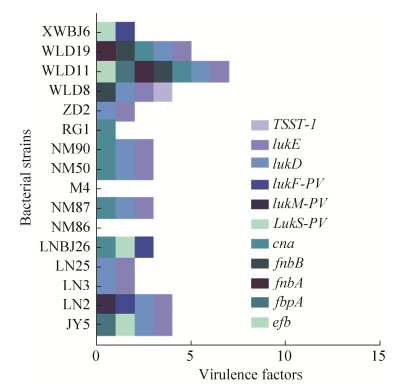 |
| 图 3 16株分离株部分重要毒力因子的携带情况 Figure 3 Carriage of some important virulence factors in 16 isolated strains. |
| 图选项 |
2.5 基因岛预测 通过分析软件对16株试验菌株基因组序列上的基因岛进行预测分析,除JY 5外其余15株菌株都预测到不同数目的基因岛,基因岛编码的产物多是调节因子、假定蛋白、蛋白酶、转座酶,LA-MRSA菌株WLD 11、WLD 19、XWBJ 6携带基因岛数目及毒力基因岛较多,但耐药基因岛数目与MSSA差异不明显(图 4)。
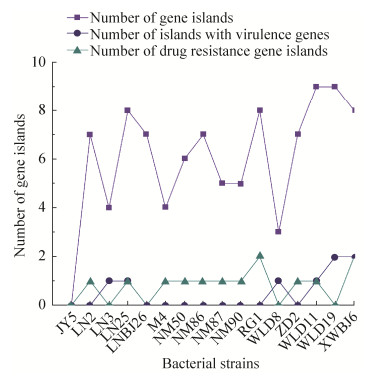 |
| 图 4 16株分离株部分重要毒力因子的携带情况 Figure 4 Carriage of some important virulence factors in 16 isolated strains. |
| 图选项 |
此外,不同菌株携带不同的耐药基因岛与毒力基因岛,毒力岛基因产物主要有致病性岛蛋白、毒素-抗毒素系统、杀白细胞毒素(panton-valentine leucocidin,PVL)假定蛋白、金黄色葡萄球菌致病性岛(Staphylococcus aureus pathogenic islands,SAPIs)、胞外肠毒素Ⅰ型前体等;耐药基因岛产物种类较多,主要有耐甲氧西林调节蛋白mecI、BlaR1、Bla2家族-内酰胺传感器、氯霉素/氟苯尼考MFS运转体FexA、博来霉素耐药蛋白、多药耐药蛋白等(表 4)。
表 4. 16株分离株毒力岛、耐药基因岛的主要基因产物 Table 4. Main gene products of 16 isolated strains of virulence island and drug resistance gene island
| Bacterial strains | Gene products of virulence gene islands | Gene products of drug resistance gene islands |
| JY 5 | – | – |
| LN 2 | Pathogenicity island protein | Multidrug resistance protein、tetracycline transferase TcmP |
| LN 3 | Tandem lipoprotein within pathogenicity island, toxin-antitoxin system | – |
| LN 25 | Tandem lipoprotein within pathogenicity island, toxin-antitoxin system, pathogenicity island SaPI | Methicillin resistance regulatory protein mecI、regulatory protein BlaR1, Bl2a |
| LNBJ 26 | – | – |
| M 4 | – | Chloramphenicol/florfenicol efflux MFS transporter FexA |
| NM 50 | Staphylococcal enterotoxin-like toxin | Bleomycin resistant protein |
| NM 86 | Putative phi PVL-like protein, toxin-antitoxin system, pathogenicity island protein | Multidrug ABC transporter ATP-binding protein |
| NM 87 | – | Methicillin resistant regulatory protein mecI |
| NM 90 | – | Bleomycin resistant protein |
| RG 1 | – | Chloramphenicol/florfenicol efflux MFS transporter FexA, Methicillin resistant regulatory protein mecI, Beta-lactamase, BlaR1 family beta-lactam sensor |
| ZD 2 | Toxin-antitoxin system, putative phi PVL-like protein | Methicillin resistant regulatory protein mecI, BlaR1 family beta-lactam sensor, beta-lactamase |
| WLD 8 | Pathogenicity island protein, toxin-antitoxin system | – |
| WLD 11 | Pathogenicity island SaPI | Beta-lactamase, BlaR1 family beta-lactam sensor, Methicillin resistant regulatory protein mecI |
