 , 童裳伦
, 童裳伦

浙江大学环境与资源学院, 污染环境修复与生态健康教育部重点实验室, 杭州 310058
收稿日期: 2021-02-05; 修回日期: 2021-03-25; 录用日期: 2021-03-25
基金项目: 国家自然科学基金面上项目(No.21677120)
作者简介: 周莉(1995-), 女, E-mail: 21814025@zju.edu.cn
通讯作者(责任作者): 童裳伦, E-mail: cltong@zju.edu.cn
摘要:为开发高效抗生素吸附剂,将钨酸铜通过静电作用负载到碘氧化铋晶体表面,制备了新型多金属氧酸盐复合材料(BiOI/CuWO4),并以扫描电镜、傅里叶红外光谱与X射线能谱等对其进行表征.选用环丙沙星(CIP)、恩诺沙星(ENR)、诺氟沙星(NOR)、氧氟沙星(OFL)这4种典型氟喹诺酮类抗生素(FQs)作为研究对象,在建立同时测定这4种FQs的高效液相色谱分析方法基础上,研究了BiOI/CuWO4对FQS的吸附性能及溶液pH值和吸附剂量等对吸附的影响.结果表明,实验浓度范围内BiOI/CuWO4对NOR、CIP、ENR和OFL的最大吸附量分别为277.8、149.3、113.6和112.4 mg·g-1,且能够在3 h内达到吸附平衡,吸附动力学符合准二级吸附动力学模型,对CIP、ENR和OFL的吸附等温线基本复合Langmuir模型,而对NOR的吸附则更加符合Freundlich模型.与其它吸附剂相比,BiOI/CuWO4对4种典型FQs具有较高的吸附量及较好的重复利用性,更符合实际应用需求.
关键词:钨酸铜氟喹诺酮吸附动力学吸附剂吸附等温
The preparation of BiOI/CuWO4 composite and its adsorption performance for fluoroquinolone antibiotics
ZHOU Li
 , TONG Changlun
, TONG Changlun

Key Laboratory of Environmental Remediation and Ecological Health of Ministry of Education, College of Environmental and Resource Sciences, Zhejiang University, Hangzhou 310058
Received 5 February 2021; received in revised from 25 March 2021; accepted 25 March 2021
Abstract: Copper tungstate was electrostatically loaded onto the surface of iodine bismuth oxide crystals to obtain a novel polyoxometallate composite of BiOI/CuWO4,whihc was characterized with scanning electron microscopy,Fourier transform infrared spectrometer,energy dispersive X-ray spectroscopy,and etc. The simultaneous determination of ciprofloxacin (CIP),enrofloxacin (ENR),norfloxacin (NOR) and oxfloxacin (OFL) with a high performance liquid chromatography was first developed,and the absorption performance of BiOI/CuWO4 for the four fluoroquinolones and its influening factors such as pH value and adsorbent dosage were also investigated. The results show that BiOI/CuWO4 presents favorable absorption towards FQs,with the maximum adsorption capacities for NOR,CIP,ENR and OFL being 277.8,149.3,113.6 and 112.4 mg·g-1,respectively. The absorption equilibrium can be obtained in 3 h,and the data well fit the pseudo second-order kinetic model. The adsorption isotherms of CIP,ENR and OFL basically fit with Langmuir model,and the adsorption of NOR better fits with Freundlich model. Compared with other adsorbents,BiOI/CuWO4 has high adsorption capacities for four FQs with good reusability,and is suitable for practical application.
Keywords: copper tungstatefluoroquinolonesadsorption kineticsadsorbentadsorption isotherm
1 引言(Introduction)氟喹诺酮类(FQs)抗生素作为一种治疗人类和动物多种细菌感染的合成抗生素, 因使用广泛已在世界范围内引起了高度关注(Meersche et al., 2016).在国内FQs也是使用十分广泛的抗生素, 其中, 诺氟沙星(NOR)、环丙沙星(CIP)和氧氟沙星(OFL)的使用量约占98%, 并可能使生物种群产生耐药性, 最终导致药效逐渐下降并显著增加人类的健康风险(Doorslaer et al., 2014).据文献报道, 某畜牧养殖厂排出的动物粪便有大量抗生素残留, 其中, 环丙沙星与恩诺沙星(ENR)含量分别为34.8~190928 μg·kg-1和13.3~32799 μg·kg-1(Yue et al., 2021);在某地区垃圾填埋场样本中检出的氟喹诺酮类药物含量高达1.4 mg·kg-1(Yao et al., 2020).同时, 在国内许多河流中都能检测到氟喹诺酮类抗生素, 并成为主要的新型污染物之一(Liu et al., 2013;Bu et al., 2013), 因此, 从水中去除氟喹诺酮类抗生素尤为重要.
