王昌宇1,2,3, 汪铭书1,2,3, 程安春1,2,3


1. 四川农业大学动物医学院, 禽病防治研究中心, 四川 成都 611130;
2. 四川农业大学动物医学院, 预防兽医研究所, 四川 成都 611130;
3. 动物疫病与人类健康四川省重点实验室, 四川 成都 611130
收稿日期:2018-05-29;修回日期:2018-10-06;网络出版日期:2018-11-29
基金项目:“十三五”国家重点研发计划(2017YFD0500800);国家现代农业(水禽)产业技术体系专项(CARS-42-17);国家农业产业体系四川兽药创新团队(CARS-SVDIP)
*通信作者:程安春, E-mail: chenganchun@vip.163.com.
摘要:膜间质蛋白酶(DegP),是一种广泛存在于真核生物和原核生物细胞中的蛋白。DegP同时具有酶活性和分子伴侣活性,并通过多聚体构成胶囊状结构执行其分子伴侣功能。DegP的酶活性依赖酶切位点与PDZ1结构域双重识别方式识别底物,这种识别模式被称为"分子量尺"。在革兰氏阴性菌中,DegP主要位于膜间质,通过分子伴侣活性与酶活性帮助保护错误折叠蛋白或降解变性蛋白。DegP也参与外膜蛋白的转运,是DegP胞内活性的研究重点。DegP也可以被分泌到胞外,帮助宿主对抗恶劣环境,并参与调节生物被膜的形成。本文将从DegP的结构与活性、胞内功能与胞外功能三大方面对DegP的研究进展进行总结,为革兰氏阴性菌周质中蛋白质质量控制与DegP体外功能的进一步研究提供参考。
关键词:革兰氏阴性菌DegP分子伴侣蛋白酶蛋白质质量控制系统
Research progress of DegP in Gram-negative bacteria
Changyu Wang1,2,3, Mingshu Wang1,2,3, Anchun Cheng1,2,3


1. Research Center of Avian Disease, College of Veterinary Medicine, Sichuan Agricultural University, Chengdu 611130, Sichuan Province, China;
2. Institute of Preventive Veterinary Medicine, College of Veterinary Medicine, Sichuan Agricultural University, Chengdu 611130, Sichuan Province, China;
3. Key Laboratory of Animal Disease and Human Health of Sichuan Province, Chengdu 611130, Sichuan Province, China
*Corresponding author: Anchun Cheng, E-mail: chenganchun@vip.163.com.
Foundation item: Supported by Grants from National Key Research and Development Program of China (2017YFD0500800), by the China Agricultural Research System (CARS-42-17) and by the Sichuan Veterinary Medicine and Drug Innovation Group of China Agricultural Research System (CARS-SVDIP)
Abstract: DegP, also known as HtrA, is a protein widely distributed in eukaryotic and prokaryotic cells. It has both protease activity and chaperone activity. On one hand, DegP forms a capsule-like structure to perform its chaperone function, called "Cage assembly". On the other hand, the protease activity of DegP depends on protease active site and PDZ1 domain, called "molecular ruler". In Gram-negative bacteria periplasm, DegP protects misfolded proteins or degrades denatured proteins. For example, DegP takes part in the transport of outer membrane proteins has been well-studied to prove its functions. Additionally, DegP can be secreted to extracellular and participates in the regulation of biofilm formation. In this way, DegP endows the ability to survive from the harsh environments on bacteria. In this review we summarized the structures and functions of DegP. It provides new insights into vitro activities of DegP and how DegP works as the final step in protein quality control system.
Keywords: Gram negative bacteriaDegPmolecular chaperonesproteaseprotein quality control system
膜间质蛋白酶DegP,是一种广泛存在于真核生物和原核生物细胞中的蛋白,同时具有酶活性和分子伴侣活性,其多聚体结构的组装与酶切识别方式独一无二,被称为“笼式组装”与“分子量尺”,在周质中起到蛋白质质量控制的作用。革兰氏阴性菌中的DegP最初在1983年由Swamy等于大肠杆菌中与其他几种蛋白酶一起被分离,当时被命名为蛋白酶Do (Protease Do)[1]。1988年Stranch和Beckwith在外膜蛋白运输的研究中通过遗传学方式再次筛选出DegP,并确认DegP能够降解错误折叠的膜间质蛋白,因此命名为膜间质蛋白酶(Degradation of periplasmic proteins,DegP)[2]。随后1989年Lipinska等的研究发现DegP是高温生长时的必需基因。由于这个发现DegP也被称为高温必需蛋白(High-temperature requirement A,HtrA)[3]。在大肠杆菌中具有和DegP类似结构的还有DegS和DegQ,由于结构存在相似性,且均为大肠杆菌高温生存必需蛋白,DegP、DegS、DegQ被归类为HtrA家族[4]。经生物信息学分析,HtrA家族隶属于PA家族,SIC亚类蛋白酶。在其他革兰氏阴性菌中发现DegP时,由于基因组只存在这一种与大肠杆菌degP同源且参与细菌高温生存的基因,很多革兰氏阴性菌中发现的DegP也被称为HtrA。但从功能与命名的匹配度上看,DegP最为符合这种蛋白的关键活性与功能描述,故文中将名称统一,称为DegP。本文将从DegP的结构与活性,以及DegP的胞内功能与胞外功能三方面介绍DegP的研究进展。