| WLD 19 | Staphylococcal enterotoxin type Ⅰ, pathogenicity island SaPI, toxin-antitoxin system, putative phi PVL-like protein | – |
| XWBJ 6 | Immunoglobulin G binding protein A precursor, pathogenicity island protein, toxin-antitoxin system, pathogenicity island SaPI, Staphylococcal enterotoxin type Ⅰ | Telithromycin regulatory protein, TetR family regulatory proteins, bleomycin resistant protein |
表选项
2.6 种系进化分析 以WLD 19为参考序列,对16株分离株全基因组进行系统发育树的构建结果如图 5所示,结果表明16株分离株可大致分为6个分支,同一分支同源性较高,JY 5、LN 25、ZD 2、LN 3为一个分支,M 4、NM 86为一个分支,WLD 11、WLD 19同为一个分支,NM 50、NM 87、NM 90为一个分支,WLD 8、LNBJ 6、RG 1、XWBJ 6分别单独为一个分支。SNP分析结果显示部分分离株同源性较高。2株MRSA分离株(WLD 11、WLD 19)的全基因组序列同源性较高,且基因组基本序列特征差异较小,携带的耐药、毒力基因情况相似,与另一株MRSA(XWBJ 6)分离株亲缘关系较远,携带的耐药、毒力基因也具有一定差异。
 |
| 图 5 16株分离株的核心基因组系统发育树 Figure 5 Phylogenetic tree of core genome of 16 isolates. |
| 图选项 |
3 讨论 3.1 SA的耐药性分析 规范动物使用抗菌药物是各界关注的问题,动物使用抗菌药物的不规范不仅会导致动物性食品存在安全隐患,还可以造成细菌发生多药耐药,严重威胁人类健康。对宁夏地区奶牛乳腺炎分离出的97株SA进行药敏试验,结果显示,SA对抗菌药物存在不同程度的耐药。本研究中的LA-MRSA与MSSA的耐药性与孟丹[11]、张行[12]、陈云等[13]报道的牛源SA的耐药性相似,对青霉素、红霉素、四环素有较高耐药性,说明这些地区的SA对青霉素、红霉素和四环素都存在耐药,应不再使用这几种药物;但均未检出万古霉素、利奈唑胺、达托霉素的耐药菌株,主要原因可能是由于万古霉素、利奈唑胺、达托霉素并未投入兽医临床使用,对细菌没有产生选择性压力。本研究中菌株对磺胺异噁唑耐药性较高,主要原因可能是由于本地区磺胺类抗菌药物使用频率较高。对16株牛源SA进行全基因组测序及数据库比对分析发现,16株代表菌株携带21种耐药基因,2株LA-MRSA菌株WLD 11、WLD 19携带耐药基因高达14种,进一步验证了LA-MRSA菌株的多重耐药性。药敏试验结果及全基因组耐药基因携带情况反映了各类抗菌药物的耐药程度,警示需要加强抗生素使用规范,监测各类临床抗菌药物的耐药情况,指导临床用药,减缓耐药性的产生。
3.2 SA的分型及毒力因子携带情况 最初对LA-MRSA的研究主要是单一的克隆复合物CC398,目前已经扩展到多个克隆复合物,并且克隆复合物中的亚型多样性也不断增加。不同地理位置流行的类型也不同,欧洲最流行的LA-MRSA克隆复合物是CC398,而亚洲则是CC9,其多样性还在不断增加,并且随着时间的推移正在迅速变化[14]。相同克隆群的菌株携带的毒力情况具有一定的相似性,如亚洲国家和德国LA-MRSA ST9分离株携带至少一种肠毒素基因[15],并且杀白细胞素(panton-valentine leucocidin,PVL)、毒性休克综合征毒素(toxic shock syndrome toxin-1,TSST-1)的检出率较高[16];在人源分离的CC1携带PVL毒素的检出率高,并且PVL阳性的MRSA CC1菌株大多数携带肠毒素基因sea[17]。
本研究中对97株SA进行spa、MLST分型,一共鉴定出16种spa型、11种ST型,其中t 224、t 518、t 359是MSSA菌株的常见spa型,t 037是9株LA-MRSA菌株的主要spa型;ST 50、ST 4053是MSSA菌株的主要ST型,ST 239为LA-MRSA菌株的主要ST型。与陈程[18]报道的宁夏地区牛源SA分型结果较为一致,鉴定出t 224、ST 50分别为宁夏地区牛源SA最常见的spa、MLST型。ST 239作为HA-MRSA的重要类型,在此次研究中检出,提示SCCmec在动物中的传播。不同分型SA携带的毒力因子无明显区别,但LA-MRSA菌株较MSSA菌株携带较多种重要毒力因子。
