 , 梁锦芝1, 肖开棒1, 李富华2, 江学顶2
, 梁锦芝1, 肖开棒1, 李富华2, 江学顶2
 , 许伟城2
, 许伟城2
 , 王海龙2, 陈忻2, 胡芸3, 梁小明4
, 王海龙2, 陈忻2, 胡芸3, 梁小明41. 佛山科学技术学院交通与土木建筑学院, 佛山 528000;
2. 佛山科学技术学院环境与化学工程学院, 佛山 528000;
3. 华南理工大学环境与能源学院, 广州 510006;
4. 生态环境部华南环境科学研究所, 国家环境保护城市生态环境模拟与保护重点实验室, 广州 510655
收稿日期: 2020-08-24; 修回日期: 2020-09-24; 录用日期: 2020-09-24
基金项目: 广东省自然科学基金(No.2018A030313734);广州市科技计划项目(No.201804020026)
作者简介: 赖树锋(1995-), 男, E-mail: shufenglai@163.com
通讯作者(责任作者): 江学顶, E-mail: jiangxueding@fosu.edu.cn
许伟城, E-mail: weichengxu@fosu.edu.cn
摘要:采用煅烧法和光还原法制备出具有高活性的Ag/g-C3N4催化剂,并将其应用于可见光下活化过一硫酸盐(PMS)降解罗丹明B(RhB)废水.系统研究了实际因素RhB浓度、催化剂投加量、PMS剂量、pH值和可溶性无机阴离子对RhB降解效果的影响.结果表明,RhB的降解率随着催化剂投加量、PMS浓度的增加而增大,随着初始RhB浓度的增加而减小.弱酸性条件有利于反应活化PMS降解RhB,而中性或碱性条件都会减缓催化反应的进行.Ag/g-C3N4-2/Vis/PMS催化体系30 min内对RhB的去除率最高可达93.2%,分别是Ag/g-C3N4/Vis和单独PMS催化体系的4.0和3.7倍.体系催化活性的提高归因于Ag的表面等离子共振效应及基于硫酸根自由基的高级氧化技术与光催化技术的协同作用.不同阴离子对催化反应的影响不同,溶液中的Cl-会对反应产生轻微的抑制作用,而H2PO4-和HCO3-的出现大大抑制了催化性能.催化剂具有良好的稳定性,5次循环后仍能在30 min之内降解77.4%的RhB.此外,捕获实验和ESR测试结果表明,Ag/g-C3N4-2/Vis/PMS催化体系中存在·O2-、h+、1O2、SO4-·和·OH活性物种,并协同降解RhB污染物.
关键词:Ag/g-C3N4过一硫酸盐光催化罗丹明B协同作用
Visible light assisted peroxymonosulfate activation on Ag modified graphite phase carbon nitride (g-C3N4) for Rhodamine B degradation
LAI Shufeng1
 , LIANG Jinzhi1, XIAO Kaibang1, LI Fuhua2, JIANG Xueding2
, LIANG Jinzhi1, XIAO Kaibang1, LI Fuhua2, JIANG Xueding2
 , XU Weicheng2
, XU Weicheng2
 , WANG Hailong2, CHEN Xin2, HU Yun3, LIANG Xiaoming4
, WANG Hailong2, CHEN Xin2, HU Yun3, LIANG Xiaoming41. School of Transportation and Civil Engineering, Foshan University, Foshan 528000;
2. School of Environmental and Chemical Engineering, Foshan University, Foshan 528000;
3. School of Environment and Energy, South China University of Technology, Guangzhou 510006;
4. State Environmental Protection Key Laboratory of Urban Ecological Environment Simulation and Protection, South China Institute of Environmental Science, Ministry of Ecology and Environment, Guangzhou 510655
Received 24 August 2020; received in revised from 24 September 2020; accepted 24 September 2020
Abstract: In this study, Ag/g-C3N4 catalysts (Ag/g-C3N4) with high activity were prepared by calcination and photoreduction process. The as-prepared catalysts were studied for the degradation of organic pollutant Rhodamine B (RhB), and were also used for peroxymonosulfate (PMS) activation in the presence and absence of visible light. The effects of RhB concentration, catalyst dosage, PMS concentration, pH value and soluble inorganic anions on RhB degradation were systematically investigated. The results showed that the degradation rate of RhB was positively correlated with catalyst dosage and PMS concentration, but negatively correlated with initial RhB concentration. The optimal pH for the reaction was achieved and weak acidic conditions could promote RhB degradation, while the neutral or basic conditions played negative effects on the reaction. The removal rate of RhB in Ag/g-C3N4-2/Vis/PMS catalyst system was 93.2% within 30 min, which was 4.0 and 3.7 times higher than that of Ag/g-C3N4/Vis and independent PMS catalysis system. The improvement of catalytic activity can be attributed to the surface plasmon resonance effect of Ag and the synergistic effect of advanced oxidation technology based on the sulfate radical and photocatalytic technology. Anions influence experiments showed that different anions played different effects on catalytic reaction. The influence of Cl- on the removal of RhB was insignificant, while H2PO4- and HCO3- seriously inhibited the degradation. Cycle experiments showed that Ag/g-C3N4-2 catalyst has good stability and 77.4% RhB can be removed within 30 min after 5 cycles. In addition, the capture experiments and ESR tests showed that different active species including ·O2-, h+, 1O2, SO4-· and ·OH can be found in Ag/g-C3N4/Vis/PMS catalyst system, and synergistically degraded RhB pollutants.