1 DegP的结构与活性 1.1 DegP单体结构与酶活性 以大肠杆菌为例,DegP包含5个主要功能单元(图 1-A),分别是信号肽(Signal peptide,SP)、酶活性结构域(Protease domain)、PDZ1结构域、PDZ2结构域和LA回路(LA Loop)[5]。信号肽在分泌过程中帮助DegP定位到周质,同时对酶活性起到抑制作用,在DegP执行蛋白质质量控制时会被DegP自行切除。酶活性结构域包含典型的丝氨酸-组氨酸-天冬氨酸催化三联体(图 1-C),发挥活性时不需要ATP水解供能,受温度激活,高于30℃即发挥酶活性,最佳酶活温度在44 ℃左右[6]。PDZ结构域的命名来源于3个真核蛋白的首字母缩写(Post_synaptic density protein,Disc large和Zonula occludens),参与蛋白质之间的相互作用。DegP中,PDZ1结构域负责识别底物序列的降解决定子(Degron),PDZ2负责在形成多聚体时与其他DegP单体结合,形成稳定的多聚体结构[7-9]。LA loop位于酶活性结构域的氨基酸序列中,负责在DegP形成六聚体时阻止酶活位点与底物的结合并维持六聚体结构的稳定[10]。本课题组研究的是鸭疫里默氏杆菌中DegP的酶活特性,发现鸭疫里默氏杆菌中DegP的最佳酶活温度也为44 ℃,其活性同样不受二价阳离子激活,但其酶活性可以被钙离子和镁离子促进(未发表)。
 |
| 图 1 DegP的结构域与空间结构[9, 14] Figure 1 Schematic diagram of DegP domain and its structure[9, 14]. A: Schematic diagram of DegP domains; B: Spatial structure of DegP; C: Ser-Asp-His catalytic triad in protease domain. |
| 图选项 |
DegP单体中,酶活性结构域与PDZ1之间形成独特的酶活工作单元,被称为“分子量尺(Molecular ruler)”(图 2-A)。这一结构由酶活性结构域与PDZ1结构域组成,DegP会先通过PDZ1识别降解决定子,再由催化三联体识别Val/Xaa或Ile/Xaa (其中Xaa为任意氨基酸),酶切底物,直至将底物分解为肽段[6]。因此DegP会将底物切割成固定长度的肽段(图 2-B)。这一特殊的工作单元限制了DegP的底物范围,不是所有含有降解决定子或Val/Xaa与Ile/Xaa酶切位点的蛋白都能被降解,两个条件都需具备[7-8]。DegP可以体外降解变性的牛血清白蛋白(BSA)[11]、酪蛋白(Casein)[8, 11-12]、球蛋白(Globin)以及溶菌酶(Lysozyme)[8, 11, 13]。而对于DegP在活细胞内究竟会分解哪些变性蛋白,与细菌生理功能存在哪些联系并没有深入研究。目前已有研究证实大肠杆菌中DegP在周质中可降解错误折叠的外膜蛋白,起到质量控制作用[14]。在志贺氏菌中发现DegP通过分解变性烷基氢过氧化物还原酶ahpC (Alky hydroperoxide reductase,C22 subunit)、琥珀酰胺辅酶A合成酶sucC (Succinyl-CoA synthetase)帮助稳定周质能量环境;分解变性Fe3+负调控蛋白fur (Ferric uptake regulator)帮助维持铁获取系统稳定[15]。
 |
| 图 2 DegP的分子量尺工作模式[16] Figure 2 The molecular ruler in DegP[16]. A model for DegP conbinds and degrades substrate. A: Molecular ruler model of DegP, explain how DegP conbinds with substrate by protese domain and PDZ2; B: Technological process of DegP degradation of its substrate. ①–③ shows DegP combinds with substrate, cut substrate up. ④ shows DegP combinds with the same subtrate again in differert site, ⑤–⑥ shows DegP degrades substrate step by step. |
| 图选项 |
1.2 DegP多聚体结构与分子伴侣活性 DegP能形成多种多聚体结构,主要为三聚体、六聚体、12聚体、24聚体。形成12及24聚体结构时DegP会通过PDZ2连接各个蛋白单体,包裹底物,形成一个胶囊状球形结构。DegP这种独特的底物结合模式被称为“笼式组装”(Cage assembly)[11, 13, 17]。在DegP的多聚体结构中,三聚体结构是基本单元。DegP的三聚体结构具有酶活区集中聚拢,PDZ结构域分散分布的特点。在不发挥酶活性功能时,2个三聚体通过LA loop连接,构成类双盘状的六聚体结构;温度高于30℃时,LA loop结构变形缩短,使得三聚体与三聚体分开[18]。而后在识别到底物之后,三聚体与三聚体之间通过PDZ2结构域识别、结合、建立连接,形成更大的多聚体结构[10]。DegP构成12聚体时,每个DegP三聚体作为一个亚结构单位,构成一个类四面体的胶囊结构;构成24聚体时,则是由8个DegP三聚体构成一个类八面体的胶囊结构(图 3)[13, 19]。
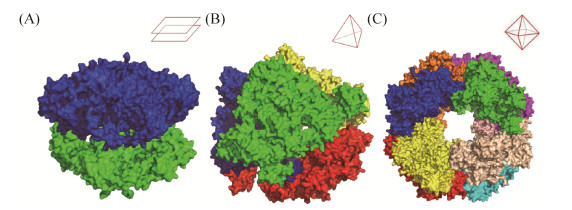 |
| 图 3 DegP六聚体(PDB:1KY9)(A)、12聚体(PDB:3OTP)(B)和24聚体(PDB:3CS0)(C)空间结构示意图[13, 19] Figure 3 Structures of DegP. A: hexamer (PDB:1KY9); B: DegP 12mer (PDB:3OTP); C: DegP 24mer (PDB:3CS0)[13, 19]. |
| 图选项 |
DegP的多聚体结构是DegP发挥分子伴侣活性的空间基础,是DegP在周质中发挥酶活性的结构支撑。DegP通过PDZ结构域构成的笼式结构内部具有相对稳定的环境,可以帮助空间结构发生变化的底物蛋白复性,同时帮助运输底物蛋白。若底物蛋白结构变形无法复性,则通过酶活性将其分解成肽段,避免失活蛋白聚集影响周质内稳态。
