胡琼1, 唐洁1

 , 刘波1, 陈廷廷1, 孙擎1, 张庆2
, 刘波1, 陈廷廷1, 孙擎1, 张庆2 1.西华大学食品与生物工程学院, 四川 成都 610039;
2.西华大学食品与生物工程学院, 古法发酵生物技术研究所, 四川 成都 610039
收稿日期:2018-03-09;修回日期:2018-04-18;网络出版日期:2018-05-10
基金项目:教育部春晖计划项目(Z2015122);四川省食品生物技术重点实验室开放基金(SZJJ2014-011);西华大学人才培养重点项目(z1310525);四川省教育厅自然科学项目(114ZB0122);西华大学研究生创新基金(ycjj2018021)
*通信作者:唐洁。Tel/Fax:+86-28-87720552;E-mail:tangjie1225@mail.xhu.edu.cn
摘要:[目的] 从长期受拟除虫菊酯类农药污染的白菜根系土壤分离1株3-苯氧基苯甲酸(3-phenoxybenzoic acid,3-PBA)降解菌,并探究其与Bacillus licheniformis G-04协同作用对高效氯氰菊酯(beta-cypermethrin,Beta-CP)的降解及污染土壤的生物修复,为土壤农药残留危害处理提供优良菌种。[方法] 采用富集驯化、筛选纯化方法,筛选3-PBA降解菌,并通过形态和生理生化特征以及16S rRNA序列分析进行鉴定。利用Origin 8.0分析3-PBA降解菌与B.licheniformis G-04的生长降解动力学过程。同时,采用高效液相色谱法评估两菌株协同降解Beta-CP的能力及其对受Beta-CP污染土壤的修复作用。[结果] 筛选得到1株3-PBA高效降解菌HA516,48 h对3-PBA(100 mg/L)的降解率达到87.73%,经鉴定为皮特不动杆菌(Acinetobacter pittii);构建了该菌株和B.licheniformis G-04的生长降解动力学方程,结果表明模型与实验数据能较好拟合;以6.7:3.3的接种比例先接种B.licheniformis G-04,24 h后再接入A.pittii HA516协同作用,在48 h,Beta-CP(50 mg/L)的降解率达78.37%,较单菌株(B.licheniformis G-04)的降解率(40.47%)提高了37.90%,半衰期从58.39 h缩短为24.51 h。土壤修复实验表明,第7天协同组对Beta-CP(30 mg/kg)的降解率较单菌株提高了33.26%,达到79.27%。[结论] A.pittii HA516是1株3-PBA高效降解菌,能与B.licheniformis G-04协同增效降解Beta-CP,可作为修复3-PBA或拟除虫菊酯类农药污染的优良微生物资源。
关键词:3-苯氧基苯甲酸高效氯氰菊酯协同降解Acinetobacter pittii HA516Bacillus licheniformis G-04土壤生物修复
Screening of a 3-phenoxybenzoic acid degrading strain and its co-degradation with Bacillus licheniformis G-04 to degrade beta-cypermethrin
Hu Qiong1, Jie Tang1

 , Bo Liu1, Tingting Chen1, Qing Sun1, Qing Zhang2
, Bo Liu1, Tingting Chen1, Qing Sun1, Qing Zhang2 1.School of Food and Bioengineering, Xihua University, Chengdu 610039, Sichuan Province, China;
2.Institute of Ancient Brewing, School of Food and Biotechnology, Xihua University, Chengdu 610039, Sichuan Province, China
Received 9 March 2018; Revised 18 April 2018; Published online 10 May 2018
*Corresponding author: Jie Tang, Tel/Fax: +86-28-87720552; E-mail: tangjie1225@mail.xhu.edu.cn
Supported by the Chunhui Program of Ministry of Education of China (Z2015122), by the Open Research Subject of Key Laboratory of Food Biotechnology of Sichuan Province of China (SZJJ2014-011), by the Key Scientific Research Fund of Xihua University (z1310525), by the Natural Science Foundation of Sichuan Provincial Department of Education (114ZB0122) and by the Graduate Student Innovation Fund of Xihua University (ycjj2018021)
Abstract: [Objective] The aim of this study was to isolate an efficient 3-phenoxybenzoic acid (3-PBA) degrading strain from the cabbage rhizosphere contaminated by pyrethroid pesticides. Furthermore, the co-degradation of beta-cypermethrin and soil bioremediation by co-culture of Bacillus licheniformis G-04 and 3-PBA-degrading strain were investigated. [Methods] 3-PBA-degrading strain was screened using enrichment domestication, isolation and purification methods, then identified by morphological, physio-biochemical tests and 16S rRNA sequence analysis. The growth and degradation kinetics of 3-PBA-degrading strain and B. licheniformis G-04 were analysised by Origin 8.0. The synergistic degradation ability and soils bioremediation of these two strains were evaluated by high performance liquid chromatography (HPLC). [Results] A novel 3-PBA-degrading strain HA516 was screened and identified as Acinetobacter pittii, the degradation rate of 3-PBA (100 mg/L) was 87.73% after 48 h of incubation. The growth and degradation kinetics of these two strains were established, which have the better fitting of predicted and the experimental value. When the inoculation proportion of the biomass of these two strains was 6.7:3.3, B. licheniformis G-04 was inoculated first, and A. pittii HA516 was inoculated after 24 h of cultivation, 78.37% Beta-CP (50 mg/L) was degraded at 48 h, which was 37.90% higher than only using strain B. licheniformis G-04, and half-life was shortened from 58.39 h to 24.51 h. Soil remediation test showed that the degradation rate of Beta-CP (30 mg/L) reached 79.27% on the seventh day, which was 33.26% higher than only using strain B. licheniformis G-04. [Conclusion] A. pittii HA516, a highly efficient 3-PBA-degrading strain, can be used as a potential strain resource for bioremediation of environment polluted with 3-PBA or pyrethroid pesticides. Beta-CP could be efficiently co-degraded by B. licheniformis G-04 and A. pittii HA516.