Keywords: Ag/g-C3N4peroxymonosulfatephotocatalysisRhodamine Bsynergistic effect
1 引言(Introduction)随着全球工业化的迅速发展, 涂料、纺织等行业产生的印染废水的环境污染问题日趋严重.这类有机污染物具有毒性大、色度高等特点, 会对水生态环境造成污染和破坏(Vellaichamy et al., 2018; Rovira et al., 2019).因此, 迫切需求一种高效、快速、安全的方法去除水体中的染料污染物.先前的研究报道证实一些传统的水处理方法能够有效地去除水中的染料污染物, 如吸附、生物处理和膜过滤等(Vandevivere et al., 1998; Pearce et al., 2003; Katheresan et al., 2018; Luo et al., 2019).然而, 这些技术普遍存在效率不高且易产生二次污染物等缺点(Han et al., 2009).近年来, 基于硫酸根自由基(SO4-·)的可见光催化活化过一硫酸盐(PMS)技术作为一种快速、有效和绿色的方法已被广泛用于消除水体环境中的有机污染物.在该催化体系中, PMS可以被活化形成具有强氧化能力的SO4-·.与羟基自由基(·OH, 氧化还原点位1.8~2.7 V, 半衰期20 ns)相比, SO4-·具有更高的氧化还原电位(2.5~3.1 V)和更长的半衰期(30~40 μs), 这更有利于去除水中的有机污染物(Liu et al., 2017; 胡优优等, 2019).在活化PMS的过程中还可能会产生一种具有强氧化性的非自由基单线态氧(1O2), 1O2也可以有效地降解有机污染物(Cheng et al., 2017).另外, 光催化材料在可见光照射下能够产生空穴(h+)和羟基自由基(·OH)等活性物种, 能与SO4-·相互协同降解水中的有机污染物.然而, 这种技术的处理效果很大程度上取决于所制备光催化材料的催化性能.因此, 构建合适、绿色、高效的光催化材料是十分重要的.
石墨相氮化碳(g-C3N4)作为一种新型的非金属光催化材料, 由于具有原料成本低、物理化学性质稳定、无毒性和适当的带隙(~2.70 eV)等特点而备受关注(Wang et al., 2018; 李航等, 2020; 赖树锋等, 2020).但该催化剂也存在可见光利用率低和光生载流子复合效率高等缺点, 从而严重限制了其在实际中的应用(Vidyasagar et al., 2018; 彭小明等, 2019).例如, Sun等(2012)利用g-C3N4-D/UV在100 min内仅能降解约40%的对十溴二苯醚.Tao等(2015)合成的g-C3N4在30 min可见光照射下活化PMS能降解86%的酸性橙7有机污染物.尽管在PMS的辅助下催化性能得到提高, 但由于g-C3N4低的光利用率和高的光生电子-空穴对复合率, 导致PMS的活化受到限制.为了进一步提高g-C3N4的光催化性能, 研究人员对其进行多种改性, 包括元素掺杂、异质结构构建、贵金属沉积等(Li et al., 2018; Song et al., 2018; Wang et al., 2018).在这些改性策略中, 银(Ag)的沉积由于表面等离子体共振(SPR)效应受到了广泛关注.沉积在g-C3N4上的Ag诱导的SPR效应有助于拓宽可见光的吸收范围, 增强光生载流子的分离效率, 提高光催化性能(Song et al., 2018).Min等(2016)通过前驱体的配位驱动组装与煅烧过程成功地构建出Ag/C3N4催化剂, 在可见光照射60 min内能够降解85%的RhB, 其光催化性能比单一g-C3N4更优越.Nagajyothi等(2017)采用简便的绿色化学方法合成Ag/g-C3N4复合光催化材料, 在紫外光照射下, 在100 min内能去除80%的孔雀绿染料, 其光催化活性是g-C3N4的2.5倍.尽管如此, Ag/g-C3N4复合材料光催化降解染料废水的能力还有待进一步提高.通过光催化技术结合PMS的优点, 可以进一步提高光催化材料的催化降解性能, 从而更快速、更高效地去除水中有机染料污染物.而目前有关Ag/g-C3N4/Vis/PMS体系催化降解RhB的研究及实际环境因素(各种无机阴离子、初始溶液PH值、PMS的浓度等)对该系统去除RhB效果的影响仍有待深入探究.
因此, 本文采用煅烧-光还原法制备Ag改性g-C3N4(Ag/g-C3N4)光催化剂, 采用X射线衍射光谱(XRD)、紫外-可见光谱(UV-vis DRS)、光致发光光谱(PL)等技术手段对光催化剂进行表征, 考察Ag的引入对g-C3N4结构和光学性质的影响.同时, 在可见光照射下, 通过催化活化PMS降解水中RhB污染物来考察所制备材料的光催化性能, 并研究初始RhB浓度、催化剂投加量、PMS投加量、初始pH值及常见无机阴离子对RhB降解效果的影响.另外, 通过捕获实验和电子自旋顺磁共振测试(ESR)鉴定该反应体系中的活性物种, 并详细地探讨Ag/g-C3N4/Vis/PMS催化体系降解RhB的反应机理.
2 材料与方法(Materials and methods)2.1 实验材料三聚氰胺和RhB购自上海麦克林生化科技有限公司, 过硫酸氢钾(2KHSO5·KHSO4·K2SO4, PMS)、对苯醌(BQ)、乙二胺四乙酸二钠(EDTA-2Na)、糠醇(FFA)、叔丁醇(TBA)、乙醇(EtOH)、氯化钠(NaCl)、碳酸钠(Na2CO3)、磷酸二氢钠(NaH2PO4)均采购自阿拉丁试剂有限公司, 硝酸银(AgNO3)和盐酸(HCl)购自国药集团化学试剂有限公司, 以上试剂均为分析纯, 可直接使用.
2.2 光催化材料的制备将4 g三聚氰胺放入加盖氧化铝坩埚并置于马弗炉中, 以10 ℃·min-1的升温速率升温至550 ℃并持续煅烧4 h.待冷却至室温后取出, 将所得产物研磨成粉末, 并标记为g-C3N4.将0.5 g g-C3N4粉末加入到30 mL去离子水中并超声30 min形成浑浊溶液, 在混浊溶液中加入一定量的AgNO3, 在黑暗条件下搅拌10 min, 接着在300 W氙灯(λ>420 nm)照射下连续搅拌2 h.最后, 将所得悬浮液离心并分别用无水乙醇和去离子水洗涤3次, 在60 ℃下干燥得到一系列Ag/g-C3N4光催化剂.通过改变AgNO3投加量, 制备出质量分数分别为1%、2%和3%的Ag/g-C3N4复合材料, 并分别标记为Ag/g-C3N4-1、Ag/g-C3N4-2和Ag/g-C3N4-3.g-C3N4在不添加AgNO3的情况下采用相同的合成方法制备出纯g-C3N4.