2 DegP的胞内功能 2.1 DegP参与蛋白质质量控制 DegP分解错误折叠蛋白,作为周质中蛋白质质量控制效应最终一环参与蛋白质质量控制系统。蛋白质质量控制的启动依赖双组分调控系统(Two-component signal transduction system),系统中包含Sigma E因子与Cpx通路。Sigma E因子启动蛋白质质量控制分为DegS依赖和DegS非依赖途径两种。DegS依赖途径由DegS识别错误折叠的外膜蛋白,修饰反Sigma E因子RseA (Regulator of SigE A),使RseA释放Sigma E因子[20];DegS非依赖途径则是由反Sigma E因子RseB (Regulator of SigE B)识别错误折叠的P菌毛黏附素PapG (P pili adhesin G),或直接受到环境压力,将信号呈递给RseP,由RseP修饰RseA[21]。RseA释放Sigma E因子,促进degP等压力应答基因表达,DegP从6聚体转化为12聚体或24聚体分解错误折叠蛋白控制周质中蛋白质质量[22]。
Cpx通路由感受蛋白CpxP、接受蛋白CpxA和传导蛋白CpxR构成[23]。CpxA可以接收到两种信号,一种信号是温度升高时由外膜脂蛋白NlpE (New lipoprotein E)产生,另一种信号是温度升高和胞外环境pH变成碱性时CpxP感受P菌毛黏附素PapE (P pili adhesin E)折叠发生变化产生[24-25, 27]。信号呈递给CpxA后,CpxA自磷酸化,并将CpxR磷酸化。激活的CpxR使degP等压力应答基因的表达上调,DegP从6聚体转化为12聚体或24聚体,控制周质中蛋白质质量。控制蛋白质质量过程中,DegP分解CpxP与刺激通路产生信号的错误折叠蛋白,终止蛋白质质量控制系统的启动(图 4)[25]。
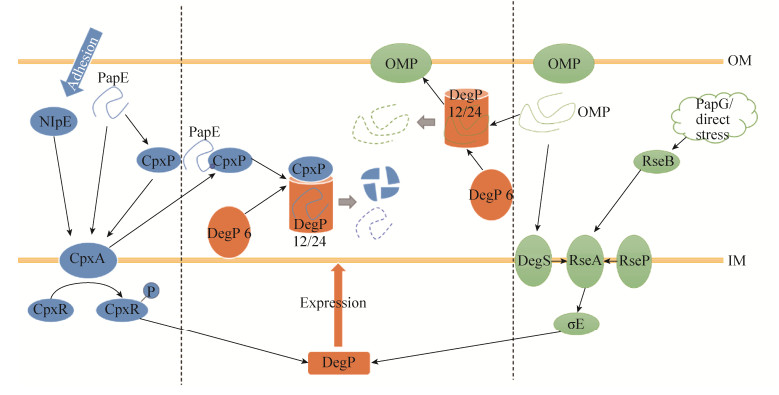 |
| 图 4 DegP通过表达调控参与蛋白质质量控制[22, 25] Figure 4 DegP take part in protein quality control via expression regulation[22, 25]. |
| 图选项 |
最近对于DegP在周质中执行蛋白质质量控制的研究中,还发现一种小分子量的周质蛋白YjfN,可以激活DegP的酶活性,并提高DegP分解OMPA的能力[26]。研究发现YjfN的表达受Cpx信号通路调节的细胞压力应答控制,且YjfN很容易被DegP分解,DegP可藉此激活酶活性。而YjfN还会和错误折叠的OMPA形成复合物,阻止错误折叠的OMPA形成毒性聚集体,方便DegP识别并分解错误折叠的OMPA[26]。这说明DegP作为周质中蛋白质质量控制的执行者,在受Cpx通路与Sigma因子双重调控完成细胞压力应答的过程中,有多种蛋白进行配合。
2.2 DegP参与外膜蛋白的运输 DegP参与外膜蛋白的运输。在大肠杆菌中,周质伴侣蛋白Skp (Seventeen-Kilodalton protein)、SurA (Survival protein A)和DegP作为周质分子伴侣帮助外膜蛋白穿过周质,其运输机制早期有三种观点:第一种观点认为此过程以SurA为主要分子伴侣,Skp和DegP构成一条补充路径。这一观点以三种基因对细菌生长的必需情况为依据,Skp和DegP双突变不致死,但SurA和DegP或SurA和Skp的双突变都致死,据此推测SurA为主要分子伴侣,Skp和DegP构成一条补充路径[28-29]。第二种观点认为Skp与SurA共同构成外膜蛋白的转运途径,但Skp先于SurA与外膜蛋白作用,二者之间存在接续性。DegP只作为质量控制因子,在外膜蛋白出现严重的折叠错误时发挥作用。因为Skp与外膜蛋白的作用在外膜蛋白通过SEC分泌系统(Secretory translocation system)穿过内膜时就已经发生,且Skp和外膜蛋白OmpA形成可溶的周质中间体,作为只结合而不折叠蛋白的分子伴侣防止OmpA聚集[30]。SurA与β-桶状装配机器Bam系统(β-barral assembly machinery)的核心蛋白BamA直接作用[31-33],帮助外膜蛋白从周质抵达外膜进行折叠与锚定。第三种观点认为SurA和Skp是主要的分子伴侣,功能发挥不分先后,DegP是二者功能的补充。此观点基于体外蛋白与底物的竞争性结合以及体外动力学方法测算得到的研究结果。研究中分别使用预先与SurA和Skp孵育的外膜蛋白作为底物,在体外适宜环境下再与DegP进行反应,分别监测SurA与Skp对外膜蛋白的保护情况,并建立体外动力学模型[34]。该结论暂无细胞内实验数据支撑。而最新的荧光能量共转移实验提出新的观点,认为SurA、Skp、DegP三者在转运外膜蛋白的过程中构成了一个复杂的系统(图 5)。SurA单独构成一条通路,Skp和DegP构成另一条通路,转运任务由两条通路共同完成。若未完全折叠的外膜蛋白在周质中发生错误折叠,则都由DegP进行分解,完成对蛋白质的质量控制[16]。
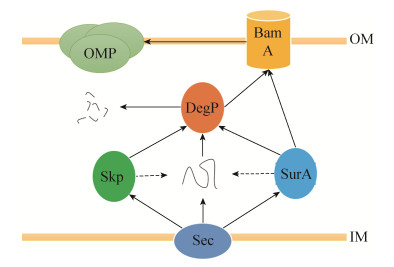 |
| 图 5 DegP、SurA、Skp共同构成对外膜蛋白的转运系统[14] Figure 5 Transportation system of OMPs in periplasm is composed of DegP, SurA and Skp[14]. |
| 图选项 |
3 DegP的胞外功能 DegP蛋白属于SIC亚类蛋白,此类蛋白多为分泌型。根据近几年的研究报道,已经发现部分革兰氏阴性菌中的DegP具有此类种属特性,能够被细菌分泌到体外参与多种生命活动。现今DegP分泌到胞外的报道多见于肠道菌群。在革兰氏阴性菌生物被膜形成的研究中,DegP也开始频繁被提及。目前在细菌抵抗恶劣环境的研究中,发现多种肠道细菌中的DegP能够被分泌到体外,并参与细菌的生命活动。如空肠弯曲杆菌中DegP被分泌到细菌体外后能够分解肠道细胞表面的降钙素,破坏肠道细胞之间的紧密连接,使得空肠弯曲杆菌可以在细胞间隙定殖,躲避肠道中的酶与酸性物质[35]。类似的,幽门螺旋杆菌中也发现了DegP可以分泌到体外帮助定殖[36]。此类细菌充分利用了DegP的酶活性,分解异源蛋白与自身的错误折叠蛋白,保护自身不受恶劣环境的侵害。
DegP的体外功能也与生物被膜息息相关。Fang等在大肠杆菌生物被膜形成的研究中,发现益生大肠杆菌可以将DegP分泌到胞外,竞争性抑制肠出血性大肠杆菌、共生大肠杆菌、铜绿假单胞杆菌、金黄色葡萄球菌及表皮葡萄球菌的生物被膜形成,从而减少致病菌感染的几率[37]。实验中通过益生大肠杆菌DegP缺失株与野生株对上述致病菌生物被膜的抑制情况的区别,以及纯化的益生大肠杆菌DegP蛋白与上述致病菌共孵育后生物被膜的生成情况变化,证明分泌到体外的DegP参与生物被膜形成与复杂环境下的种间竞争。其中益生大肠杆菌分泌DegP对肠出血型大肠杆菌的生物被膜形成抑制最为明显[37]。生物被膜的骨架是由各类蛋白与肽聚糖构成的,DegP分泌到细菌体外后依然能发挥酶活性并破坏生物被膜骨架蛋白从而抑制生物被膜的形成。DegP与骨架蛋白的相互作用依然在研究当中。