目前, 国内外****已经报道了多种去除FQs的技术和方法, 其中包括高级氧化(Witte et al., 2009)、吸附(Chen et al., 2015)、光降解(Babi? et al., 2009)和生物降解(Girardi et al., 2011)等.与其它处理技术相比, 吸附处理具有方便、易操作等优点, 并已广泛应用于此类抗生素的去除(Wu et al., 2013;Ma et al., 2015).迄今, 各种FQs吸附剂已相继被开发, 主要有磁性碳(Mao et al., 2016)、活性炭(Fu et al., 2017)、生物炭(Ma et al., 2021)、蒙脱石(Wang et al., 2011)、壳聚糖(Wang et al., 2019)等, 但其中大多数存在不能完全与水分离、易团聚, 或对FQs的吸附能力有限等问题.在过去几年中, 碘氧化铋(BiOI)已被成功合成为3D花状结构(Zhang et al., 2008;Lei et al., 2010;Zhang et al., 2010)、纳米片(An et al., 2008;Chang et al., 2009)和纳米棒(Fang et al., 2009), 其吸附特性也引起了人们的广泛关注, 并被证明对甲基橙(MO)、罗丹明B(RhB)、亚甲基蓝(MB)和苯酚等有机染料废水处理具有良好的去除效果(Wang et al., 2014).Ren等(2012)研究发现, 采用化学沉淀法可快速制备比表面积大的花形空心结构的BiOI, 对20 mg·L-1罗丹明B的吸附去除率可达85%;Gondal等(2011)研究表明, BiOBr对罗丹明B也具有很好的吸附去除率;Hao等(2012)使用聚乙烯吡咯烷酮(PVP)作为结构导向剂在室温下成功合成了新型微球状BiOI, 且合成的BiOI对TC具有更高的吸附能力.
有****通过一种简便的水热方法合成了分级微晶纳米多孔Bi2WO6, 其3D球形多孔Bi2WO6微晶对MB表现出优异的吸附能力(Wang et al., 2016).由于过渡金属钨酸盐是一种重要的无机材料, 所以在许多领域都有潜在的应用(Saranya et al., 2012).与贵金属相比, 过渡金属Cu价格便宜且毒性更低.Gan等(2018)采用直接沉淀法并通过调节前驱体铜的量来调节纳米铜的尺寸, 成功制备了装饰有纳米铜的TiO2(Cu@TiO2), 并发现提高Cu@TiO2组分中铜含量可以提高抗生素的吸附效率;Lin(2015)合成了基于铜的HKUST-1 MOFs并用于吸附去除苯酚和对硝基苯酚, 处理效果良好是由于合成材料的高表面积及铜的不饱和金属离子位点;同样, Hao等(2019)将磁性CuZnFe2O4生物炭用于双酚A(BPA)和磺胺甲恶唑(SMX)处理, 其良好的处理效果也是由于含铜的金属结构.
基于此, 本文采用简单的化学沉淀法制备新型多金属氧酸盐复合材料(BiOI/CuWO4), 并将其应用于4种典型FQs(环丙沙星(CIP)、恩诺沙星(ENR)、诺氟沙星(NOR)、氧氟沙星(OFL))的吸附去除. 同时, 采用傅里叶红外光谱(FTIR)、X射线衍射仪(XRD)、扫描电镜(SEM)与Zeta电位等手段对复合材料进行表征, 并通过分析吸附等温线与动力学参数研究其吸附机理, 以期为吸附去除污水中FQs类抗生素提供参考.
2 材料与方法(Materials and methods)2.1 试剂与材料环丙沙星(CIP)、恩诺沙星(ENR)、诺氟沙星(NOR)、碘化钾(KI)、乙腈(色谱纯)、乙醇(色谱纯)、三乙胺购自阿拉丁试剂(上海)有限公司;氧氟沙星(OFL)购自麦克林生化科技(上海)有限公司;二水合钨酸钠(Na2WO4·2H2O)购自Bidepharm(上海)有限公司;腐殖酸(HA)购自源叶生物科技(上海)有限公司;其他试剂如五水合硫酸铜(CuSO4·5H2O)、硝酸铋(Bi (NO3) 3·5H2O)、H3PO4、NaCl、HCl和NaOH购自国药集团化学试剂有限公司(上海), 均为AR级.
2.2 实验仪器Agilent 1200高效液相色谱(Agilent公司, 美国);扫描电子显微镜(Hitachi, 日本);能谱仪(Hitachi, 日本);D8 Advance X射线衍射仪(XRD)(布鲁克公司, 美国);AVATAR 370傅里叶变换红外光谱仪(Thermo Nicolet, 美国);ZEN360电位分析仪(Malvern公司, 英国).
2.3 液相色谱分离条件4种典型FQs的分离测定在Agilent 1200 HPLC上进行, 其分离测定条件为: Eclipse XDB-C18柱(4.6 mm×250 mm, 5 μm), 温度为30 ℃, 流动相为磷酸-三乙胺溶液(pH=2.4)、甲醇和乙腈(79∶10∶11, V/V), 流速为1.0 mL·min-1, 进样体积为20 μL, 紫外检测波长为275 nm.
2.4 BiOI/CuWO4复合材料的制备准确称量0.2 mmol的Bi(NO3)3·5H2O和0.2 mmol KI, 将其一起溶解于30 mL水溶液中, 通过磁力搅拌器进行均匀混合, 并加热至70 ℃, 并在70 ℃保持2 h;然后, 准确称量1.0 mmol的Na2WO4·2H2O和1.0 mmol的CuSO4·5H2O, 添加至上述搅拌中的混合溶液中, 并在70 ℃保持48 h;反应结束后, 将其冷却至室温, 通过离心分离出褐色沉淀物, 分别用水和乙醇洗涤3次;最后将褐色粉末在70 ℃下干燥12 h, 得到BiOI/CuWO4.