2.3 催化剂的表征样品的物相结构通过X-射线衍射仪(XRD, Rigaku D/max-2500型)进行表征分析.通过场发射扫描电子显微镜(SEM, S-4800型)、透射电子显微镜(TEM, JEM-2100HR型)和能量色散X射线光谱(EDX)对样品的形貌和元素组成进行表征.采用分光光度计(UV-vis DRS, UV-2550)对紫外-可见漫反射进行分析.采用日本日立公司的F-4600荧光光谱仪在室温条件下对催化剂样品进行光致发光测试, 激发波长为375 nm.
2.4 RhB降解实验本实验以RhB为目标污染物, 所制备的催化剂在可见光照射下活化PMS对RhB进行降解.将50 mg催化剂投加到50 mL浓度为10 mg·L-1的RhB溶液中, 在黑暗条件下搅拌60 min以达到吸附-解吸平衡.接着向RhB溶液投加0.2 g·L-1的PMS, 开启300 W氙灯(λ>420 nm), 在温度25 ℃条件下进行光催化反应.光催化反应过程中, 在一定时间间隔内提取溶液, 用0.45 μm的滤膜过滤, 滤液利用紫外-可见分光光度计在RhB的最大吸收波长554 nm处测其吸光度, 降解率(η)可表示为:
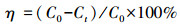 | (1) |
3 结果与讨论(Results and analysis)3.1 不同体系RhB降解效果在相同条件下(RhB浓度10 mg·L-1, 催化剂投加量1.0 g·L-1, 初始pH=4.65, PMS投加量0.2 g·L-1, 温度25 ℃), 研究不同催化体系对RhB的降解效果, 结果如图 1a所示.在没有添加光催化剂和PMS的条件下, RhB浓度在30 min内几乎没变化(Blank为单独可见光照射下的实验结果).在可见光照射和仅投加PMS的条件下, RhB降解率为25.2%.这表明在可见光照射下PMS对污染物具有一定的降解能力, 这可能归因于在激发态下PMS可以被RhB激活产生活性物质, 从而分解有机污染物(Heidarpour et al., 2020).在仅投加光催化剂的情况下, g-C3N4和Ag/g-C3N4-2对RhB的光催化降解率分别为11.3%和23.4%, 这意味着Ag的引入有利于提高g-C3N4的光催化活性.在Ag/g-C3N4/Vis/PMS催化体系中, RhB的降解效率随着Ag负载量的增加呈先提升后下降的趋势, 2%Ag负载量表现出最佳的降解效果, 30 min时RhB的去除率达到93.2%.而当Ag负载量从2%增加到3%时, RhB的去除率略有下降, 这可能是由于过多的Ag作为电荷复合中心, 从而导致催化性能降低.在相同条件下, Ag/g-C3N4-2/Vis/PMS体系的降解效率分别是Ag/g-C3N4-2/Vis(23.0%)、g-C3N4/Vis/PMS(29.2%)和Ag/g-C3N4-2/Dark/PMS(27.3%)体系的4.0、3.2和3.4倍.上述结果表明, Ag的引入有助于提高g-C3N4的光催化活性及活化PMS的能力, 从而提高RhB去除率.
图 1(Fig. 1)
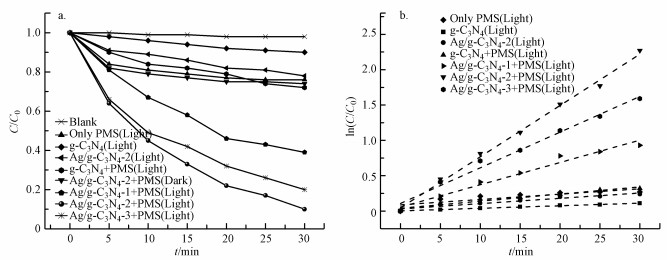 |
| 图 1 不同材料在可见光下降解RhB的光催化活性(a)及RhB降解的伪一级动力学(b)([Ag/g-C3N4-2]=1.0 g·L-1, [PMS]=0.2 g·L-1, [RhB]=10 mg·L-1, 初始pH=4.65, T=25 ℃) Fig. 1Photocatalytic activities of different composites for degradation of RhB under visible light irradiation(a) and pseudofirst-order kinetics of RhB degradation(b) |
为了进一步量化比较不同催化体系中RhB去除效率的不同, 利用伪一级动力学方程(式(2))计算相应的动力学常数(Juntrapirom et al., 2017).
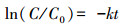 | (2) |
3.2 材料的表征3.2.1 XRD分析利用XRD对所制备的g-C3N4和Ag/g-C3N4-2材料的晶体结构进行表征, 结果如图 2所示.从图 2可以看出, 所制备的催化剂都存在位于2θ=12.8°和27.5°处的两个g-C3N4化合物典型特征峰.第1个特征峰对应于由平面结构内的堆垛单元引起的(100)晶面, 而第2个特征峰归属于芳香环堆垛形成的特征峰, 标记为(002)晶面(Chen et al., 2018).可以发现, 与g-C3N4相比, Ag/g-C3N4-2的特征衍射峰强度相对较弱, 这可归因于Ag与g-C3N4之间强烈的相互作用及对其聚合的抑制(Jin et al., 2017).此外, 在Ag/g-C3N4-2图谱中没有观察到Ag物种相关的衍射峰, 这可能是由于Ag含量相对较低(Fu et al., 2015).