4 问题和展望 目前对于DegP的研究,依然存在很多问题。首先是DegP两种活性的关系,现在依然存在争论。现在的主流观点认为DegP的分子伴侣活性与DegP多聚体的“笼式组装”有关,而酶活性为单体具备的能力,两者的功能发挥情景存在交集,但二者相互独立。另一种新提出的观点认为DegP的分子伴侣活性为副属性,在温度不足以使DegP发挥酶活性时DegP依然包裹底物,则DegP表现为分子伴侣活性,因此分子伴侣活性为DegP的副属性[38]。本课题组现阶段主要研究鸭疫里默氏杆菌DegP的蛋白酶性质,现已在温度和二价阳离子对DegP酶活性影响上有所进展。本课题组在研究过程中发现,现阶段对DegP的研究并没有把DegP的蛋白酶功能这一基础属性研究透彻,比如DegP在胞内的天然底物除去引起周质蛋白质质量控制的各种因子外,还知之甚少;DegP作为蛋白酶的一些基本属性,如Km值,迄今均无报道。这些基础属性对于DegP的分子伴侣功能与酶活功能的发挥有重要的指示作用,但就现在的研究进展来看,这些基础性质的研究程度远没有DegP参与外膜蛋白作用等与信号通路相关的研究深入。
生物被膜是近年来逐渐兴起的研究热点,现已发现生物被膜可以提高细菌的耐药性。DegP在生物被膜调控中起到作用,对于生物被膜调控的机理解释与对生物被膜生成的防控具有极大的潜在价值,非常值得挖掘。我们可以推测DegP通过酶活性调控生物被膜的形成,影响的是蛋白构成的骨架,这对革兰氏阴性菌生物被膜形成原理以及阻止生物被膜生成的研究都有极大助力。目前对于一些重要的革兰氏阴性菌如鸭疫里默氏杆菌中的DegP在胞内的天然底物以及对生物被膜的形成影响都知之甚少,但根据鸭疫里默氏杆菌中对铁获取系统和抗生素耐性的研究进展和外膜蛋白及生物被膜的研究进展,以及DegP在周质中执行蛋白质质量控制的特性,可以推测DegP与以上众多系统中的蛋白稳定有关[39-60]。综上所述,DegP胞内外功能的研究重点都在DegP的天然底物上,故鉴定DegP的天然底物,通过研究DegP的作用底物从而解释DegP如何帮助细菌抵抗恶劣环境,保持周质稳定是革兰氏阴性菌DegP今后的主要研究方向。
References
| [1] | Swamy KHS, Chung CH, Goldberg AL. Isolation and characterization of protease do from Escherichia coli, a large serine protease containing multiple subunits. Archives of Biochemistry and Biophysics, 1983, 224(2): 543-554. DOI:10.1016/0003-9861(83)90242-4 |
| [2] | Strauch KL, Beckwith J. An Escherichia coli mutation preventing degradation of abnormal periplasmic proteins. Proceedings of the National Academy of Sciences of the United States of America, 1988, 85(5): 1576-1580. DOI:10.1073/pnas.85.5.1576 |
| [3] | Lipinska B, Fayet O, Baird L, Georgopoulos C. Identification, characterization, and mapping of the Escherichia coli htrA gene, whose product is essential for bacterial growth only at elevated temperatures. Journal of Bacteriology, 1989, 171(3): 1574-1584. DOI:10.1128/jb.171.3.1574-1584.1989 |
| [4] | Waller PR, Sauer RT. Characterization of degQ and degS, Escherichia coli genes encoding homologs of the DegP protease. Journal of Bacteriology, 1996, 178(4): 1146-1153. DOI:10.1128/jb.178.4.1146-1153.1996 |
| [5] | Kim DY, Kim KK. Structure and function of HtrA family proteins, the key players in protein quality control. Journal of Biochemistry & Molecular Biology, 2005, 38(3): 266-274. |
| [6] | Ge X, Wang R, Ma J, Liu Y, Ezemaduka AN, Chen PR, Fu XM, Chang ZY. DegP primarily functions as a protease for the biogenesis of β-barrel outer membrane proteins in the Gram-negative bacterium Escherichia coli. FEBS Journal, 2014, 281(4): 1226-1240. DOI:10.1111/febs.12701 |
| [7] | Krojer T, Pangerl K, Kurt J, Sawa J, Mechtler K, Huber R, Ehrmann M, Clausen T. Interplay of PDZ and protease domain of DegP ensures efficient elimination of misfolded proteins. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(22): 7702-7707. DOI:10.1073/pnas.0803392105 |
| [8] | Iwanczyk J, Damjanovic DJ, Leong V, Jomaa A, Ghirlando R, Ortega J. Role of the PDZ domains in Escherichia coli DegP protein. Journal of Bacteriology, 2007, 189(8): 3176-3186. DOI:10.1128/JB.01788-06 |
| [9] | Harris BZ, Lim WA. Mechanism and role of PDZ domains in signaling complex assembly. Journal of Cell Science, 2001, 114(18): 3219-3231. |
| [10] | Hansen G, Hilgenfeld R. Architecture and regulation of HtrA-family proteins involved in protein quality control and stress response. Cellular and Molecular Life Sciences, 2013, 70(5): 761-775. DOI:10.1007/s00018-012-1076-4 |