2.5 氟喹诺酮类抗生素的吸附实验吸附实验在100 mL锥形瓶中进行, 每个锥形瓶中包含50 mL 4种氟喹诺酮类抗生素浓度均为10 mg·L-1的混合溶液和40 mg吸附剂, 该锥形瓶置于30 ℃、180 r·min-1的恒温振荡器中振荡.用稀HCl或稀NaOH溶液调节溶液的pH, 用配备有UV检测器的HPLC在275 nm下测定氟喹诺酮的浓度.吸附容量根据公式(1)计算.
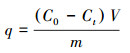 | (1) |
3 结果与讨论(Results and discussion)3.1 BiOI/CuWO4复合材料的表征3.1.1 SEM分析不同材料的SEM如图 1所示.可见BiOI呈片状晶体, BiOI微球尺寸为4~6 μm(图 1a), 而CuWO4具有均一的微球结构(图 1b).对于制备的BiOI/CuWO4复合材料(图 1c), 尺寸在20~40 nm之间的CuWO4颗粒均匀地分散在板状的BiOI表面上, 复合材料在吸附FQs(以CIP试验)后并无显著变化(图 1d).
图 1(Fig. 1)
 |
| 图 1 BiOI(a)、CuWO4(b)、BiOI/CuWO4(c)和BiOI/CuWO4-CIP(d)的扫描电镜图 Fig. 1SEM images of BiOI (a), CuWO4 (b), BiOI/CuWO4 (c) and BiOI/CuWO4-CIP (d) |
3.1.2 XRD分析复合材料的XRD图谱如图 2a所示.从图可以看出, 所有的衍射峰都对应BiOI及CuWO4的标准卡片, 证明两者共存, 且没有杂质存在.另外, 从图谱中还可以看出, 所有物质对应的衍射峰都比较宽, 峰强不高, 说明制备的材料粒径较小, 且结晶性相对较弱.
图 2(Fig. 2)
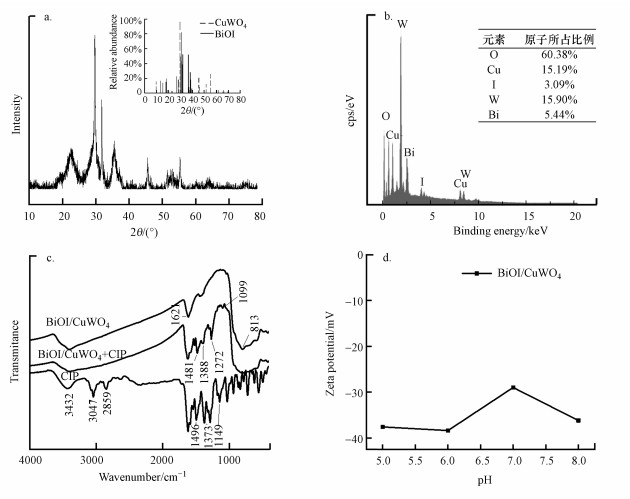 |
| 图 2 BiOI/CuWO4的X射线衍射图(a)、EDS能谱图(b)、傅里叶红外光谱图(c)和不同pH下的ζ电位(d) Fig. 2XRD spectra of the synthesized BiOI/CuWO4 (a), EDS spectra (b), FTIR spectra (c), and zeta potential of BiOI/CuWO4 suspension (d) |
3.1.3 EDS分析为了确定BiOI/CuWO4复合材料中各种元素的百分比组成, 对复合材料进行EDS能谱分析, 结果如图 2b所示.可以看出, BiOI/CuWO4复合材料中Bi、I、Cu、W和O元素的百分比分别为5.44%、3.09%、15.19%、15.90%、60.38%.
3.1.4 FTIR分析对于BiOI/CuWO4复合材料吸附FQs前后的变化, 本文以CIP为例进行FTIR表征, 结果如图 2c所示. CIP图谱中, 3432 cm-1处的吸收峰为—COOH中的O—H基团, 3047 cm-1和2859 cm-1处的吸收峰为—CH2—反对称伸缩振动与对称伸缩振动, 1621 cm-1处的吸收峰为芳香环中的CC键伸缩振动所致, 1496 cm-1处的吸收峰对应C—N键的伸缩振动, 1149 cm-1处的吸收峰对应C—C键的伸缩振动.而BiOI/CuWO4图谱中, 813 cm-1处的峰对应于WO42-的伸缩振动.吸附后的BiOI/CuWO4+CIP的光谱与未吸附的BiOI/CuWO4类似, 仅在1496 cm-1(C—N)、1149 cm-1(C—C键伸缩振动)、1275 cm-1(O—H单键弯曲振动)处有所不同, 表示样品中存在吸附的CIP, 即CIP成功地吸附在BiOI/CuWO4上.
3.1.5 Zeta电位分析图 2d结果显示, BiOI/CuWO4复合材料在pH=5~8时都是负电位, 具有强大的负电势.
3.2 BiOI/CuWO4复合材料吸附性能的影响因素3.2.1 BiOI/CuWO4材料组成比的影响考察了BiOI/CuWO4复合材料中CuWO4与BiOI物质的量比对FQs吸附性能的影响, 结果如图 3所示.结果显示, CuWO4/BiOI比为5∶1时对FQs的去除效果最佳.