Keywords: 3-phenoxybenzoic acidbeta-cypermethrinco-degradationAcinetobacter pittii HA516Bacillus licheniformis G-04soil bioremediation
拟除虫菊酯类农药(pyrethroid pesticides, PP)是以天然除虫菊素为先导物合成的一类仿生型杀虫剂[1],具有广泛性、高效性及低残留等特点[2],是未来几十年难以替代的农药类型之一。高效氯氰菊酯(beta-cypermethrin, Beta-CP)是PP的一种,广泛应用于农业生产和室内卫生害虫防治等[3],其代谢产物可通过食物链富集于动物和人体,对非靶标生物具有神经毒性、生殖毒性和蓄积毒性[4-5]。3-苯氧基苯甲酸(3-phenoxybenzoic acid, 3-PBA)是Beta-CP的主要代谢产物之一,属雌激素类物质[6],具有一定的生殖毒性,易被动植物吸收[7]。Heudorf等[8]报道,德国成年人和儿童尿液中的3-PBA含量高达0.30 μg/L。而且3-PBA在造成二次污染的同时[9],也导致农药生物矿化作用受阻,从而限制了农药的生物降解途径[10]。因此,如何同时消除环境中的Beta-CP及其主要代谢产物3-PBA已受到广泛关注。
生物降解法以其条件温和、高效无毒、无二次污染及生态协调等特点被认为是一种有效的农药污染“绿色”解决方案[11],其中微生物协同代谢是污染物去除的主要方法之一。所以从环境中筛选获得高效降解Beta-CP和3-PBA的微生物是Beta-CP得以持续降解的关键。目前,国内外****已筛选到大量Beta-CP降解菌,主要有铜绿假单胞菌(Pseudomonas aeruginosa)[12]、蜡样芽孢杆菌(Bacillus cereus)[13]、苏云金杆菌(Bacillus thuringiensis)[14]、枯草芽孢杆菌(Bacillus subtilis)[15]、黑曲霉(Aspergillus niger)[16]等;关于3-PBA降解菌株,如米曲霉(Aspergillus oryzae)[17]、假单胞菌(Pseudomonas sp.)[18]、芽孢杆菌(Bacillus sp.)[19]、鞘氨醇单胞菌(Sphingomonas sp.)[20]、嗜麦芽寡养单胞菌(Stenotrophomonas sp.)[21]等也有报道。然而,单一微生物往往不具备降解矿化所需所有酶系,产生的有毒代谢中间产物会抑制微生物生长进而降低降解效率[22],导致不完全的生物降解发生[23]。有研究表明,多种微生物协同降解过程中,微生物对环境具有更强的适应性,可提高污染物降解效率[24-25]。Tran[25]等也证明了微生物的共培养可以有效地降解有机污染物和共代谢过程产生的相关代谢物。而目前通过多菌株协同作用同时降解Beta-CP和3-PBA的报道较少[26-27]。
本研究以实验室保藏的1株Beta-CP降解菌——Bacillus licheniformis G-04[28],与从长期受拟除虫菊酯类农药污染的白菜根系土壤中分离出的1株3-PBA高效降解菌——Acinetobacter pittii HA516协同降解Beta-CP,并对两菌株的生长降解动力学、菌株协同作用降解Beta-CP及污染土壤生物修复进行分析,为其进一步应用于受3-PBA或PP污染环境的生物修复提供理论基础。
1 材料和方法 1.1 材料与仪器
1.1.1 试剂与培养基: Beta-CP,南京荣诚化工有限公司;3-PBA,梯希爱(上海)化学化工发展有限公司;乙腈(色谱纯),美国Adamas-beta公司;Bacteria DNA Kit提取试剂盒、多重PCR扩增试剂盒,天根生化科技有限公司;基础盐培养基(Mineral Salt Medium, MSM)和Luria-Bertani培养基(LB Medium)参照文献[29]进行配制。
1.1.2 样品来源: B. licheniformis G-04为实验室保藏的1株Beta-CP降解菌,分离自成都郫县某一长期施用拟除虫菊酯农药的菜园,培养72 h对Beta-CP (50 mg/L)的降解率为83.86%[28];分离3-PBA降解菌的土样采自川北地区长期受PP污染的白菜根系土壤(10–20 cm),生物修复土壤采自同一地区未受污染土壤。
1.1.3 仪器: Agilent 1200型高效液相色谱仪、Agilent 1200型DAD紫外检测器,美国Agilent公司;JSM7500F型扫描电镜,日本电子株式会社;UNICO 7200型紫外分光光度计,美国UNICO公司;Heraeus Multifuge X1R高速冷冻离心机,美国Thermo Scientific公司。
1.2 Beta-CP和3-PBA的检测及其标准曲线方程建立 Beta-CP和3-PBA的样品处理及检测方法参照文献[28, 30]进行,采用高效液相色谱法(High Performance Liquid Chromatography,HPLC),紫外检测器波长为210 nm。配制Beta-CP标准溶液(50 mg/L)和3-PBA标准溶液(100 mg/L),依次进样1、2、4、6、8、10 μL进行标准曲线绘制,拟合Beta-CP和3-PBA质量-峰面积的标准曲线分别为:YBeta-CP=10638425.352x+548384.701(R2=0.9997);Y3-PBA =83.4563x+0.7695(R2=1.0000)。
1.3 3-PBA降解菌的筛选与鉴定 3-PBA降解菌的富集驯化、筛选纯化和菌株鉴定参照文献[29]进行,纯化后的菌株转接到含3-PBA (100 mg/L)的MSM培养基中,空白对照不接菌而接等体积无菌水,30 ℃、180 r/min培养48 h后取样测定3-PBA残留量[30],筛选高降解率[按公式(1)计算]菌株,将最高效降解菌命名为HA516用于后续实验。
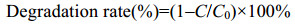 | 公式(1) |
1.4 降解菌的生长降解动力学 将两菌株种子液(OD600≈1.0)[30]按5.0% (V/V)的接种量分别接种到对应含Beta-CP (50 mg/L)和3-PBA (100 mg/L)的MSM培养基中,在30 ℃、180 r/min条件下培养,定时取样测定其OD600值、Beta-CP和3-PBA残留量。
Logistic方程是由Verhulst-Peal提出,常用来描述菌体生长动力学过程[23],其生长动力学方程如公式(2)所示;其一级降解动力学方程[31]如公式(3)所示;按公式(4)计算底物降解半衰期t1/2(h)。
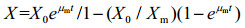 | 公式(2) |
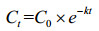 | 公式(3) |
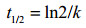 | 公式(4) |
1.5.1 不同接种比例对Beta-CP降解率的影响: 在LB培养基中加入定量的Beta-CP使其终浓度为50 mg/L,按5.0% (V/V)的接种量接入OD600≈1.0的B. licheniformis G-04和菌株HA516的种子液,其接种比例分别为10:0、7.5:2.5、6.7:3.3、5:5、3.3:6.7、2.5:7.5、0:10 (V/V),在30 ℃、180 r/min条件下培养48 h,取样测定Beta-CP降解率。