| [11] | Kim S, Grant RA, Sauer RT. Covalent linkage of distinct substrate degrons controls assembly and disassembly of DegP proteolytic cages. Cell, 2011, 145(1): 67-78. DOI:10.1016/j.cell.2011.02.024 |
| [12] | Jiao XD, Zhang M, Cheng S, Sun L. Analysis of Edwardsiella tarda DegP, a serine protease and a protective immunogen. Fish & Shellfish Immunology, 2010, 28(4): 672-677. |
| [13] | Jiang JS, Zhang XF, Chen Y, Wu Y, Zhou ZH, Chang ZY, Sui SF. Activation of DegP chaperone-protease via formation of large cage-like oligomers upon binding to substrate proteins. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(33): 11939-11944. DOI:10.1073/pnas.0805464105 |
| [14] | Lyu ZX, Zhao XS. Periplasmic quality control in biogenesis of outer membrane proteins. Biochemical Society Transactions, 2015, 43(2): 133-138. |
| [15] | 马姗姗.志贺氏菌HtrA蛋白的功能研究.中国人民解放军军事医学科学院硕士学位论文, 2011. |
| [16] | Clausen T, Kaiser M, Huber R, Ehrmann M. HTRA proteases: regulated proteolysis in protein quality control. Nature Reviews Molecular Cell Biology, 2011, 12(3): 152-162. DOI:10.1038/nrm3065 |
| [17] | Kim S, Sauer RT. Cage assembly of DegP protease is not required for substrate-dependent regulation of proteolytic activity or high-temperature cell survival. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(19): 7263-7268. |
| [18] | Li SS, Wang R, Li DY, Ma J, Li H, He XC, Chang ZY, Weng YX. Thermal-triggerd proteinquake leads to disassembly of DegP hexamer as an imperative activation step. Scientific Reports, 2014, 4: 4834. |
| [19] | Subrini O, Betton JM. Assemblies of DegP underlie its dual chaperone and protease function. FEMS Microbiology Letters, 2009, 296(2): 143-148. DOI:10.1111/fml.2009.296.issue-2 |
| [20] | Barchinger SE, Ades SE. Regulated proteolysis: control of the Escherichia coli σE-dependent cell envelope stress response//Dougan D. Regulated Proteolysis in Microorganisms. Dordrecht: Springer, 2013, 66: 129. |
| [21] | Kim DY. Two stress sensor proteins for the expression of sigmaE regulon: DegS and RseB. Journal of Microbiology, 2015, 53(5): 306-310. DOI:10.1007/s12275-015-5112-6 |
| [22] | Ruiz N, Silhavy TJ. Sensing external stress: watchdogs of the Escherichia coli cell envelope. Current Opinion in Microbiology, 2005, 8(2): 122-126. DOI:10.1016/j.mib.2005.02.013 |
| [23] | MacRitchie DM, Acosta N, Raivio TL. DegP is involved in Cpx-mediated posttranscriptional regulation of the type Ⅲ secretion apparatus in enteropathogenic Escherichia coli. Infection and Immunity, 2012, 80(5): 1766-1772. |
| [24] | Strozen TG, Langen GR, Howard SP. Adenylate cyclase mutations rescue the degP temperature-sensitive phenotype and induce the Sigma E and Cpx extracytoplasmic stress regulons in Escherichia coli. Journal of Bacteriology, 2005, 187(18): 6309-6316. DOI:10.1128/JB.187.18.6309-6316.2005 |
| [25] | Isaac DD, Pinkner JS, Hultgren SJ, Silhavy TJ. The extracytoplasmic adaptor protein CpxP is degraded with substrate by DegP. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(49): 17775-17779. DOI:10.1073/pnas.0508936102 |
| [26] | Kim S, Song I, Eom G, Kim S. A small periplasmic protein with a hydrophobic C-terminal residue enhances DegP proteolysis as a suicide activator. Journal of Bacteriology, 2018, 200(3): 00519-00517. |