图 2(Fig. 2)
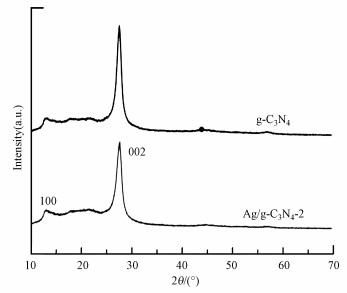 |
| 图 2 催化剂的XRD谱图 Fig. 2XRD patterns of the catalysts |
3.2.2 SEM/TEM/EDX分析通过SEM对g-C3N4和Ag/g-C3N4-2的形貌进行分析, 结果如图 3a、3b所示.g-C3N4和Ag/g-C3N4-2均呈现出表面光滑的、聚集的且形状不规则的形态, 这是通过热缩合方法合成的g-C3N4的典型结构特征(Yang et al., 2016).通过TEM(图 3c)进一步观察Ag/g-C3N4-2的形貌结构, 从图中可以发现Ag/g-C3N4-2为层状堆积结构.此外, 在其表面没有观察到Ag物种的存在, 这可能是由于Ag的高度分散所致(Ling et al., 2019).为了进一步研究复合材料的组成, 通过EDX对其元素组成进行分析.如图 3d~3h所示, 该复合催化剂中含有C、N、Ag元素, 且Ag在复合材料中的含量为1.06%.另外, 各元素均具有良好的分散度.上述结果表明通过煅烧-光还原法成功地合成了Ag/g-C3N4复合材料.
图 3(Fig. 3)
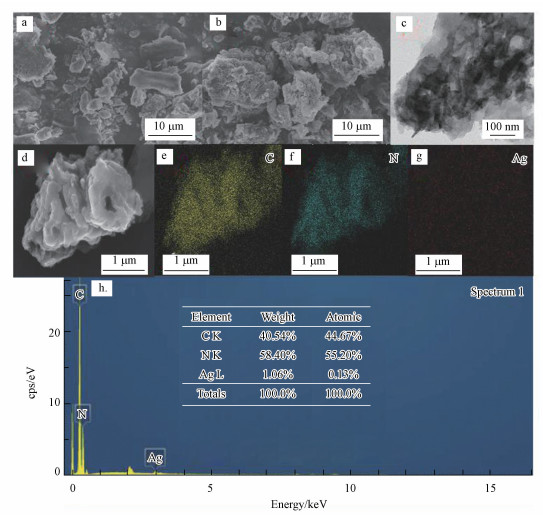 |
| 图 3 g-C3N4和Ag/g-C3N4-2的SEM图(a, b)及Ag/g-C3N4-2的TEM图(c)和EDX谱图(d~h) Fig. 3SEM images of g-C3N4 and Ag/g-C3N4-2(a, b), TEM image(c) and EDX spectrum of Ag/g-C3N4-2(d~h) |
3.2.3 DRS与PL分析通过UV-vis DRS和PL对g-C3N4和Ag/g-C3N4-2的光学性质进行研究.图 4a显示, g-C3N4的吸收边缘位于458 nm左右, 在引入Ag物种后, 材料的吸收边缘发生了红移(Ag/g-C3N4-2, 464 nm).这种现象归因于Ag的SPR效应, 有利于拓宽可见光的吸收范围, 提高光催化性能(Vidyasagar et al., 2018).光催化剂的带隙可由Eg=1240/λg(λg为催化剂的吸收边缘)计算所得(Zada et al., 2017).经计算得出g-C3N4和Ag/g-C3N4-2的Eg分别约为2.71 eV和2.67 eV, 带隙的减小证明Ag/g-C3N4-2具有更好的可见光吸收能力.采用光致发光(PL)光谱研究所制备材料的光生电子和空穴分离效率.由图 4b可知, Ag/g-C3N4-2材料的PL强度比g-C3N4弱, 这意味着Ag的引入抑制了光生电子和空穴的复合率, 提高了光生载流子的分离效率, 这可能有助于增强光催化活性和活化PMS的能力.
图 4(Fig. 4)
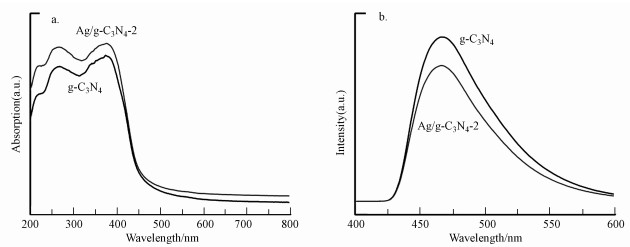 |
| 图 4 所制备材料的紫外可见漫反射光谱(a)和光致发光光谱(b) Fig. 4UV-vis diffuse reflectance spectra(a) and photoluminescence spectra (b) of the as-prepared catalysts |
3.3 影响因素的研究3.3.1 RhB浓度的影响在实际废水中, 水中污染物浓度会发生变化, 因此, 本研究通过改变RhB初始浓度来研究其对Ag/g-C3N4/Vis/PMS催化体系的影响.在Ag/g-C3N4-2浓度为1.0 g·L-1, PMS浓度为0.2 g·L-1, 初始pH为4.65, 温度为25 ℃的条件下, 4种不同初始RhB浓度下Ag/g-C3N4-2/Vis/PMS催化体系对RhB的降解效率见图 5a.由图可知, 当RhB初始浓度从10 mg·L-1提高到40 mg·L-1时, 污染物去除率由93.2%降至31.5%, 其相应的动力学常数从0.0756 min-1降至0.0128 min-1.造成这种现象的原因可能是随着RhB浓度增加, 降解中间产物增多, 形成了污染物、产物与活性物种间的相互竞争关系, 削弱了RhB与活性物种相互接触的机率, 从而导致RhB去除效率下降.
图 5(Fig. 5)
 |
| 图 5 不同初始RhB浓度(a)、Ag/g-C3N4-2光催化剂投加量(b)、PMS投加浓度(c)、初始溶液pH(d)及无机阴离子(e)条件下Ag/g-C3N4-2/Vis/PMS体系中RhB的降解效率和速率常数 (插图为相应的动力学常数) Fig. 5The RhB degradation efficiency and rate constant in the Ag/g-C3N4-2/Vis/PMS system under various initial RhB concentration(a), dosage of Ag/g-C3N4-2 photocatalyst (b), initial PMS concentration (c), initial solution pH (d) and inorganic anions (e) conditions (inset: corresponding kinetic constants) |
3.3.2 催化剂投加量的影响在PMS浓度为0.2 g·L-1, RhB浓度为10 mg·L-1, 初始pH为4.65, 温度为25 ℃的条件下, 通过改变反应体系中Ag/g-C3N4-2的投加量来考察催化材料用量对降解效果的影响, 结果如图 5b所示.由图可知, RhB的降解效果随着Ag/g-C3N4-2投加量的增加而提升, 当催化剂投加量提高到1.0 g·L-1或更多时, RhB的去除率在30 min内达到93.2%以上.这可能归因于催化系统能够吸收更多的光子能量, 产生更多的光生电子和空穴, 有助于活化更多的PMS和产生更多的活性物种, 从而提高光催化降解效果.然而, 当投加量为2.0 g·L-1时, RhB的降解效果(97.6%)并没有进一步明显加快或提升.这可能归因于催化体系的活性位点随着催化剂投加量的增加而增加, 从而产生更多的活性氧化物种和活化更多的PMS, 但当催化剂投加量过于饱和时, 水中的催化剂易发生团聚, 从而导致催化剂可接触的比表面积降低, 进而减少活性点位数(赵浩迪等, 2018).因此, 在催化降解过程中选择合适的催化剂投加量是十分重要的, 本研究选择1.0 g·L-1作为催化剂的最佳投加量.