图 3(Fig. 3)
 |
| 图 3 BiOI/CuWO4复合材料中物质的量比对吸附的影响 Fig. 3Effect of BiOI/CuWO4 molar ratio on its absorption behavior |
3.2.2 吸附剂量的影响通过将不同剂量的吸附剂加入到50 mL FQs(10 mg·L-1)溶液中, 来考察吸附剂量对吸附效果的影响.由图 4a可知, 当吸附剂的剂量从0.04 g·L-1增加到0.24 g·L-1时, NOR的吸附容量从107.5 mg·g-1降低到41.7 mg·g-1 (图 4a).这可能是因为少量吸附剂的所有吸附位点都暴露在外, 所以吸附剂表面的吸附更快地达到饱和, 从而导致更高的吸附容量(Li et al., 2017).相反, 较高剂量的吸附剂意味着在吸附过程中存在大量未充分利用的吸附位点, 从而导致较低的吸附容量.比较吸附剂容量和去除效率, 选择0.2 g·L-1的吸附剂量用于后续实验.
图 4(Fig. 4)
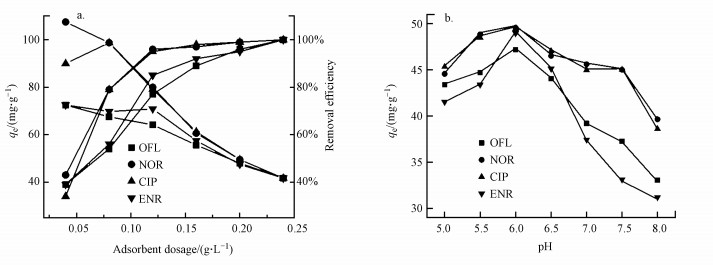 |
| 图 4 吸附剂量(a)与pH(b)对BiOI/CuWO4复合材料吸附效果的影响 Fig. 4Effects of adsorbent dosage (a) and pH (b) on the adsorption of FQs by BiOI/CuWO4 composite |
3.2.3 pH的影响溶液的pH值会影响水中污染物分子的分配系数, 并对吸附剂的电性能产生一定的影响.因此, 进行分批吸附实验以评估pH对吸附行为的影响.如图 4b所示, pH在5.0~6.0之间时, BiOI/CuWO4吸附FQs的能力增强, 而pH在6.0~8.0范围内, 吸附能力下降, 在pH=6.0时吸附量达到最大值.以CIP为例进行分析, 当溶液pH在5.0~8.0时, 吸附剂对CIP的吸附能力发生了很大变化, 平衡吸附量从45 mg·g-1降低到37.6 mg·g-1, 在pH=6.0时达到49.5 mg·g-1的最大吸附容量.在水溶液中, CIP具有氨基(—NH2)和羧基(—COOH)两性基团, 可以形成3种形态物质, 即CIP +(pH < 5.9)、CIP-+(5.9 < pH < 8.9)和CIP-(pH>8.9).因此, 在pH=6.0时, 溶液中存在大量的阳离子和两性离子, 由于静电作用和疏水作用使得BiOI/CuWO4对CIP的吸附更有利.相反, 当pH<6.0时, 由于两性离子数量的减少, 所以疏水作用减弱.同时, 当pH>6.0时, CIP-增加会增强CIP与BiOI/CuWO4吸附剂之间的静电排斥力, 从而导致CIP吸附能力下降.因此, 静电作用和疏水作用在BiOI/CuWO4对CIP的吸附中起重要作用.
3.2.4 腐殖酸的影响图 5a显示了腐殖酸对FQs吸附性能的影响.随着腐殖酸浓度从5 mg·L-1增加到50 mg·L-1, FQs在BiOI/CuWO4上的吸附量几乎无增加, 腐殖酸浓度对FQs的吸附能力几乎没有影响.
图 5(Fig. 5)
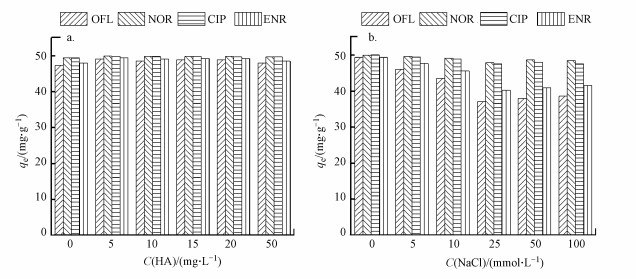 |
| 图 5 腐殖酸(a)与氯化钠(b)对BiOI/CuWO4复合材料吸附性能的影响 Fig. 5Effects of humic acid (a) and NaCl (b) on the adsorption of FQs by BiOI/CuWO4 composite |
3.2.5 盐度的影响在pH=6.0条件下, 考察了NaCl浓度对BiOI/CuWO4复合材料吸附FQs的影响.如图 5b显示, 随着NaCl浓度增加到100 mmol·L-1, FQs在BiOI/CuWO4上的吸附量略有降低.目前的研究已经证实了无机阳离子, 如Na+的抑制作用(Xu et al., 2019), 主要原因为额外添加的离子可能改变了BiOI/CuWO4的Zeta电位, 从而导致吸附剂表面负电荷降低, 静电引力变弱.此外, 较高的离子强度会导致BiOI/CuWO4聚集, 从而抑制吸附.
3.3 BiOI/CuWO4复合材料的吸附机理3.3.1 吸附动力学本文考察了不同吸附时间与吸附容量之间的变化关系, 并研究了NOR、CIP、ENR和OFL在BiOI/CuWO4复合材料上的吸附动力学.从图 6中可以看出, 在1.5 h时就可达到平衡吸附容量90%的水平, 在3 h时可达到吸附平衡.