| [27] | Buelow DR, Raivio TL. Cpx signal transduction is influenced by a conserved N-terminal domain in the novel inhibitor CpxP and the periplasmic protease DegP. Journal of Bacteriology, 2005, 187(19): 6622-6630. DOI:10.1128/JB.187.19.6622-6630.2005 |
| [28] | Kornd rfer IP, Dommel MK, Skerra A. Structure of the periplasmic chaperone Skp suggests functional similarity with cytosolic chaperones despite differing architecture. Nature Structural & Molecular Biology, 2004, 11(10): 1015-1020. |
| [29] | Sklar JG, Wu T, Kahne D, Silhavy TJ. Defining the roles of the periplasmic chaperones SurA, Skp, and DegP in Escherichia coli. Genes & Development, 2007, 21(19): 2473-2484. |
| [30] | Purdy GE, Fisher CR, Payne SM. IcsA surface presentation in Shigella flexneri requires the periplasmic chaperones DegP, Skp, and SurA. Journal of Bacteriology, 2007, 189(15): 5566-5573. DOI:10.1128/JB.00483-07 |
| [31] | Noinaj N, Fairman JW, Buchanan SK. The crystal structure of BamB suggests interactions with BamA and its role within the BAM complex. Journal of Molecular Biology, 2011, 407(2): 248-260. DOI:10.1016/j.jmb.2011.01.042 |
| [32] | Hagan CL, Silhavy TJ, Kahne D. β-barrel membrane protein assembly by the bam complex. Annual Review of Biochemistry, 2011, 80(1): 189-210. |
| [33] | Fardini Y, Trotereau J, Bottreau E, Souchard C, Velge P, Virlogeux-Payant I. Investigation of the role of the BAM complex and SurA chaperone in outer-membrane protein biogenesis and type Ⅲ secretion system expression in Salmonella. Microbiology, 2009, 155(5): 1613-1622. DOI:10.1099/mic.0.025155-0 |
| [34] | Wu S, Ge X, Lv ZX, Zhi ZY, Chang ZY, Zhao XS. Interaction between bacterial outer membrane proteins and periplasmic quality control factors: a kinetic partitioning mechanism. Biochemical Journal, 2011, 438(3): 505-511. DOI:10.1042/BJ20110264 |
| [35] | Hoy B, Geppert T, Boehm M, Reisen F, Plattner P, Gadermaier G, Sewald N, Ferreira F, Briza P, Schneider G, Backert S, Wessler S. Distinct roles of secreted HtrA proteases from gram-negative pathogens in cleaving the junctional protein and tumor suppressor E-cadherin. Journal of Biological Chemistry, 2012, 287(13): 10115-10120. DOI:10.1074/jbc.C111.333419 |
| [36] | Tegtmeyer N, Moodley Y, Yamaoka Y, Pernitzsch SR, Schmidt V, Traverso FR, Schmidt TP, Rad R, Yeoh KG, Bow H, Torres J, Gerhard M, Schneider G, Wessler S, Backert S. Characterisation of worldwide Helicobacter pylori strains reveals genetic conservation and essentiality of serine protease HtrA. Molecular Microbiology, 2016, 99(5): 925-944. DOI:10.1111/mmi.13276 |
| [37] | Fang KL, Jin X, Hong SH. Probiotic Escherichia coli inhibits biofilm formation of pathogenic E. coli via extracellular activity of DegP. Scientific Reports, 2018, 8: 4939. DOI:10.1038/s41598-018-23180-1 |
| [38] | Chang ZY. The function of the DegP (HtrA) protein: Protease versus chaperone. IUBMB Life, 2016, 68(11): 904-907. DOI:10.1002/iub.v68.11 |