1.5.2 不同接种顺序对Beta-CP降解率的影响: 在LB培养基中加入一定量的Beta-CP使其终浓度为50 mg/L,根据1.5.1的实验结果设置两菌株的最佳接种比例,分别按以下3种不同接种顺序接入OD600≈1.0的B. licheniformis G-04和菌株HA516,在30 ℃、180 r/min条件下培养48 h,取样测定Beta-CP降解率。
接种顺序1:先接种B. licheniformis G-04,培养24 h后再接种菌株HA516。
接种顺序2:同时接种B. licheniformis G-04和菌株HA516。
接种顺序3:先接种菌株HA516,培养24 h后再接种B. licheniformis G-04。
1.6 菌株协同作用生物修复污染土壤 对采集回来的土样进行理化指标测定[32],该土壤呈弱碱性,有机质含量为15.90–21.10 g/kg,总氮为2.30–2.80 g/kg,总磷为0.38–0.51 g/kg,含水量为30.50%–36.10% (W/W)。烘干后过2 mm滤筛,去除土壤中可见杂质。称取Beta-CP原农药,添加到土壤中使其含量为30 mg/kg,过夜备用。将土样分为协同组、单菌株和空白组3组,每组设3个重复,每组土样1 kg。协同组根据1.5的实验结果,按5% (V/W)添加量,在最佳接种比例和接种顺序下将混合菌株添加到污染土壤中;单菌株按5% (V/W)添加量将OD600≈1.0的B. licheniformis G-04添加到污染土壤中;空白组添加等量无菌水,3组实验中土壤含水量保持为35% (W/W),温度为20 ℃,每隔1 d取样测定Beta-CP残留量[29]。
1.7 数据分析 实验结果采用Origin 8.0统计软件进行分析,数据用均数±标准差(x±s)表示。
2 结果和分析 2.1 3-PBA降解菌的筛选鉴定 经富集驯化和分离筛选,共获得10株可耐受3-PBA (1600 mg/L)的菌株,8株具备有效降解3-PBA的能力。经方差分析(图 1),其中一株菌株降解率与其他7株降解率差异性显著(P < 0.01),达87.73%,命名为HA516。将菌株HA516划线接种于MSM培养基中,呈圆形、乳白色、中央凸起、粘稠、表面光滑、边缘整齐的菌落。菌体(图 2)呈长圆杆状,单个排列,大小为(0.6–1.0) μm× (0.8–2.6) μm。革兰氏镜检、甲基红、蛋白酶、吲哚和硫化氢实验均呈阴性;发酵葡萄糖、V-P反应均呈阳性,表明菌株HA516可能属于肠杆菌科细菌[33-34]。将菌株HA516的16S rRNA扩增产物回收测序获得1358 bp的序列。利用BLAST检索分析将菌株HA516的序列提交至GenBank (accession number: KY474547)进行同源性分析,发现菌株HA516与Acinetobacter pittii ATCC 19004 (accession number: NR117621)、Acinetobacter pittii BR12 (accession number: KT748634)的同源性达99%。对于16S rRNA序列同源性大于97%,认为属于同种[35],可以推测菌株HA516为不动杆菌属细菌(Acinetobacter sp.)。利用MEGA 7.0软件及其Neighbor-Joining法构建系统发育树(图 3)进一步确定菌株HA516的种属地位,发现菌株HA516与Acinetobacter pittii ATCC 19004、Acinetobacter pittii BR12、Acinetobacter pittii W26均处于同一分支,进一步证实了菌株HA516为皮特不动杆菌(Acinetobacter pittii)。结合形态学、生理生化特征[36]、16S rRNA基因序列和系统发育分析,可以确定菌株HA516为皮特不动杆菌(Acinetobacter pittii)。
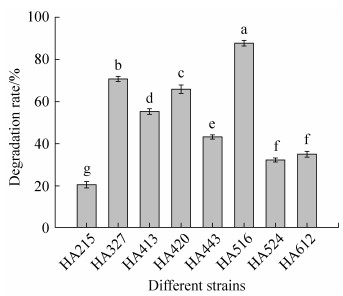 |
| 图 1 不同菌株对3-PBA的降解率 Figure 1 Degradation rate of 3-PBA by different strains. Different letters indicate significant difference. P < 0.01. |
| 图选项 |
 |
| 图 2 菌株HA516扫描电镜形态 Figure 2 Scanning electron microscopic morphology of strain HA516. |
| 图选项 |
 |
| 图 3 菌株HA516基于16S rRNA的菌株系统发育分析 Figure 3 Phylogenetic tree of strain HA516 based on 16S rRNA sequence. Node number represents the confidence level of relatives; The length of branch represents the evolutionary distance and the coefficient is 0.0050. |
| 图选项 |
2.2 B. licheniformis G-04和A. pittii HA516的生长降解动力学模型拟合 利用Origin 8.0对菌株生物量与菌株降解动力学模型进行非线性拟合,分别得到两菌株生长动力学方程:XG-04=0.04346e0.08927t/[1-0.03959 (1-0.04346e0.08927t)],μm=0.09927 h–1,X0=0.04346及Xm= 1.0978;XHA516=0.09133e0.09344t/[1-0.08051 (1–e0.09344t)],μm=0.09344 h–1,X0=0.09133及Xm=1.1344。图 4-A表明B. licheniformis G-04能在以Beta-CP为唯一碳源的MSM培养基中良好生长,0–10 h处于生长延滞期,10 h后OD600急剧上升,迅速进入生长对数期,细胞数量显著增加,48 h达到稳定期。图 4-B表明A. pittii HA516具有较短的生长延滞期,在生长对数期菌体呈几何级数增长。
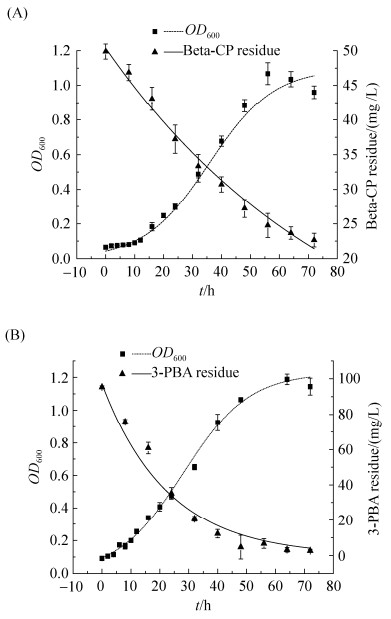 |
| 图 4 B. licheniformis G-04 (A)和A. pittii HA516 (B)的生长和降解曲线 Figure 4 Growth and degradation curve of strain B. licheniformis G-04(A) and A. pittii HA516(B). P < 0.01. |
| 图选项 |