图 6(Fig. 6)
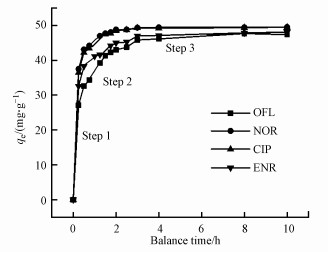 |
| 图 6 BiOI/CuWO4对FQs的吸附动力学 Fig. 6Adsorption kinetics of BiOI/CuWO4 for FQs |
采用准一级吸附动力学方程(式(2))和准二级吸附动力学方程(式(3))拟合了BiOI/CuWO4对FQs的吸附动力学, 结果见图 7, 相关动力学参数和可决系数(R2)见表 1.
图 7(Fig. 7)
 |
| 图 7 BiOI/CuWO4吸附FQs的动力学模型(a.准一级动力学模型, b.准二级动力学模型) Fig. 7The kinetic models for adsorption of FQs on BiOI/CuWO4 (a.pseudo-first-order kinetic model, b. pseudo-second-order kinetic model) |
表 1(Table 1)
| 表 1 准一级和准二级模型的动力学参数 Table 1 Kinetic parameters for the pseudo-first-order and pseudo-second order models | |||||||||||||||||||||||||||||||||||||||||||
表 1 准一级和准二级模型的动力学参数 Table 1 Kinetic parameters for the pseudo-first-order and pseudo-second order models
| |||||||||||||||||||||||||||||||||||||||||||
 | (2) |
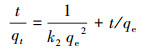 | (3) |
 | (4) |
结果表明, 准二级吸附动力学模型的拟合效果(R2=0.999)比准一级吸附动力学模型(R2=0.9189~0.9737)更佳.从表 1可以看出, 对于NOR, 两个吸附模型拟合的吸附容量分别为18.7 mg·g-1和50.0 mg·g-1, 而实验获得的吸附容量为49.6 mg·g-1.根据准二级模型拟合结果计算的qe值与实验数据吻合良好, 表明NOR、CIP、ENR和OFL在复合材料上的吸附为化学吸附.因此, 准二级吸附动力学模型更适合描述FQs的吸附行为.
表 2(Table 2)
| 表 2 粒子内扩散模型参数 Table 2 Parameters of intraparticle diffusion model | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
表 2 粒子内扩散模型参数 Table 2 Parameters of intraparticle diffusion model
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
研究表明(Kera et al., 2017), 被吸附物从液相转移到固体吸附剂中一般需要3个过程: 膜扩散阶段、内扩散阶段、平衡阶段. 粒子内扩散模型(式(4))拟合的吸附过程如图 6所示, 图中步骤1、2和3分别与外表面扩散中吸附剂内的颗粒内扩散和平衡阶段有关.步骤1的斜率高于步骤2和步骤3, 步骤1阶段速率最快, FQs快速进入吸附剂内部后, 受到更大的阻力, 使得吸附速率变慢, 表明颗粒内扩散不是唯一的限速步骤.
3.3.2 吸附等温线为了进一步研究吸附剂与FQs之间的相互作用, 在pH=6.0和25 ℃条件下进行了等温吸附实验, 结果如图 8所示.对于NOR, 随着NOR浓度从10 mg·L-1增加到80 mg·L-1, 其吸附容量从49.5 mg·g-1增加到177.1 mg·g-1.这可能是因为较高的初始浓度意味着较高的浓度梯度, 从而导致较高的驱动力(Calagui et al.2014), 使得NOR分子快速转移至复合材料的表面.
图 8(Fig. 8)
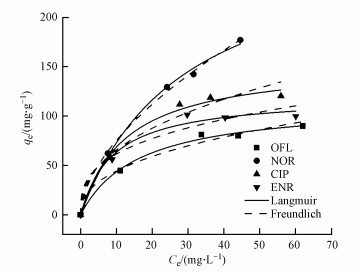 |
| 图 8 FQs起始浓度对吸附效果的影响 Fig. 8Effect of the initial FQs concentrations on the adsorption performance |
通常不采用简单的线性分布模型拟合结果来解释疏水性有机物, 而是采用非线性的Langmuir(式(5))和Freundlich(式(6))吸附等温线模型, 其中, Langmuir等温线假定吸附剂的吸附表面均匀, 其中所有吸附位点都相同且在能量上相等, 而Freundlich等温线描述的是非均相吸附, 吸附剂的吸附位点是多种多样的.Langmuir和Freundlich等温线模型拟合结果分别如图 9a和9b所示, 吸附等温线模型参数如表 3所示.通过对比R2可以发现, 复合材料对CIP、ENR和OFL的吸附更符合Langmuir模型, 表明吸附过程是均匀表面的单分子层吸附.对于NOR, 复合材料对其吸附更符合Freundlich模型, 说明这是一种多层吸附.同时根据Langmuir模型可以得出, NOR、CIP、ENR和OFL在BiOI/CuWO4上的最大吸附容量分别为277.8、149.3、113.6、112.4 mg·g-1, 吸附能力顺序为NOR>CIP>ENR>OFL.此外, BiOI/CuWO4对FQs的吸附能力与其他文献报道的吸附剂(表 4)相比, BiOI/CuWO4对FQs具有更好的吸附能力与潜在的应用前景.