由于SA存在大量的毒力决定因子且具有不同的作用机制,因此其致病性的分子基础是多因素的,通过全基因组测序全面了解其毒力相关基因,分析SA感染的特征,为临床防控提供理论依据[19]。本研究中的16株代表菌株携带多种编码与疾病相关的毒力基因,这些毒力基因的调控可增强细菌的生存、传播能力及致病力。16株代表菌株基因组除了鉴定出可造成巨噬细胞溶解的杀白细胞毒素基因lukE和lukD[20]外,还发现了具有能够引起葡萄球菌烫伤皮肤综合征的剥脱性毒素eta[21]、PVL、TSST-1、多种肠毒素基因、类毒素及胶原粘附蛋白基因cna。VFDB数据库分析表明16株代表菌株有较高的毒力水平。3株LA-MRSA分离株基因组携带的耐药基因与Sun等[19]报道的山东猪源ST 398 MRSA基因组分析耐药基因携带结果类似,但毒力基因携带结果存在一定差异,山东猪源MRSA白细胞毒素基因lukE和lukD等检测结果为阴性,而本研究中的2株MRSA ST 239均携带lukE和lukD,1株MRSA ST 59同时携带LukS-PV和LukM-PV两种杀白细胞素素,主要原因可能是不同的MRSA克隆群体具有不同的病原体特征,携带的毒力因子具有一定差异。MRSA ST 239作为HA-MRSA重要的MLST类型,在本次研究中检出,与周佳维等[22]报道的HA-MRSA ST 239基因组分析毒力携带结果相似,均携带eta、lukE与lukD等重要毒力相关基因,提示SCCmec在动物中的传播。16株代表菌株基因组毒力基因对比结果显示,3株LA-MRSA携带毒力因子种类较多,LukS-PV、LukF-PV、LukM-PV、TSST-1等重要毒力因子携带情况与MSSA差异较小。毒力结果分析表明LA-MRSA、MSSA均有较高的毒力水平,应与HA-MRSA、CA-MRSA同样引起各界人员的重视,相关研究人员应加强其毒力监测及控制。
基因岛可以编码多种功能,主要涉及共生关系、发病机理及提高生物的适应性等[23]。LA-MRSA预测的基因岛中耐药基因岛数目与MSSA菌株无明显区别,但毒力基因岛数目较MSSA多。预测的基因岛编码的基因产物大部分是假定蛋白与蛋白酶,毒力基因岛编码的产物中发现了毒素、毒素-抗毒素系统、胞外肠毒素Ⅰ型前体、致病性岛的超抗原SaPI、PVL假定蛋白等的存在。毒素-抗毒素系统是细菌适应特异性环境胁迫的重要调控系统[24],肠毒素可导致食物中毒而损害人体[25],SAPIs是一种可移动遗传元件,在发病过程和毒力调控中起着重要作用。SAPIs编码毒力因子,如超抗原、表皮溶解毒素、生物被膜形成基因、铁转运或抗生素耐药基因(青霉素、磷霉素和富西酸),可通过某些噬菌体[26-27]在细菌中传播,有研究表明tst、sec、sel基因与超抗原SaPI经常同时在SA中被发现,且携带tst基因、SaPI阳性MRSA比SaPI阴性MRSA更容易扩散到各种器官组织,通过促进MRSA的毒力表达,诱导巨噬细胞分泌IL-6参与MRSA感染的病理过程,从而引起更严重的组织和细胞损伤[28-29]。耐药基因岛编码的基因产物主要有耐甲氧西林调节蛋白mecI、β-内酰胺传感器BlaRI、β-内酰胺酶Bla2、氯霉素/氟苯尼考MFS运转体FexA、博来霉素耐药蛋白、多药耐药蛋白等。基因岛预测分析结果提示16株分离株耐药情况严重,且具有较强的致病潜能、适应能力及传播能力。对菌株基因组序列中的基因岛进行研究与分析,使研究人员从分子水平了解SA的多重耐药性和毒力产生、传播的原因,做好预防措施以减少SA感染所造成的损失。
4 结论 宁夏地区牛源SA耐药情况严峻且携带大量耐药基因、毒力相关基因,基因岛预测分析提示16株菌株具有较强的毒力水平及传播能力。研究结果表明,目前兽医临床应该加强对SA的监测及抗菌药物的合理使用,防止耐药情况的加重,遏制LA-MRSA的感染与传播。
References
| [1] | 易明梅. 上海地区奶牛乳房炎主要致病菌分析及基因治疗初探. 上海交通大学硕士学位论文, 2008. |
| [2] | Wellenberg GJ, van der Poel WHM, Van Oirschot JT. Viral infections and bovine mastitis: a review. Veterinary Microbiology, 2002, 88(1): 27-45. DOI:10.1016/S0378-1135(02)00098-6 |
| [3] | Osumi T, Kishimoto Y, Kano R, Maruyama H, Onozaki M, Makimura K, Ito T, Matsubara K, Hasegawa A. Prototheca zopfii genotypes isolated from cow barns and bovine mastitis in Japan. Veterinary Microbiology, 2008, 131(3/4): 419-423. |
| [4] | Halasa T, Nielen M, Huirne RBM, Hogeveen H. Stochastic bio-economic model of bovine intramammary infection. Livestock Science, 2009, 124(1/3): 295-305. |
| [5] | Pérez VKC, da Costa GM, Guimar?es AS, Heinemann MB, Lage AP, Dorneles EMS. Relationship between virulence factors and antimicrobial resistance in Staphylococcus aureus from bovine mastitis. Journal of Global Antimicrobial Resistance, 2020, 22: 792-802. DOI:10.1016/j.jgar.2020.06.010 |