分析B. licheniformis G-04降解Beta-CP (图 4-A)和A. pittii HA516降解3-PBA (图 4-B)的动力学过程中得到菌株对底物的一级降解动力学方程:CG-04=50.35474e–0.01187t,k=0.01187,t1/2=58.39;CHA516=97.03633e–0.04371t,k=0.04371,t1/2=15.86。48 h A. pittii HA516对3-PBA的降解率达到87.73%,优于Zhu等[17]分离到的Aspergillus oryzae M4培养5 d对3-PBA (100 mg/L)的降解率,表明A. pittii HA516具有3-PBA高效降解能力。
得到B. licheniformis G-04生长降解动力学模型的拟合度分别为0.9719和0.9880;A. pittii HA516生长降解动力学模型的拟合度分别为0.9886和0.9918。表明2种模型能较好反映B. licheniformis G-04和A. pittii HA516的实际生长规律以及分别对Beta-CP和3-PBA的降解作用。
2.3 两菌株协同作用降解高效氯氰菊酯的特性
2.3.1 不同接种比例对Beta-CP降解率的影响: 基于B. licheniformis G-04和A. pittii HA516作用底物不同,考察其协同作用的最适接种比例。由图 5可知,48 h时不同比例的B. licheniformis G-04和A. pittii HA516对Beta-CP的降解率不同。随着B. licheniformis G-04接种量的减少、A. pittii HA516接种量的增加,Beta-CP降解率呈现先增加后降低的趋势,当B. licheniformis G-04和A. pittii HA516接种比例为6.7:3.3 (V/V)时,Beta-CP的降解率最高。这与Zhao等[26]的研究相一致,可能是A. pittii HA516降解了B. licheniformis G-04代谢Beta-CP产生的有毒中间产物3-PBA,减少了降解体系中3-PBA的累积,从而加速Beta-CP的降解。而A. pittii HA516是一株高效3-PBA降解菌,对Beta-CP的降解率不高,所以当B. licheniformis G-04的接种量降低后,Beta-CP的降解率也随之下降。因此,B. licheniformis G-04和A. pittii HA516能通过协同的方式提高对Beta-CP的降解率,其最适接种比例为6.7:3.3 (V/V)。
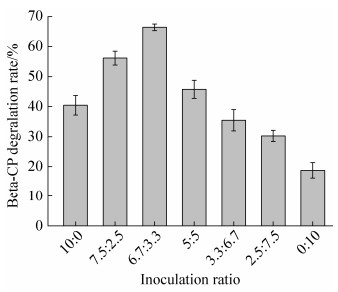 |
| 图 5 菌株接种比例(B. licheniformis G-04:A. pittii HA516)对Beta-CP降解的影响 Figure 5 The effect of inoculation ratio of two strains on the degradation of Beta-CP (B. licheniformis G-04:A. pittii HA516). P < 0.01. |
| 图选项 |
2.3.2 不同接种顺序对Beta-CP降解率的影响: 基于B. licheniformis G-04和A. pittii HA516的生长特性不同,考察两菌株的最适接种顺序对Beta-CP降解的影响。从图 6可以看出,先接种B. licheniformis G-04,24 h后再接种A. pittii HA516的降解体系中,培养终点时Beta-CP的残留量最少,说明先接种B. licheniformis G-04对Beta-CP的降解效率最高。这可能是由于先接入的B. licheniformis G-04降解Beta-CP为3-PBA,解除了Beta-CP对A. pittii HA516的部分抑制作用。而生成的3-PBA被后接入的A. pittii HA516迅速降解,从而降低了3-PBA对B. licheniformis G-04代谢的反馈抑制,这与Liu等[27]研究结果相一致。因此两菌株降解Beta-CP的最适接种顺序为先接种B. licheniformis G-04,24 h后再接种A. pittii HA516。
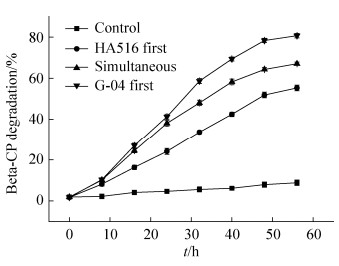 |
| 图 6 菌株接种顺序对Beta-CP降解的影响 Figure 6 The effect of inoculation sequence of two strains on the degradation of Beta-CP. P < 0.01. |
| 图选项 |
在最佳接种比例和接种顺序下,得出协同组对底物(Beta-CP)的一级降解动力学方程为:Cco-degrading strains=52.51510e–0.02828t,k=0.02828,t1/2=24.51,拟合度为0.9609。48 h对Beta-CP (50 mg/L)的降解率为78.37%,与2.3.1中的单菌株(B. licheniformis G-04)降解率作比较,协同组较单菌株(40.47%)提高37.90%。表明B. licheniformis G-04和A. pittii HA516协同作用有效提高了Beta-CP的降解效率。
2.4 污染土壤生物修复 以6.7:3.3 (V/V)的接种比例,先接种B. licheniformis G-04,24 h后再接种A. pittii HA516协同修复土壤结果见图 7。前2 d协同组和单菌株降解效率差异较小,第7天协同组对Beta-CP降解效率达到了79.27%,较单菌株提高了33.26%,这与在培养基中的趋势一致,随着时间的延长,菌株对土壤的修复作用逐渐增强,但比在培养基中的降解效率低,可能是因为土壤中缺乏B. licheniformis G-04和A. pittii HA516生长的营养元素[27]。土壤修复实验结果表明B. licheniformis G-04和A. pittii HA516的协同作用在污染土壤的修复应用方面有很大的潜能。
 |
| 图 7 土壤生物修复 Figure 7 Soil remediation, Beta-CP content in the soil by using single strain (B. licheniformis G-04), co-degrading strains (B. licheniformis G-04 and A. pittii HA516), and in the uninoculated soil (control). P < 0.01. |
| 图选项 |
3 讨论 3-PBA是绝大多数拟除虫菊酯类农药降解的有毒中间产物,会抑制微生物的生长和农药的持续降解[9, 26],因此,3-PBA的消除是母体农药得以持续矿化降解的关键。本课题组首先从长期受PP污染的白菜根系土壤中分离得到具有高效3-PBA降解能力的菌株HA516,经鉴定为皮特不动杆菌(Acinetobacter pittii)。近年来关于皮特不动杆菌的报道较少,但不动杆菌(Acinetobactersp.)广泛应用于生物医疗、食品工业和环境治理等领域[37-38],特别是在环境治理方面,如刘玉华[34]等从油井溢油污染土壤分离到高效石油降解菌Acinetobactersp. BZ-15;王迪等[39]从污水处理厂筛选到以双酚A为唯一碳源的高效降解菌Acinetobacter calcoaceticus D-17;Acinetobacter sp. YN3也能在废水处理过程中快速高效地完成异养硝化和好氧反硝化脱氮作用[36]。因此,不动杆菌在环境治理方面具有潜在的生物修复作用。同时,分析A. pittii HA516的生长降解动力学过程发现,其48 h对3-PBA (100 mg/L)的降解率达到87.73%,比从活性淤泥分离到的苍白杆菌DG-S-01 (培养9 d)对3-PBA (100 mg/L)的降解率高3.03%[7],比从酱油曲料分离到的Aspergillus oryzae M4 (培养5 d)对3-PBA (100 mg/L)的降解率高7.11%[17]。并且在该菌的作用下3-PBA的半衰期缩短为15.86 h,远低于Chen等[21]从活性淤泥分离出的Stenotrophomonas sp. ZS-S-01 (2.3–4.9 d),表明A. pittii HA516是1株高效3-PBA降解菌。