图 9(Fig. 9)
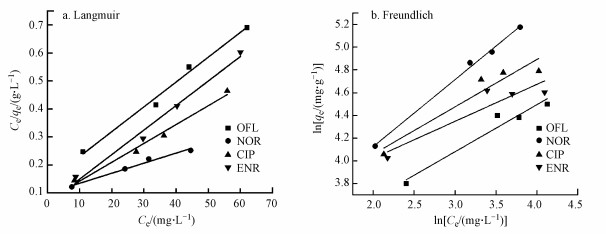 |
| 图 9 Langmuir模型(a)和Freundlich模型(b)拟合结果 Fig. 9Adsorption isotherms fitted by Langmuir (a) and Freundlich (b) models |
表 3(Table 3)
| 表 3 Langmuir和Freundlich模型的参数 Table 3 Parameters for the Langmuir and Freundlich models | |||||||||||||||||||||||||||||||||||||||||||
表 3 Langmuir和Freundlich模型的参数 Table 3 Parameters for the Langmuir and Freundlich models
| |||||||||||||||||||||||||||||||||||||||||||
表 4(Table 4)
| 表 4 BiOI/CuWO4与其他材料的吸附效果对比 Table 4 Comparison of the adsorption caability between BiOI/CuWO4 with other materials | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
表 4 BiOI/CuWO4与其他材料的吸附效果对比 Table 4 Comparison of the adsorption caability between BiOI/CuWO4 with other materials
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
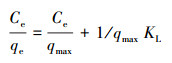 | (5) |
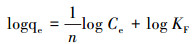 | (6) |
3.4 BiOI/CuWO4重复利用性在实际应用中, 基于成本考虑, 通常要求吸附剂是可回收的.吸附结束时, 用0.22 μm的滤膜分离吸附剂, 用25 mL甲醇(含80%, 0.1 mol·L-1盐酸)作为再生剂, 超声清洗10 min, 操作重复5次;然后用水洗涤2次, 并将获得的固体在70 ℃下干燥, 以用于下一次吸附.由图 10可知, 在相同条件下, 重复吸附3次后, BiOI/CuWO4对NOR、CIP、ENR和OFL的吸附去除率虽有所下降, 但仍保持在75%以上.造成这一现象的原因可能是每次洗脱仍有少量的FQs没有被洗脱下来导致空余吸附位点减少.
图 10(Fig. 10)
 |
| 图 10 BiOI/CuWO4的重复利用性 Fig. 10The reusability of BiOI/CuWO4 composite |
4 结论(Conclusions)1) BiOI/CuWO4对FQs的吸附遵循准二级吸附动力学, 但对于吸附等温线模型, CIP、ENR、OFL 3种氟喹诺酮类抗生素遵循Langmiur模型, 而NOR更符合Freundlich模型, 表明其在吸附剂表面不像其它3种氟喹诺酮类抗生素为单层吸附, 而是具有更复杂的吸附机理, 从而使其具有更高的吸附容量.
2) 静电作用和疏水作用在BiOI/CuWO4吸附剂对FQs的吸附中起重要作用.
3) BiOI/CuWO4对FQs的吸附顺序为NOR>CIP>ENR>OFL, 脱附再生后的BiOI/CuWO4对FQs仍具有稳定的吸附性能, 可多次循环使用.
参考文献
| An H Z, Du Y, Wang T M, et al. 2008. Photocatalytic properties of BiOX(X=Cl, Br, and I)[J]. Rare Metals, 27(3): 243-250. DOI:10.1016/S1001-0521(08)60123-0 |
| Andrew Lin K Y, Hsieh Y T. 2015. Copper-based metal organic framework (MOF), HKUST-1, as an efficient adsorbent to remove p-nitrophenol from water[J]. Journal of the Taiwan Institute of Chemical Engineers, 50: 223-228. DOI:10.1016/j.jtice.2014.12.008 |
| Antonelli R, Malpass G R P, Silva M G C D, et al. 2020. Adsorption of ciprofloxacin onto thermally modified bentonite clay: experimental design, characterization, and adsorbent regenera-tion[J]. Journal of Environmental Chemical Engineering, 8(6): 104553. DOI:10.1016/j.jece.2020.104553 |
| Antonelli R, Martins F R, Malpass G R P, et al. 2020. Ofloxacin adsorption by calcined Verde-lodo bentonite clay: Batch and fixed bed system evaluation[J]. Journal of Molecular Liquids, 315: 113718. DOI:10.1016/j.molliq.2020.113718 |
| Babi? S, Peri?a M, ?kori? I. 2009. Photolytic degradation of norfloxacin, enrofloxacin and ciprofloxacin in various aqueous media[J]. Chemosphere, 91(11): 1635-1642. |
| Bu Q, Wang B, Huang J, et al. 2013. Pharmaceuticals and personal care products in the aquatic environment in China: a review[J]. Journal of Hazardous Materials, 262(15): 189-211. |
| Calagui M J C, Senoro D B, Kan C C, et al. 2014. Adsorption of indium(III) ions from aqueous solution usingchitosan-coated bentonite beads[J]. Journal of Hazardous Materials, 277: 120-126. DOI:10.1016/j.jhazmat.2014.04.043 |
| Chang X F, Huang J, Tan Q Y, et al. 2009. Photocatalytic degradation of PCP-Na over BiOI nanosheets under simulated sunlight irradiation[J]. Catalysis Communications, 10(15): 1957-1961. DOI:10.1016/j.catcom.2009.06.023 |
| Chen H, Gao B, Li H. 2015. Removal of sulfamethoxazole and ciprofloxacin from aqueous solutions by graphene oxide[J]. Journal of Hazardous Materials, 282: 201-207. DOI:10.1016/j.jhazmat.2014.03.063 |
| Doorslaer X V, Dewulf J, Langenhove H V, et al. 2014. Fluoroquinolone antibiotics: an emerging class of environmental micropollutants[J]. Science of the Total Environment, 500-501: 250-269. DOI:10.1016/j.scitotenv.2014.08.075 |
| Fang F, Chen L, Wu L M. 2009. Syntheses, morphologies and properties of BiOI nanolamellas and BiSI nanorods[J]. Chinese Journal of Structural Chemistry, 28(11): 1399-1406. |
| Fang X, Wu S, Wu Y, et al. 2020. High-efficiency adsorption of norfloxacin using octahedral UIO-66-NH2 nanomaterials: Dynamics, thermodynamics, and mechanisms[J]. Applied Surface Science, 518: 146226. DOI:10.1016/j.apsusc.2020.146226 |