3.3.3 PMS投加浓度的影响在Ag/g-C3N4-2浓度为1.0 g·L-1, RhB浓度为10 mg·L-1, 初始pH为4.65, 温度为25 ℃条件下, 不同PMS投加浓度对RhB去除效率的影响见图 5c.由图可知, 随着PMS投加浓度的增加, RhB的去除效率也随之提高, 出现这种现象可能是由于体系中SO4-·和/或其他活性物种增加.当PMS投加浓度从0.1 g·L-1增加到0.2 g·L-1时, 反应速率从0.0411 min-1增加到0.0756 min-1.然而, 当PMS投加浓度由0.3 g·L-1增加至0.4 g·L-1时, 速率常数仅从0.0886 min-1提高到0.0934 min-1, 反应速率常数并没有加速增大.这可能归因于以下两个方面:首先, 在高浓度PMS下, PMS与部分·OH和SO4-·反应生成反应性较小的SO5-·, 和/或自由基的自结合反应, 如式(3)~式(5)所示(Xie et al., 2015; Zhu et al., 2016);其次, 受到催化剂用量的限制, 只有部分PMS可被活化, 所以随着PMS浓度加大, 污染物去除效率提升速率减缓.从实用性和经济性考虑, 本研究选择1.0 g·L-1(Ag/g-C3N4-2剂量)和0.2 g·L-1(PMS剂量)作为实验用量.
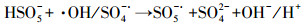 | (3) |
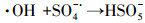 | (4) |
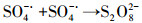 | (5) |
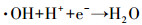 | (6) |
 | (7) |
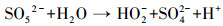 | (8) |
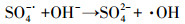 | (9) |
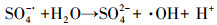 | (10) |
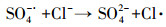 | (11) |
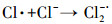 | (12) |
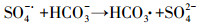 | (13) |
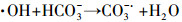 | (14) |
图 6(Fig. 6)
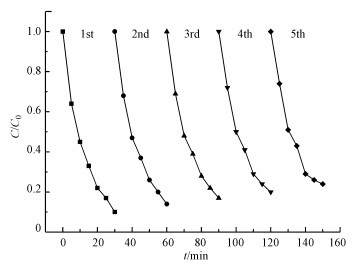 |
| 图 6 Ag/g-C3N4-2光催化剂活化PMS降解RhB的循环实验 Fig. 6Cyclic utilization of the Ag/g-C3N4-2 photocatalyst for RhB degradation with PMS addition |
3.5 催化机理分析为鉴定Ag/g-C3N4-2/Vis/PMS催化体系在降解污染物过程中的活性物种, 采用乙二胺四乙酸二钠(EDTA-2Na)、对苯醌(BQ)、叔丁醇(TBA)、糠醇(FFA)、乙醇(EtOH)分别作为h+、·O2-、·OH, 1O2和·OH与SO4-·的清除剂来进行捕获实验.如图 7a~7b所示, 添加FFA(72.3%)轻微抑制了RhB降解, 而BQ(21.0%)和EDTA-2Na(32.5%)则会明显抑制RhB的降解.TBA可作为·OH的清除剂, 而EtOH能同时猝灭·OH和SO4-·(Xie et al., 2019).在反应体系中分别投入等量的TBA和EtOH, 可以发现EtOH(61.2%)比TBA(82.1%)更抑制Ag/g-C3N4-2/Vis/PMS催化体系降解RhB的效果, 这表明反应过程中存在SO4-·.上述结果表明, 在Ag/g-C3N4-2/Vis/PMS催化体系中存在h+、·O2-、1O2、SO4-·和·OH, 不同活性物种均参与了RhB的氧化分解反应.在催化系统中分别添加EDTA-2Na、BQ、TBA、FFA、EtOH清除剂后, 催化反应系统相应的降解速率常数k清除分别为0.0123、0.0082、0.0571、0.0410和0.0331 min-1(图 7c).而在没添加任何清除剂时, 催化反应系统的降解速率常数k为0.0756 min-1.为了进一步对活性物种的贡献进行定量化, 采用公式(15)对清除剂相应的活性物种在光催化反应过程中的贡献率CR进行计算(李国亭等, 2012).