生物降解本质是酶促降解[40],而单一微生物往往不具备矿化有机污染物的完整酶系,部分降解中间产物的毒性还高于母体农药,进一步抑制单一微生物的生长,限制其降解速率[29];基于此,本文以B. licheniformis G-04和A. pittii HA516协同作用降解Beta-CP,以6.7:3.3的接种比例先接种B. licheniformis G-04,24 h后再接入A. pittii HA516,协同降解Beta-CP较B. licheniformis G-04单独作用,其降解效率提高了37.90%,可能是由于3-PBA抑制了Beta-CP代谢过程中关键酶的表达,或限制代谢途径中关键酶的活性,进一步降低整个降解代谢进程,这与Liu等[27]利用Bacillus licheniformis B-1和Sphingomonas sp. SC-1协同降解氯氰菊酯较Bacillus licheniformis B-1单独作用其降解效率高的结果相一致。同时Zhao等[26]研究也表明,当有毒中间产物3-PBA被降解,其农药降解速率显著提高;本研究中,在接种量相同的条件下,3-PBA降解菌A. pittii HA516的介入可减少降解体系中3-PBA的累积,消除3-PBA对B. licheniformis G-04代谢的反馈抑制,从而加速Beta-CP的高效降解,使得B. licheniformis G-04和A. pittii HA516协同作用降解Beta-CP的半衰期缩短至24.51 h,远低于Bacillus licheniformis B-1和Aspergillus oryzae M-4协同作用降解Beta-CP[26],表明B. licheniformis G-04和A. pittii HA516协同作用能快速高效地利用Beta-CP。Chen等[41]研究表明共代谢具有更高的适应性,利用蜡样芽胞杆菌和金色链霉菌构成的复合体系降解氯氰菊酯与单一菌株相比,其降解效果显著提高;在实验室模拟生物修复Beta-CP污染土壤,B. licheniformis G-04和A. pittii HA516的协同修复较单一B. licheniformis G-04的修复更有效,且该结果较Zhao等[26]利用Bacillus licheniformis B-1和Sphingomonas sp. SC-1的修复效果更佳,表明B. licheniformis G-04和A. pittii HA516协同作用在污染土壤的修复应用方面有很大的潜能。同时,由于自然条件下田间微生物情况较实验室模拟条件更为复杂,而Beta-CP在自然微生物降解的情况下可能已处在不同降解阶段,降解中间产物3-PBA可能先于Beta-CP成为降解的抑制因子;因此,在自然条件下的污染土壤生物修复需根据不同土壤受此类农药污染的具体情况,重新考虑协同作用菌株的接种顺序及比例,以期达到更好的生物修复效果。
研究表明A. pittii HA516是1株高效3-PBA降解菌,可与B. licheniformis G-04协同增效降解Beta-CP,且具有很好的污染土壤修复效果,在生物修复受Beta-CP或PP污染的环境中有良好的应用前景。
References
| [1] | Cycoń M, Piotrowska-Seget Z. Pyrethroid-degrading microorganisms and their potential for the bioremediation of contaminated soils:a review. Frontiers in Microbiology, 2016, 7: 1463. |
| [2] | Wang P, Zhou ZQ, Jiang SR, Yang L. Chiral resolution of cypermethrin on cellulose-tris (3, 5-dimethylphenyl-carbamate) chiral stationary phase. Chromatographia, 2004, 59(9/10): 625-629. |
| [3] | Weston DP, Holmes RW, Lydy MJ. Residential runoff as a source of pyrethroid pesticides to urban creeks. Environmental Pollution, 2009, 157(1): 287-294. DOI:10.1016/j.envpol.2008.06.037 |
| [4] | Perry MJ, Venners SA, Barr DB, Xu XP. Environmental pyrethroid and organophosphorus insecticide exposures and sperm concentration. Reproductive Toxicology, 2007, 23(1): 113-118. DOI:10.1016/j.reprotox.2006.08.005 |
| [5] | Chen SH, Luo JJ, Hu MY, Huang HS, Zhang F. Degradation characteristics and kinetics of beta-cypermethrin by Streptomyces sp. HP-S-01. Microbiology China, 2011, 38(8): 1207-1215. (in Chinese) 陈少华, 罗建军, 胡美英, 黄华盛, 张芳. 链霉菌HP-S-01降解高效氯氰菊酯的特性及其动力学. 微生物学通报, 2011, 38(8): 1207-1215. |
| [6] | Liu Y, Wu AH, Hu J, Lin MM, Wen MT, Zhang X, Xu CX, Hu XD, Zhong JF, Jiao LX, Xie YJ, Zhang CZ, Yu XY, Liang Y, Liu XJ. Detection of 3-phenoxybenzoic acid in river water with a colloidal gold-based lateral flow immunoassay. Analytical Biochemistry, 2015, 483: 7-11. DOI:10.1016/j.ab.2015.04.022 |