| [6] | Amoako DG, Bester LA, Somboro AM, Baijnath S, Govind CN, Essack SY. Plasmid-mediated resistance and virulence mechanisms in the private health sector in KwaZulu-Natal, South Africa: an investigation of methicillin resistant Staphylococcus aureus (MRSA) clinical isolates collected during a three month period. International Journal of Infectious Diseases, 2016, 46: 38-41. DOI:10.1016/j.ijid.2016.03.019 |
| [7] | Miyasaki Y, Rabenstein JD, Rhea J, Crouch ML, Mocek UM, Kittell PE, Morgan MA, Nichols WS, Van Benschoten MM, Hardy WD, Liu GY. Isolation and characterization of antimicrobial compounds in plant extracts against multidrug-resistant Acinetobacter baumannii. PLoS One, 2013, 8(4): e61594. DOI:10.1371/journal.pone.0061594 |
| [8] | Magro G, Biffani S, Minozzi G, Ehricht R, Monecke S, Luini M, Piccinini R. Virulence genes of S. aureus from dairy cow mastitis and contagiousness risk. Toxins (Basel), 2017, 9(6): 195. DOI:10.3390/toxins9060195 |
| [9] | Ito T, Okuma K, Ma XX, Yuzawa H, Hiramatsu K. Insights on antibiotic resistance of Staphylococcus aureus from its whole genome: genomic island SCC. Drug Resistance Updates, 2003, 6(1): 41-52. DOI:10.1016/S1368-7646(03)00003-7 |
| [10] | 李君. 猪源耐甲氧西林金黄色葡萄球菌流行病学特征及遗传进化分析. 中国农业大学博士学位论文, 2017. |
| [11] | Meng D, Meng QL, Qiao J, Cai KJ, Wang DF, Ma S, Wu YH, Cai XP. Study on resistance characteristics, virulence genes and molecular classification of Staphylococcus aureus isolated from cows in Xinjiang. Acta Veterinaria et Zootechnica Sinica, 2018, 49(1): 181-194. (in Chinese) 孟丹, 孟庆玲, 乔军, 蔡扩军, 王登峰, 马帅, 伍晔晖, 才学鹏. 奶牛源金黄色葡萄球菌新疆流行株的耐药特性、毒力基因及分子分型. 畜牧兽医学报, 2018, 49(1): 181-194. |
| [12] | Zhang H, Yang F, Li XP, Wang XR, Luo JY, Li HS. Study on relationship between antimicrobial resistance and efflux pump related genes of methicillin-resistant Staphylococcus aureus in dairy cow. Acta Veterinaria et Zootechnica Sinica, 2019, 50(11): 2326-2332. (in Chinese) 张行, 杨峰, 李新圃, 王旭荣, 罗金印, 李宏胜. 牛源耐甲氧西林金黄色葡萄球菌耐药性与外排泵相关基因的检测及相关性研究. 畜牧兽医学报, 2019, 50(11): 2326-2332. DOI:10.11843/j.issn.0366-6964.2019.11.017 |
| [13] | 陈云. 牛源耐甲氧西林金黄色葡萄球菌耐药性和毒力基因的检测. 内蒙古农业大学硕士学位论文, 2019. |