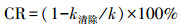 | (15) |
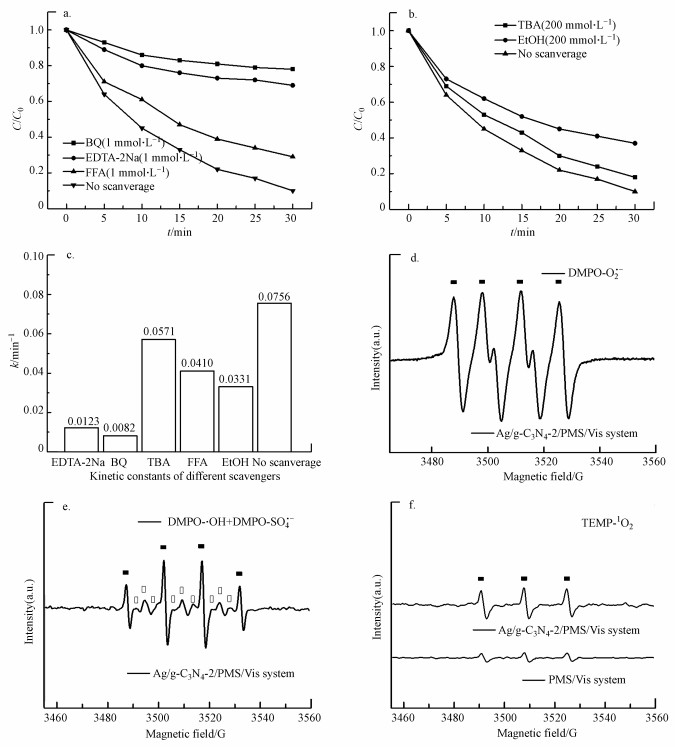 |
| 图 7 可见光照射下Ag/g-C3N4-2活化PMS的反应物捕获实验(a、b)和不同清除剂下的动力学常数(c)及DMPO-·O2(d)、DMPO-SO4·-和DMPO-·OH(e)和TEMP-1O2(f)的ESR谱 ([Ag/g-C3N4-2]=1.0 g·L-1, [PMS]=0.2 g·L-1, [RhB]=10 mg·L-1, 初始pH=4.65, T=25 ℃, [DMPO]=50 mmol·L-1, [TEMP]=20 mmol·L-1) Fig. 7Reactive species trapping experiments of Ag/g-C3N4-2 with PMS under visible light irradiation(a, b), kinetic constants of different scavengers(c) and ESR spectra of the DMPO-·O2- (d), DMPO-SO4·- and DMPO-·OH (e) and TEMP-1O2 (f) adduct |
式中, k清除为催化反应体系在添加清除剂时的降解速率常数, k为催化反应体系在没添加清除剂时的降解速率常数.
由计算结果可知, 各活性物种对氧化RhB的贡献(贡献率)由大到小顺序为:·O2-(89.1%)>h+(83.7%)> 1O2(45.7%)>SO4-·(31.8%)>·OH(24.4%).为了进一步确定Ag/g-C3N4-2/Vis/PMS体系光催化降解RhB过程中存在的活性氧化物种, 以DMPO和TEMP为自旋捕获剂, 进行了一系列的ESR测试.在Ag/g-C3N4-2/PMS/Vis体系中检测到DMPO-·O2-的ESR信号(图 7d), 表明·O2-存在于反应过程中.从图 7e中可以观察到·OH/DMPO和SO4-·/DMPO的信号峰, 表明PMS在反应过程中被活化形成·OH和SO4-·.此外, 在图 7f中可以明显观察到1O2/TEMP的1∶1∶1三重态信号特征, 这意味着反应系统中存在1O2.与PMS/Vis系统相比, Ag/g-C3N4-2/Vis系统具有更强的1O2/TEMP信号峰强度, 这意味着催化剂的投入有助于催化反应系统产生更多的1O2.ESR测试结果与捕获实验结果一致.为了更好地阐明催化反应机理, 根据如下经验公式计算得出催化剂的能带结构(Xu et al., 2015; Chandrashekar et al., 2018):
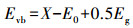 | (16) |
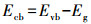 | (17) |
图 8(Fig. 8)
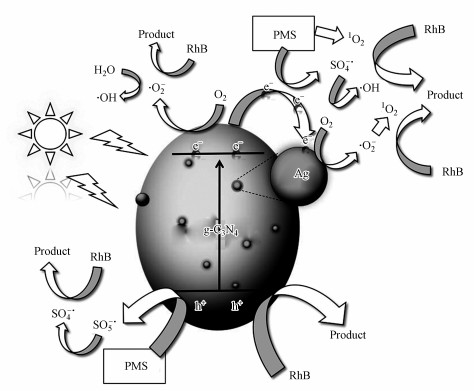 |
| 图 8 PMS辅助光催化降解RhB机理示意图 Fig. 8Schematic diagram of mechanism for RhB degradation by PMS assisted photocatalytic system |
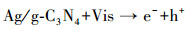 | (18) |
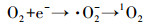 | (19) |
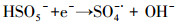 | (20) |
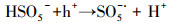 | (21) |
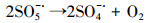 | (22) |
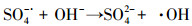 | (23) |
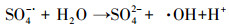 | (24) |
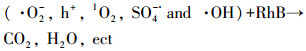 | (25) |
2) 在Ag/g-C3N4-2/PMS/Vis催化体系中, RhB的降解率随着催化剂投加量、PMS浓度的增加而增大, 随着初始RhB浓度的增加而减小.弱酸性条件有利于反应活化PMS降解RhB, 中性或碱性条件都会抑制催化反应的进行.溶液中的Cl-会对反应产生轻微的抑制作用, 而H2PO4-和HCO3-表现出强烈的抑制作用.
3) 捕获实验和ESR测试表明, 催化体系中存在自由基和非自由基(·O2-、h+、1O2、SO4-·和·OH)并协同降解RhB污染物.此外, Ag/g-C3N4-2具有良好的可重复性和稳定性.