| [39] | Luo HY, Liu MF, Wang MS, Zhao XX, Jia RY, Chen S, Sun KF, Yang Q, Wu Y, Chen XY, Francis B, Zou YF, Jing B, Cheng AC, Zhu DK. A novel resistance gene, lnu(H), conferring resistance to lincosamides in riemerella anatipestifer CH-2. International Journal of Antimicrobial Agents, 2018, 51(1): 136-139. DOI:10.1016/j.ijantimicag.2017.08.022 |
| [40] | Liu MF, Huang M, Zhu DK, Wang MS, Jia RY, Chen S, Sun KF, Yang Q, Wu Y, Biville F, Cheng AC. Identifying the genes responsible for iron-limited condition in Riemerella anatipestifer CH-1 through RNA-Seq-based analysis. BioMed Research International, 2017, 2017: 8682057. |
| [41] | Yi HB, Yuan B, Liu JB, Zhu DK, Wu Y, Wang MS, Jia RY, Sun KF, Yang Q, Chen S, Liu MF, Chen XY, Cheng AC. Identification of a wza-like gene involved in capsule biosynthesis, pathogenicity and biofilm formation in Riemerella anatipestifer. Microbial Pathogenesis, 2017, 107: 442-450. DOI:10.1016/j.micpath.2017.04.023 |
| [42] | Liu MF, Zhang L, Huang L, Biville F, Zhu DK, Wang MS, Jia RY, Chen S, Sun KF, Yang Q, Wu Y, Chen XY, Cheng AC. Use of Natural transformation to establish an easy knockout method in Riemerella anatipestifer. Applied and Environmental Microbiology, 2017, 83(9): e00127-17. |
| [43] | Huang L, Yuan H, Liu MF, Zhao XX, Wang MS, Jia RY, Chen S, Sun KF, Yang Q, Wu Y, Chen XY, Cheng AC, Zhu DK. Type B Chloramphenicol acetyltransferases are responsible for chloramphenicol resistance in Riemerella anatipestifer, China. Frontiers in Microbiology, 2017, 8: 297. |
| [44] | Wang MY, Zhang PY, Zhu DK, Wang MS, Jia RY, Chen S, Sun KF, Yang Q, Wu Y, Chen XY, Biville F, Cheng AC, Liu MF. Identification of the ferric iron utilization gene B739_1208 and its role in the virulence of R. anatipestifer CH-1. Veterinary Microbiology, 2017, 201: 162-169. DOI:10.1016/j.vetmic.2017.01.027 |
| [45] | Liu MF, Wang MY, Zhu DK, Wang MS, Jia RY, Chen S, Sun KF, Yang Q, Wu Y, Chen XY, Biville F, Cheng AC. Investigation of TbfA in Riemerella anatipestifer using plasmid-based methods for gene over-expression and knockdown. Scientific Reports, 2016, 6: 37159. DOI:10.1038/srep37159 |
| [46] | Zhu DK, Yang XQ, He Y, Zhou WS, Song XH, Wang JB, Zhang Y, Liu MF, Wang MS, Jia RY, Chen S, Sun KF, Yang Q, Wu Y, Chen XY, Cheng AC. Comparative genomic analysis identifies structural features of CRISPR-Cas systems in Riemerella anatipestifer. BMC Genomics, 2016, 17: 689. DOI:10.1186/s12864-016-3040-4 |
| [47] | Liu JB, Zhu DK, Ma GP, Liu MF, Wang MS, Jia RY, Chen S, Sun KF, Yang Q, Wu Y, Chen XY, Cheng AC. Genome-wide analysis of the synonymous codon usage patterns in Riemerella anatipestifer. International Journal of Molecular Sciences, 2016, 17(8): 1304. DOI:10.3390/ijms17081304 |
| [48] | Liao HB, Liu MF, Cheng XJ, Zhu DK, Wang MS, Jia RY, Chen S, Sun KF, Yang Q, Biville F, Cheng AC. The detection of Hemin-Binding proteins in Riemerella anatipestifer CH-1. Current Microbiology, 2016, 72(2): 152-158. |
| [49] | Liao HB, Cheng XJ, Zhu DK, Wang MS, Jia RY, Chen S, Chen XY, Biville F, Liu MF, Cheng AC. TonB energy transduction systems of Riemerella anatipestifer are required for iron and Hemin utilization. PLoS One, 2015, 10(5): e0127506. DOI:10.1371/journal.pone.0127506 |