| [14] | Price LB, Stegger M, Hasman H, Aziz M, Larsen J, Andersen PS, Pearson T, Waters AE, Foster JT, Schupp J, Gillece J, Driebe E, Liu CM, Springer B, Zdovc I, Battisti A, Franco A, Zmudzki J, Schwarz S, Butaye P, Jouy E, Pomba C, Porrero MC, Ruimy R, Smith TC, Robinson DA, Weese JS, Arriola CS, Yu FY, Laurent F, Keim P, Skov R, Aarestrup FM. Staphylococcus aureus CC398: host adaptation and emergence of methicillin resistance in livestock. mBio, 2012, 3(1): e00305-11. |
| [15] | Chuang YY, Huang YC. Livestock-associated meticillin-resistant Staphylococcus aureus in Asia: an emerging issue?. International Journal of Antimicrobial Agents, 2015, 45(4): 334-340. DOI:10.1016/j.ijantimicag.2014.12.007 |
| [16] | Wan MT, Lauderdale TL, Chou CC. Characteristics and virulence factors of livestock associated ST9 methicillin-resistant Staphylococcus aureus with a novel recombinant staphylocoagulase type. Veterinary Microbiology, 2013, 162(2/4): 779-784. |
| [17] | Alba P, Feltrin F, Cordaro G, Porrero MC, Kraushaar B, Argudín MA, Nyk?senoja S, Monaco M, Stegger M, Aarestrup FM, Butaye P, Franco A, Battisti A. Livestock-associated methicillin resistant and methicillin susceptible Staphylococcus aureus sequence type (CC)1 in European farmed animals: high genetic relatedness of isolates from Italian cattle herds and humans. PLoS One, 2015, 10(8): e137143. |
| [18] | 陈程. 宁夏地区牛源金黄色葡萄球菌分子流行病学特征及遗传进化分析. 宁夏大学硕士学位论文, 2018. |
| [19] | Sun CT, Chen BL, Hulth A, Schwarz S, Ji X, Nilsson LE, Ma SZ, Sun Q, Bi ZW, Wang Y, Bi ZQ, Wu CM, B?rjesson S. Genomic analysis of Staphylococcus aureus along a pork production chain and in the community, Shandong Province, China. International Journal of Antimicrobial Agents, 2019, 54(1): 8-15. DOI:10.1016/j.ijantimicag.2019.03.022 |
| [20] | Yoong P, Torres VJ. The effects of Staphylococcus aureus leukotoxins on the host: cell lysis and beyond. Current Opinion in Microbiology, 2013, 16(1): 63-69. DOI:10.1016/j.mib.2013.01.012 |
| [21] | Maga?a-Lizárraga JA, Parra-Unda JR, Ahumada-Santos YP, Ahumada-Santosa YP, de J Uribe-Beltrán M, Gómez-Gil B, Vega-López IF, Prieto-Alvarado R, Báez-Flores ME. Whole-genome sequencing of Staphylococcus aureus L401, a mecA-negative community-associated methicillin-resistant strain isolated from a healthy carrier. Journal of Global Antimicrobial Resistance, 2019, 17: 260-262. DOI:10.1016/j.jgar.2019.04.011 |