参考文献
| Abdelhaleem A, Chu W. 2017. Photodegradation of 4-chlorophenoxyacetic acid under visible LED activated N-doped TiO2 and the mechanism of stepwise rate increment of the reused catalyst[J]. Journal of Hazardous Materials, 338: 491-501. DOI:10.1016/j.jhazmat.2017.05.056 |
| Ao X, Liu W. 2017. Degradation of sulfamethoxazole by medium pressure UV and oxidants: peroxymonosulfate, persulfate, and hydrogen peroxide[J]. Chemical Engineering Journal, 313: 629-637. DOI:10.1016/j.cej.2016.12.089 |
| Abdelhaleem A, Chu W. 2018. Monuron photodegradation using peroxymonosulfate activated by nonmetal-doped TiO2 under visible LED and the modeling via a parallel-serial kinetic approach[J]. Chemical Engineering Journal, 338: 411-421. DOI:10.1016/j.cej.2018.01.036 |
| Ahn Y Y, Yun E T, Seo J W, et al. 2016. Activation of peroxymonosulfate by surface-loaded noble metal nanoparticles for oxidative degradation of organic compounds[J]. Environmental Science & Technology, 50(18): 10187-10197. |
| Chan K H, Chu W. 2009. Degradation of atrazine by cobalt-mediated activation of peroxymonosulfate: different cobalt counteranions in homogenous process and cobalt oxide catalysts in photolytic heterogeneous process[J]. Water Research, 43(9): 2513-2521. DOI:10.1016/j.watres.2009.02.029 |
| Chen D D, Wu S X, Fang J Z, et al. 2018. A nanosheet-like alpha-Bi2O3/g-C3N4 heterostructure modified by plasmonic metallic Bi and oxygen vacancies with high photodegradation activity of organic pollutants[J]. Separation and Purification Technology, 193: 232-241. DOI:10.1016/j.seppur.2017.11.011 |
| Cheng X, Guo H, Zhang Y, et al. 2017. Non-photochemical production of singlet oxygen via activation of persulfate by carbon nanotubes[J]. Water Research, 113: 80-88. DOI:10.1016/j.watres.2017.02.016 |
| Chandrashekar C K, Madhusudan P, Shivaraju H P, et al. 2018. Synthesis of rare earth-doped yttrium vanadate polyscale crystals and their enhanced photocatalytic degradation of aqueous dye solution[J]. International Journal of Environmental Science and Technology, 15(2): 427-440. DOI:10.1007/s13762-017-1401-4 |
| Dong L, Xu T, Chen W, et al. 2019. Synergistic multiple active species for the photocatalytic degradation of contaminants by imidazole-modified g-C3N4 coordination with iron phthalocyanine in the presence of peroxymonosulfate[J]. Chemical Engineering Journal, 357: 198-208. DOI:10.1016/j.cej.2018.09.094 |
| Fu Y S, Huang T, Zhang L L, et al. 2015. Ag/g-C3N4 catalyst with superior catalytic performance for the degradation of dyes: a borohydridegenerated superoxide radical approach[J]. Nanoscale, 7: 13723-13733. DOI:10.1039/C5NR03260A |
| Fang G, Dionysiou D D, Zhou D, et al. 2013. Transformation of polychlorinated biphenyls by persulfate at ambient temperature[J]. Chemosphere, 90(5): 1573-1580. DOI:10.1016/j.chemosphere.2012.07.047 |
| Ghanbari F, Moradi M. 2017. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: review[J]. Chemical Engineering Journal, 310: 41-62. DOI:10.1016/j.cej.2016.10.064 |
| Heidarpour H, Padervand M, Soltanieh M, et al. 2020. Enhanced decolorization of rhodamine B solution through simultaneous photocatalysis and persulfate activation over Fe/C3N4 photocatalyst[J]. Chemical Engineering Research and Design, 153: 709-720. DOI:10.1016/j.cherd.2019.09.007 |
| 胡优优, 李正魁. 2019. 水凝胶负载BiOI活化过一硫酸盐降解尼泊金甲酯[J]. 中国环境科学, 39(8): 3249-3254. DOI:10.3969/j.issn.1000-6923.2019.08.015 |
| Han F, Kambala V S, Srinivasan M P, et al. 2009. Tailored titanium dioxide photocatalysts for the degradation of organic dyes in wastewater treatment: A review[J]. Applied Catalysis A-General, 359(1): 25-40. |
| Hu Y Y, Li Z K, Yang J H, et al. 2019. Degradation of methylparaben using BiOI-hydrogel composites activated peroxymonosulfate under visible light irradiation[J]. Chemical Engineering Journal, 360: 200-211. DOI:10.1016/j.cej.2018.11.217 |
| Juntrapirom S, Tantraviwat D, Suntalelat S, et al. 2017. Visible light photocatalytic performance and mechanism of highly efficient SnS/BiOI heterojunction[J]. Journal of Colloid and Interface Science, 504: 711-720. DOI:10.1016/j.jcis.2017.06.019 |
| Jin J, Liang Q, Ding C, et al. 2017. Simultaneous synthesis-immobilization of Ag nanoparticles functionalized 2D g-C3N4 nanosheets with improved photocatalytic activity[J]. Journal of Alloys and Compounds, 691: 763-771. DOI:10.1016/j.jallcom.2016.08.302 |
| Khan M E, Han T H, Khan M M, et al. 2018. Environmentally sustainable fabrication of Ag@g-C3N4 nanostructures and their multifunctional efficacy as antibacterial agents and photocatalysts[J]. ACS Applied Nano Materials, 1(6): 2912-2922. DOI:10.1021/acsanm.8b00548 |
| Katheresan V, Kansedo J, Lau S Y, et al. 2018. Efficiency of various recent wastewater dye removal methods: A review[J]. Journal of Environmental Chemical Engineering, 6(4): 4676-4697. DOI:10.1016/j.jece.2018.06.060 |
| 赖树锋, 肖开棒, 梁锦芝, 等. 2020. 石墨氮化碳光催化剂的制备及其改性研究进展[J]. 人工晶体学报, 49(4): 744-750. DOI:10.3969/j.issn.1000-985X.2020.04.029 |
| Liu J, Zhou J, Ding Z, et al. 2017. Ultrasound irritation enhanced heterogeneous activation of peroxymonosulfate with Fe3O4 for degradation of azo dye[J]. Ultrasonics Sonochemistry, 34: 953-959. DOI:10.1016/j.ultsonch.2016.08.005 |
| 李航, 封磊, 宋萍, 等. 2020. Cu改性石墨相氮化碳(g-C3N4)光催化灭活铜绿微囊藻的效能与机理研究[J]. 环境科学学报, 40(5): 1692-1702. |