| [50] | Luo HY, Liu MF, Wang LY, Zhou WS, Wang MS, Cheng AC, Jia RY, Chen S, Sun KF, Yang Q, Chen XY, Zhu DK. Identification of ribosomal RNA methyltransferase gene ermF in Riemerella anatipestifer. Avian Pathology, 2015, 44(3): 162-168. DOI:10.1080/03079457.2015.1019828 |
| [51] | Wang XJ, Liu WB, Zhu DK, Yang LF, Liu MF, Yin SJ, Wang MS, Jia RY, Chen S, Sun KF, Cheng AC, Chen XY. Comparative genomics of Riemerella anatipestifer reveals genetic diversity. BMC Genomics, 2014, 15: 479. DOI:10.1186/1471-2164-15-479 |
| [52] | Zhong CY, Cheng AC, Wang MS, Zhu DK, Luo QH, Chen S, Zhang SH, Chen XY. Quantitative real-time PCR study of the expression and regulation of the tetracycline resistance gene in Riemerella anatipestifer. Poultry Science, 2013, 92(6): 1552-1559. DOI:10.3382/ps.2012-02672 |
| [53] | Yuan B, Cheng AC, Wang MS. Polysaccharide export outer membrane proteins in gram-negative bacteria. Future Microbiology, 2013, 8(4): 525-535. |
| [54] | Wang XJ, Zhu DK, Wang MS, Cheng AC, Jia RY, Zhou Y, Chen ZL, Luo QH, Liu F, Wang Y, Chen XY. Complete genome sequence of Riemerella anatipestifer reference strain. Journal of Bacteriology, 2012, 194(12): 3270-3271. DOI:10.1128/JB.00366-12 |
| [55] | Liu MF, Huang Y, Liu JJ, Biville F, Zhu DK, Wang MS, Jia RY, Chen S, Zhao XX, Yang Q, Wu Y, Zhang SQ, Chen XY, Liu YY, Zhang L, You Y, Yu YL, Cheng AC. Multiple genetic tools for editing the genome of Riemerella anatipestifer using a counterselectable marker. Applied Microbiology and Biotechnology, 2018, 102(17): 7475-7488. DOI:10.1007/s00253-018-9181-4 |
| [56] | Liu JB, Wang MS, Yi HB, Liu MF, Zhu DK, Wu Y, Jia RY, Sun KF, Yang Q, Chen S, Zhao XX, Chen XY, Cheng AC. ATPase activity of GroEL is dependent on GroES and it is response for environmental stress in Riemerella anatipestifer. Microbial Pathogenesis, 2018, 121: 51-58. DOI:10.1016/j.micpath.2018.04.029 |
| [57] | Zhu DK, Luo HY, Liu MF, Zhao XX, Jia RY, Chen S, Sun KF, Yang Q, Wu Y, Chen XY, Cheng AC, Wang MS. Various Profiles of tet Genes Addition to tet(X) in Riemerella anatipestifer Isolates From Ducks in China. Frontiers in Microbiology, 2018, 9: 585. DOI:10.3389/fmicb.2018.00585 |
| [58] | He Y, Wang MS, Liu MF, Huang L, Liu CY, Zhang X, Yi HB, Cheng AC, Zhu DK, Yang Q, Wu Y, Zhao XX, Chen S, Jia RY, Zhang SQ, Liu YY, Yu YL, Zhang L. Cas1 and Cas2 from the type Ⅱ-C CRISPR-Cas system of Riemerella anatipestifer are required for spacer acquisition. Frontiers in Cellular and Infection Microbiology, 2018, 8: 195. DOI:10.3389/fcimb.2018.00195 |
| [59] | Liu MF, Huang M, Shui Y, Biville F, Zhu DK, Wang MS, Jia RY, Chen S, Sun KF, Zhao XX, Yang Q, Wu Y, Chen XY, Cheng AC. Roles of B739_1343 in iron acquisition and pathogenesis in Riemerella anatipestifer CH-1 and evaluation of the RA-CH-1ΔB739_1343 mutant as an attenuated vaccine. PLoS One, 2018, 13(5): e0197310. DOI:10.1371/journal.pone.0197310 |
| [60] | Zhang X, Wang MS, Liu MF, Zhu DK, Biville F, Jia RY, Chen S, Sun KF, Yang Q, Wu Y, Zhao XX, Chen XY, Cheng AC. Contribution of RaeB, a putative RND-type transporter to aminoglycoside and detergent resistance in Riemerella anatipestifer. Frontiers in Microbiology, 2017, 8: 2435. DOI:10.3389/fmicb.2017.02435 |