| [7] | Chen SH, Hu MY, Liu JJ, Zhong GH, Yang L, Rizwan-ul-Haq M, Han HT. Biodegradation of beta-cypermethrin and 3-phenoxybenzoic acid by a novel Ochrobactrum lupini DG-S-01. Journal of Hazardous Materials, 2011, 187(1/3): 433-440. |
| [8] | Heudorf U, Angerer J, Drexler H. Current internal exposure to pesticides in children and adolescents in Germany:urinary levels of metabolites of pyrethroid and organophosphorus insecticides. International Archives of Occupational and Environmental Health, 2004, 77(1): 67-72. DOI:10.1007/s00420-003-0470-5 |
| [9] | Sun H, Chen W, Xu XL, Ding Z, Chen XD, Wang XR. Pyrethroid and their metabolite, 3-phenoxybenzoic acid showed similar (anti) estrogenic activity in human and rat estrogen receptor α-mediated reporter gene assays. Environmental Toxicology and Pharmacology, 2014, 37(1): 371-377. DOI:10.1016/j.etap.2013.11.031 |
| [10] | White GF, Russell NJ, Tidswell EC. Bacterial scission of ether bonds. Microbiological Reviews, 1996, 60(1): 216-232. |
| [11] | Guo XQ, Wang XJ, Sun AL, Li DX, Shi XZ. Advances of studies on microbial degradation of pyrethroid insecticides. China Biotechnology, 2017, 37(5): 126-132. (in Chinese) 郭晓青, 王秀娟, 孙爱丽, 李德祥, 史西志. 环境中拟除虫菊酯类农药微生物降解技术研究进展. 中国生物工程杂志, 2017, 37(5): 126-132. |
| [12] | Tang AX, Wang BW, Liu YY, Li QY, Tong ZF, Wei YJ. Biodegradation and extracellular enzymatic activities of Pseudomonas aeruginosa strain GF31 on β-cypermethrin. Environmental Science and Pollution Research, 2015, 22(17): 13049-13057. DOI:10.1007/s11356-015-4545-0 |
| [13] | Liu J, Huang WW, Han HT, She CC, Zhong GH. Characterization of cell-free extracts from fenpropathrin-degrading strain Bacillus cereus ZH-3 and its potential for bioremediation of pyrethroid-contaminated soils. Science of the Total Environment, 2015, 523: 50-58. DOI:10.1016/j.scitotenv.2015.03.124 |
| [14] | Chen SH, Deng YY, Chang CQ, Lee J, Cheng YY, Cui ZN, Zhao JN, He F, Hu MY, Zhang LH. Pathway and kinetics of cyhalothrin biodegradation by Bacillus thuringiensis strain ZS-19. Scientific Reports, 2015, 5: 8784. DOI:10.1038/srep08784 |
| [15] | Xiao Y, Chen SH, Gao YQ, Hu W, Hu MY, Zhong GH. Isolation of a novel beta-cypermethrin degrading strain Bacillus subtilis BSF01 and its biodegradation pathway. Applied Microbiology and Biotechnology, 2015, 99(6): 2849-2859. DOI:10.1007/s00253-014-6164-y |
| [16] | Deng WQ, Lin DR, Yao K, Yuan HY, Wang ZL, Li JL, Zou LK, Han XF, Zhou K, He L, Hu XJ, Liu SL. Characterization of a novel β-cypermethrin-degrading Aspergillus niger YAT strain and the biochemical degradation pathway of β-cypermethrin. Applied Microbiology and Biotechnology, 2015, 99(19): 8187-8198. DOI:10.1007/s00253-015-6690-2 |
| [17] | Zhu YT, Li JL, Yao K, Zhao N, Zhou K, Hu XJ, Zou LK, Han XF, Liu AP, Liu SL. Degradation of 3-phenoxybenzoic acid by a filamentous fungus Aspergillus oryzae M-4 strain with self-protection transformation. Applied Microbiology and Biotechnology, 2016, 100(22): 9773-9786. DOI:10.1007/s00253-016-7847-3 |
| [18] | Halden RU, Tepp SM, Halden BG, Dwyer DF. Degradation of 3-phenoxybenzoic acid in soil by Pseudomonas pseudoalcaligenes POB310(pPOB) and two modified Pseudomonas strains. Applied and Environmental Microbiology, 1999, 65(8): 3354-3359. |