| Fu H, Li X, Wang J, et al. 2017. Activated carbon adsorption of quinolone antibiotics in water: Performance, mechanism, and modeling[J]. Journal of Environmental Sciences, 56(6): 145-152. |
| Gan Y, Zhang M, Xiong J, et al. 2018. Impact of Cu particles on adsorption and photocatalytic capability of mesoporous Cu@TiO2 hybrid towards ciprofloxacin antibiotic removal[J]. Journal of the Taiwan Institute of Chemical Engineers, 96: 229-242. |
| Girardi C, Greve J, Lamsh?ft M, et al. 2011. Biodegradation of ciprofloxacin in water and soil and its effects on the microbial communities[J]. Journal of Hazardous Materials, 198: 22-30. DOI:10.1016/j.jhazmat.2011.10.004 |
| Gondal M A, Chang X, Ali M A, et al. 2011. Adsorbtion and degradation performance of rhodamine B over BiOBr under monochromatic 532 nm pulsed laser exposure[J]. Applied Catalysis A: General, 397(1/2): 192-200. |
| Hao R, Xiao X, Zuo X, et al. 2012. Efficient adsorption and visible-light photocatalytic degradation of tetracycline hydrochloride using mesoporous BiOI microspheres[J]. Journal of Hazardous Materials, 209-210: 137-145. DOI:10.1016/j.jhazmat.2012.01.006 |
| Heo J Y, Yoon Y M, Lee G Y, et al. 2019. Enhanced adsorption of bisphenol A and sulfamethoxazole by a novel magnetic CuZnFe2O4-biochar composite[J]. Bioresource Technology, 281: 179-187. DOI:10.1016/j.biortech.2019.02.091 |
| Kera N H, Bhaumik M, Pillay K, et al. 2017. Selective removal of toxic Cr(VI) from aqueous solution by adsorption combined with reduction at a magnetic nanocomposite surface[J]. Journal of Colloid and Interface Science, 503: 214-228. DOI:10.1016/j.jcis.2017.05.018 |
| Lei Y, Wang G, Song S, et al. 2010. Room temperature, template-free synthesis of BiOI hierarchical structures: Visible-light photocatalytic and electrochemical hydrogen storage properties[J]. Dalton Transactions, 39(13): 3273-3278. DOI:10.1039/b922126c |
| Li S Q, Zhang X D, Huang Y M. 2017. Zeoliticimidazolate framework-8 derived nanoporous carbon as an effective and recyclable adsorbent for removal of ciprofloxacin antibiotics from water[J]. Journal of Hazardous Materials, 321: 711-719. DOI:10.1016/j.jhazmat.2016.09.065 |
| Li H, Wu W, Hao X, et al. 2018. Removal of ciprofloxacin from aqueous solutions by ionic surfactant-modified carbon nanotubes[J]. Environmental Pollution, 243: 206-217. DOI:10.1016/j.envpol.2018.08.059 |
| Li J F, Chen Y, Wang Z, et al. 2018. Self-templating synthesis of hollow copper tungstate spheres as adsorbents for dye removal[J]. Journal of Colloid and Interface Science, 526: 459-469. DOI:10.1016/j.jcis.2018.05.023 |
| Liu J L, Wong M H. 2013. Pharmaceuticals and personal care products (PPCPs): a review on environmental contamination in China[J]. Environment International, 59: 208-224. DOI:10.1016/j.envint.2013.06.012 |
| Liu X Y, Liu M Y, Zhang L. 2018. Co-adsorption and sequential adsorption of the co-existence four heavy metal ions and three fluoroquinolones on the functionalized ferromagnetic 3D NiFe2O4 porous hollow microsphere[J]. Journal of Colloid & Interface Science, 511: 135-144. |
| Ma F Q, Yao J W, Zhang Y F, et al. 2018. Oxygen vacancy promoting adsorption property of BiOI microspheres modified with SDS[J]. Chinese Chemical Letters, 29: 1689-1691. DOI:10.1016/j.cclet.2017.12.016 |
| Ma J, Yang M, Yu F, et al. 2015. Water-enhanced removal of ciprofloxacin from water by porous graphene hydrogel[J]. Scientific Reports, 5: 13578. DOI:10.1038/srep13578 |
| Ma Y, Li M, Li P, et al. 2021. Hydrothermal synthesis of magnetic sludge biochar for tetracycline and ciprofloxacin adsorptive removal[J]. Bioresource Technology, 319: 124199. DOI:10.1016/j.biortech.2020.124199 |
| Mahmoud M E, Saad S R, El-Ghanam A M, et al. 2020. Developed magnetic Fe3O4-MoO3-AC nanocomposite for effective removal of ciprofloxacin from water[J]. Materials Chemistry and Physics, 257: 123454. |
| Mao H, Wang S, Lin J Y, et al. 2016. Modification of a magnetic carbon composite for ciprofloxacin adsorption[J]. Journal of Environmental Sciences, 49: 179-188. DOI:10.1016/j.jes.2016.05.048 |