| [22] | Zhou JW, Li A, Jiang Y, Hua XT, Yu YS. Genomic analysis of healthcare-associated methicillin-resistant Staphylococcus aureus ZJ5499. Chinese Journal of Microecology, 2016, 28(10): 1138-1142. (in Chinese) 周佳维, 李昂, 蒋琰, 华孝挺, 俞云松. 医院相关性ST5型耐甲氧西林金黄色葡萄球菌ZJ5499的全基因测序及序列分析. 中国微生态学杂志, 2016, 28(10): 1138-1142. |
| [23] | Chen C, Wang T, Chang JW, Wan JH, Wang GQ. High-throughput sequencing analysis of a methicillin- resistant Staphylococcus aureus strain in dairy cattle in Ningxia province. Microbiology China, 2019, 46(7): 1636-1644. (in Chinese) 陈程, 王瞳, 常佳伟, 万佳宏, 王桂琴. 宁夏地区一株奶牛源耐甲氧西林金黄色葡萄球菌流行株的高通量测序分析. 微生物学通报, 2019, 46(7): 1636-1644. |
| [24] | He ZL, Wang H. Functions of bacterial toxin-antitoxin systems. Chinese Journal of Biotechnology, 2018, 34(8): 1270-1278. (in Chinese) 何志利, 王慧. 细菌毒素-抗毒素系统的功能. 生物工程学报, 2018, 34(8): 1270-1278. |
| [25] | Liu SQ, Zhang SH, Duan YQ, Niu Y, Gu HJ, Zhao ZP, Zhang SG, Yang Y, Wang XL, Gao YH, Yang PH. Transcutaneous immunization of recombinant Staphylococcal enterotoxin B protein using a dissolving microneedle provides potent protection against lethal enterotoxin challenge. Vaccine, 2019, 37(29): 3810-3819. DOI:10.1016/j.vaccine.2019.05.055 |
| [26] | Alite C, Humphrey S, Donderis J, Maiques E, Ciges-Tomas JR, Penadés JR, Marina A. Dissecting the link between the enzymatic activity and the SaPI inducing capacity of the phage 80α dUTPase. Scientific Reports, 2017, 7(1): 11234. DOI:10.1038/s41598-017-11234-9 |
| [27] | Tormo MA, Ferrer MD, Maiques E, úbeda C, Selva L, Lasa í, Calvete JJ, Novick RP, Penadés JR. Staphylococcus aureus pathogenicity island DNA is packaged in particles composed of phage proteins. Journal of Bacteriology, 2008, 190(7): 2434-2440. DOI:10.1128/JB.01349-07 |
| [28] | Long L, Zhang JX. Repressor Stl controls toxic shock-inducing staphylococcal pathogenicity islands. Chinese Journal of Pathophysiology, 2012, 28(11): 1991-1993. (in Chinese) 龙莉, 张吉翔. Stl阻遏因子控制葡萄球菌引起中毒性休克的"致病性岛". 中国病理生理杂志, 2012, 28(11): 1991-1993. DOI:10.3969/j.issn.1000-4718.2012.11.015 |
| [29] | Zheng Y, Qin CH, Zhang XF, Zhu YF, Li AQ, Wang M, Tang YW, Kreiswirth BN, Chen L, Zhang HF, Du H. The tst gene associated Staphylococcus aureus pathogenicity island facilitates its pathogenesis by promoting the secretion of inflammatory cytokines and inducing immune suppression. Microbial Pathogenesis, 2020, 138: 103797. DOI:10.1016/j.micpath.2019.103797 |