| [19] | Chen SH, Hu W, Xiao Y, Deng YY, Jia JW, Hu MY. Degradation of 3-phenoxybenzoic acid by a Bacillus sp.. PLoS One, 2012, 7(11): e50456. DOI:10.1371/journal.pone.0050456 |
| [20] | Tang J, Yao K, Liu SL, Jia DY, Chi YL, Zeng CY, Wu S. Biodegradation of 3-phenoxybenzoic acid by a novel Sphingomonas sp. SC-1. Fresenius Environmental Bulletin, 2013, 22(5): 1564-1572. |
| [21] | Chen SH, Yang L, Hu MY, Liu JJ. Biodegradation of fenvalerate and 3-phenoxybenzoic acid by a novel Stenotrophomonas sp. strain ZS-S-01 and its use in bioremediation of contaminated soils. Applied Microbiology and Biotechnology, 2011, 90(2): 755-767. DOI:10.1007/s00253-010-3035-z |
| [22] | Nzila A. Update on the cometabolism of organic pollutants by bacteria. Environmental Pollution, 2013, 178: 474-482. DOI:10.1016/j.envpol.2013.03.042 |
| [23] | Vallero DA. Chapter 7-applied microbial ecology:bioremediation. Environmental Biotechnology, 2010: 325-400. |
| [24] | Luo W, Zhu XC, Chen WT, Duan ZB, Wang L, Zhou Y. Mechanisms and strategies of microbial cometabolism in the degradation of organic compounds-chlorinated ethylenes as the model. Water Science and Technology:A Journal of the International Association on Water Pollution Research, 2014, 69(10): 1971-1983. DOI:10.2166/wst.2014.108 |
| [25] | Tran NH, Urase T, Ngo HH, Hu JY, Ong SL. Insight into metabolic and cometabolic activities of autotrophic and heterotrophic microorganisms in the biodegradation of emerging trace organic contaminants. Bioresource Technology, 2013, 146: 721-731. DOI:10.1016/j.biortech.2013.07.083 |
| [26] | Zhao JY, Chi YL, Xu YC, Jia DY, Yao K. Co-metabolic degradation of β-Cypermethrin and 3-phenoxybenzoic acid by co-culture of Bacillus licheniformis B-1 and Aspergillus oryzae M-4. PLoS One, 2016, 11(11): e0166796. DOI:10.1371/journal.pone.0166796 |
| [27] | Liu FF, Chi YL, Wu S, Jia DY, Yao K. Simultaneous degradation of cypermethrin and its metabolite, 3-phenoxybenzoic acid, by the cooperation of Bacillus licheniformis B-1 and Sphingomonas sp. SC-1. Journal of Agricultural and Food Chemistry, 2014, 33(8): 8256-8262. |
| [28] | Liu B, Tang J, Chen TT, Shi Y, Zeng L, Zeng CY. Isolation and identification of two pyrethroid pesticides degradation strains and research of degradative capabilities. Science and Technology of Food Industry, 2017, 38(4): 214-219, 224. (in Chinese) 刘波, 唐洁, 陈廷廷, 史颖, 曾林, 曾朝懿. 两株拟除虫菊酯类农药降解菌的分离鉴定及其降解能力的研究. 食品工业科技, 2017, 38(4): 214-219, 224. |
| [29] | Tang J, Yao K, Jia DY, Chi YL, Li QP. Isolation and characterization of Beta-cypermethrin degrading strain and its condition optimization. Journal of Sichuan University (Engineering Science Edition), 2013, 45(4): 176-180. (in Chinese) 唐洁, 姚开, 贾冬英, 迟原龙, 李清平. 高效氯氰菊酯降解菌的分离与鉴定及其降解条件优化. 四川大学学报(工程科学版), 2013, 45(4): 176-180. |
| [30] | Liu B, Tang J, Chen TT, Zeng L, Zeng CY, Zhang Q. Screening and characterization of a 3-phenoxybenzoic acid degrading Enterobacter ludwigii. Acta Microbiologica Sinica, 2018. (in Chinese) 刘波, 唐洁, 陈廷廷, 曾林, 曾朝懿, 张庆. 一株路德维希肠杆菌的筛选及其对3-苯氧基苯甲酸的降解特性分析. 微生物学报, 2018. DOI:10.13343/j.cnki.wsxb.20170305 |