| Meersche T V D, Pamel E V, Poucke C V, et al. 2016. Development, validation and application of an ultra high performance liquid chromatographic-tandem mass spectrometric method for the simultaneous detection and quantification of five different classes of veterinary antibiotics in swine manure[J]. Journal of Chromatography A, 1429: 248-257. DOI:10.1016/j.chroma.2015.12.046 |
| Muhammad Z A, Sun X F, Liu J, et al. 2018. Enhancement of ciprofloxacin sorption on chitosan/biochar hydrogel beads[J]. Science of the Total Environment, 639: 560-569. DOI:10.1016/j.scitotenv.2018.05.129 |
| Ren K X, Zhang K, Liu J, et al. 2012. Controllable synthesis of hollow/flower-like BiOI microspheres and highly efficient adsorption and photocatalytic activity[J]. Cryst Eng Comm, 14(13): 4384-4390. DOI:10.1039/c2ce25087j |
| Saranya S, Senthilkumar S T, Sankar K V, et al. 2012. Synthesis of MnWO4 nanorods and its electrical and electrochemical properties[J]. Electroceramics, 28(4): 220-225. DOI:10.1007/s10832-012-9714-7 |
| Tang H M, Li W Y, Jiang H S, et al. 2019. ZIF-8-derived hollow carbon for efficient adsorption of antibiotics[J]. Nanomaterials, 9(1): 117. DOI:10.3390/nano9010117 |
| Wang B, Xu X Y, Tang H, et al. 2020. Highly efficient adsorption of three antibiotics from aqueous solutions using glucose-based mesoporous carbon[J]. Applied Surface Science, 528: 147048. DOI:10.1016/j.apsusc.2020.147048 |
| Wang D, Zhang J, Guo L, et al. 2016. Synthesis of nano-porous Bi2WO6 hierarchical microcrystal with selective adsorption for cationic dyes[J]. Materials Research Bulletin, 83: 387-395. DOI:10.1016/j.materresbull.2016.06.029 |
| Wang X, Yang S, Li H, et al. 2014. High adsorption and efficient visible-light-photodegradation for cationic Rhodamine B with microspheric BiOI photocatalyst[J]. RSC Advances, 4(80): 42530-42537. DOI:10.1039/C4RA05506C |
| Hong W, Liu Y N, Dong C X, et al. 2015. Adsorption of levofloxacin onto an iron-pillared montmorillonite (clay mineral): Kinetics, equilibrium and mechanism[J]. Applied Clay Science, 117: 301-307. |
| Wang C J, Li Z, Jiang W T. 2011. Adsorption of ciprofloxacin on 2:1 dioctahedral clay minerals[J]. Applied Clay Science, 53(4): 723-728. DOI:10.1016/j.clay.2011.06.014 |
| Wang N, Xiao W, Niu B, et al. 2019. Highly efficient adsorption of fluoroquinolone antibiotics using chitosan derived granular hydrogel with 3D structure[J]. Journal of Molecular Liquids, 281: 307-314. DOI:10.1016/j.molliq.2019.02.061 |
| Witte B D, Dewulf J, Demeestere K, et al. 2009. Ozonation and advanced oxidation by the peroxone process of ciprofloxacin in water[J]. Journal of Hazardous Materials, 161(2/3): 701-708. |
| Wu S, Zhao X, Li Y, et al. 2013. Adsorption of ciprofloxacin onto biocomposite fibers of graphene oxide/calcium alginate[J]. Chemical Engineering Journal, 230: 389-395. DOI:10.1016/j.cej.2013.06.072 |
| Xu X, Liu Y, Wang T, et al. 2019. Co-adsorption of ciprofloxacin and Cu(II) onto titanate nanotubes: Speciation variation and metal-organic complexation[J]. Journal of Molecular Liquids, 292: 111375. DOI:10.1016/j.molliq.2019.111375 |
| Yao L, Li Y, Li Z, et al. 2020. Prevalence of fluoroquinolone, macrolide and sulfonamide-related resistance genes in landfills from East China, mainly driven by MGEs[J]. Ecotoxicology and Environmental Safety, 190: 110131. DOI:10.1016/j.ecoenv.2019.110131 |
| Yue Z, Zhang J, Zhou Z, et al. 2021. Pollution characteristics of livestock faeces and the key driver of the spread of antibiotic resistance genes[J]. Journal of Hazardous Materials, 409: 124957. DOI:10.1016/j.jhazmat.2020.124957 |
| Zhang X X, De W. 2010. Facile synthesis of nanostructured BiOI microspheres with high visible light-induced photocatalytic activity[J]. Journal of Materials Chemistry, 20(28): 5866-5870. DOI:10.1039/c0jm00333f |
| Zhang X, Ai Z, Jia F, et al. 2008. Generalized one-pot synthesis, characterization, and photocatalytic activity of hierarchical BiOX (X=Cl, Br, I) Nanoplate Microspheres[J]. Journal of Physical Chemistry C, 112(3): 747-753. DOI:10.1021/jp077471t |