| [31] | Ren L, Shi YH, Jia Y, Yao XS, Nahurira R, Mi CX, Yan YC. Biodegradation characteristics and kinetics of p-nitrophenol by strain Arthrobacter sp. CN2. Environmental Science, 2015, 36(5): 1757-1762. (in Chinese) 任磊, 史延华, 贾阳, 姚雪松, NahuriraR, 弥春霞, 闫艳春. 菌株Arthrobacter sp. CN2降解对硝基苯酚的特性与动力学. 环境科学, 2015, 36(5): 1757-1762. |
| [32] | Zhang C, Chen WB, Wu DJ, Wei TD. Remediation of oil-contaminated soil with mixed bacteria. Environmental Protection of Chemical Industry, 2014, 34(1): 19-23. (in Chinese) 张超, 陈文兵, 武道吉, 魏天迪. 混合菌修复石油污染土壤. 化工环保, 2014, 34(1): 19-23. DOI:10.3969/j.issn.1006-1878.2014.01.005 |
| [33] | Di FR, Song DH, Liu FL, Yang J. Isolation of marine Acinetobacter and its characteristics of petroleum hydrocarbon degradation. Marine Environmental Science, 2017, 36(6): 898-904. (in Chinese) 邸富荣, 宋东辉, 刘凤路, 杨劼. 分离海洋不动杆菌及其对石油烃降解性能研究. 海洋环境科学, 2017, 36(6): 898-904. |
| [34] | Liu YH, Hu XK. Microbial degradation of petroleum hydrocarbons by Acinetobacter sp. BZ-15, isolated from contaminated soil. Scientia Sinica Vitae, 2016, 46(9): 1091-1100. (in Chinese) 刘玉华, 胡晓珂. 高效石油烃降解菌不动杆菌(Acinetobacter sp. BZ-15)的筛选、鉴定及其降解性能研究. 中国科学:生命科学, 2016, 46(9): 1091-1100. |
| [35] | Wang ZX, Xu Y, Zhou PJ. Taxonomy of a new species of Haloalkalophilic archaeon. Acta Microbiologica Sinica, 2000, 40(2): 115-120. (in Chinese) 王振雄, 徐毅, 周培瑾. 嗜盐碱古生菌新种的系统分类学研究. 微生物学报, 2000, 40(2): 115-120. DOI:10.3321/j.issn:0001-6209.2000.02.001 |
| [36] | Yan WZ, Zhang HQ, Yu CT, Lei YF, Hao J, Shi JP. Isolation of Acinetobacter sp. YN3 and its heterotrophic nitrification-aerobic denitrification characters. Chinese Journal of Environmental Engineering, 2017, 11(7): 4419-4428. (in Chinese) 颜薇芝, 张汉强, 余从田, 雷艳芳, 郝健, 史吉平. 1株异养硝化好氧反硝化不动杆菌的分离及脱氮性能. 环境工程学报, 2017, 11(7): 4419-4428. |
| [37] | Pailhoriès H, Tiry C, Eveillard M, Kempf M. Acinetobacter pittii isolated more frequently than Acinetobacter baumannii in blood cultures:the experience of a French hospital. The Journal of Hospital Infection, 2018, 99(3): 360-363. DOI:10.1016/j.jhin.2018.03.019 |
| [38] | Purohit A, Rai SK, Chownk M, Sangwan RS, Yadav SK. Xylanase from Acinetobacter pittii MASK 25 and developed magnetic cross-linked xylanase aggregate produce predominantly xylopentose and xylohexose from agro biomass. Bioresource Technology, 2017, 244: 793-799. DOI:10.1016/j.biortech.2017.08.034 |
| [39] | Wang D, Ma XP, Meng XL, Dong WL, Dong X, Xu CB. Screening, identification of a bisphenol A-degrading strain and its growth and degradation conditions. Environmental Protection of Chemical Industry, 2017, 37(2): 189-193. (in Chinese) 王迪, 马溪平, 孟雪莲, 董文灵, 董兴, 徐成斌. 一株双酚A降解菌的筛选、鉴定及其生长、降解条件. 化工环保, 2017, 37(2): 189-193. DOI:10.3969/j.issn.1006-1878.2017.02.011 |
| [40] | Sogorb MA, Vilanova E. Enzymes involved in the detoxification of organophosphorus, carbamate and pyrethroid insecticides through hydrolysis. Toxicology Letters, 2002, 128(1/3): 215-228. |
| [41] | Chen SH, Luo JJ, Hu MY, Lai KP, Geng P, Huang HS. Enhancement of cypermethrin degradation by a coculture of Bacillus cereus ZH-3 and Streptomyces aureus HP-S-01. Bioresource Technology, 2012, 110: 97-104. DOI:10.1016/j.biortech.2012.01.106 |