| 李国亭, 宋海燕, 刘秉涛. 2012. 光催化过程中羟基自由基的产生与效能[J]. 环境工程学报, 6(10): 3388-3392. |
| Li C, Yu S, Dong H, et al. 2018. Z-scheme mesoporous photocatalyst constructed by modification of Sn3O4 nanoclusters on g-C3N4 nanosheets with improved photocatalytic performance and mechanism insight[J]. Applied Catalysis B: Environmental, 238: 284-293. DOI:10.1016/j.apcatb.2018.07.049 |
| Liu J, Zhao Z, Shao P, et al. 2015. Activation of peroxymonosulfate with magnetic Fe3O4-MnO2 core-shell nanocomposites for 4-chlorophenol degradation[J]. Chemical Engineering Journal, 262: 854-861. DOI:10.1016/j.cej.2014.10.043 |
| Luo W, Hu F, Hu Y, et al. 2019. Persulfate enhanced visible light photocatalytic degradation of organic pollutants by construct magnetic hybrid heterostructure[J]. Journal of Alloys and Compounds, 806: 1207-1219. DOI:10.1016/j.jallcom.2019.07.329 |
| Liang P, Zhang C, Duan X, et al. 2017. An insight into metal organic framework derived N-doped graphene for the oxidative degradation of persistent contaminants: formation mechanism and generation of singlet oxygen from peroxymonosulfate[J]. Environmental Science: Nano, 4(2): 315-324. DOI:10.1039/C6EN00633G |
| Ling Y, Liao G Z, Xu P, et al. 2019. Fast mineralization of acetaminophen by highly dispersed Ag-g-C3N4 hybrid assisted photocatalytic ozonation[J]. Separation and Purification Technology, 216: 1-8. DOI:10.1016/j.seppur.2019.01.057 |
| Nagajyothi P C, Pandurangan M, Vattikuti S V P, et al. 2017. Enhanced photocatalytic activity of Ag/g-C3N4 composite[J]. Separation and Purification Technology, 188: 228-237. DOI:10.1016/j.seppur.2017.07.026 |
| Oh W D, Dong Z, Lim T T. 2016. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects[J]. Applied Catalysis B: Environmental, 194: 169-201. DOI:10.1016/j.apcatb.2016.04.003 |
| Pearce C I, Lloyd J R, Guthrie J T, et al. 2003. The removal of colour from textile wastewater using whole bacterial cells: a review[J]. Dyes and Pigments, 58(3): 179-196. DOI:10.1016/S0143-7208(03)00064-0 |
| 彭小明, 罗文栋, 胡玉瑛, 等. 2019. 磷掺杂的介孔石墨相氮化碳光催化降解染料[J]. 中国环境科学, 39(8): 3277-3285. DOI:10.3969/j.issn.1000-6923.2019.08.019 |
| Rovira J, Domingo J L. 2019. Human health risks due to exposure to inorganic and organic chemicals from textiles: A review[J]. Environmental Research, 168: 62-69. DOI:10.1016/j.envres.2018.09.027 |
| Song Y, Qi J, Tian J, et al. 2018. Construction of Ag/g-C3N4 photocatalysts with visible-light photocatalytic activity for sulfamethoxazole degradation[J]. Chemical Engineering Journal, 341: 547-555. DOI:10.1016/j.cej.2018.02.063 |
| Sun C Y, Chen C C, Ma W H, et al. 2012. Photocatalytic debromination of decabromodiphenyl ether by graphiticcarbonnitride[J]. Science China-Chemistry, 55(12): 2532-2536. DOI:10.1007/s11426-012-4644-4 |
| Tao Y F, Qian N, Wei M Y, et al. 2015. Metal-free activation of peroxymonosulfate by g-C3N4 under visible-light irradiation for the degradation of organic dyes[J]. RSC Advances, 5: 185-186. |
| Vellaichamy B, Periakaruppan P. 2018. Synergistic combination of a novel metal-free mesoporous band gap-modified carbon nitride grafted polyaniline nanocomposite for decontamination of refractory pollutant[J]. Industrial & Engineering Chemistry Research, 57: 6684-6695. |
| Vandevivere P C, Bianchi R, Verstraete W. 1998. Treatment and reuse of wastewater from the textile wet-processing industry: Review of emerging technologies[J]. Journal of Chemical Technology & Biotechnology, 72: 202-289. |
| Vidyasagar D, Ghugal S G, Kulkarni A, et al. 2018. Silver/Silver(Ⅱ) oxide (Ag/AgO) loaded graphitic carbon nitride microspheres: an effective visible light active photocatalyst for degradation of acidic dyes and bacterial inactivation[J]. Applied Catalysis B: Environmental, 221: 339-348. DOI:10.1016/j.apcatb.2017.09.030 |
| Wang L, Guo X, Chen Y, et al. 2019. Cobalt-doped g-C3N4 as a heterogeneous catalyst for photo-assisted activation of peroxymonosulfate for the degradation of organic contaminants[J]. Applied Surface Science, 467: 954-962. |
| Wang M, Guo P, Zhang Y, et al. 2018. Synthesis of hollow lantern-like Eu(Ⅲ)-doped g-C3N4 with enhanced visible light photocatalytic perfomance for organic degradation[J]. Journal of Hazardous Materials, 349: 224-233. DOI:10.1016/j.jhazmat.2018.01.058 |
| Xie P C, Ma J, Liu W, et al. 2015. Removal of 2-MIB and geosmin using UV/persulfate: contributions of hydroxyl and sulfate radicals[J]. Water Research, 69: 223-233. DOI:10.1016/j.watres.2014.11.029 |
| Xie M, Tang J, Kong L, et al. 2019. Cobalt doped g-C3N4 activation of peroxymonosulfate for monochlorophenols degradation[J]. Chemical Engineering Journal, 360: 1213-1222. DOI:10.1016/j.cej.2018.10.130 |
| Xu W, Fang J, Chen Y, et al. 2015. Novel heterostructured Bi2S3/Bi2Sn2O7 with highly visible light photocatalytic activity for the removal of rhodamine B[J]. Materials Chemistry and Physics, 154: 30-37. DOI:10.1016/j.matchemphys.2015.01.040 |
| Yang X, Qian F, Zou G, et al. 2016. Facile fabrication of acidified g-C3N4/g-C3N4 hybrids with enhanced photocatalysis performance under visible light irradiation[J]. Applied Catalysis B: Environmental, 193: 22-35. DOI:10.1016/j.apcatb.2016.03.060 |
| 赵浩迪, 张静, 王梅菁, 等. 2018. 预磁化强化ZnO@Fe3O4活化PMS降解AO7[J]. 环境科学学报, 38(12): 4663-4669. |
| Zhu K, Wang J, Wang Y, et al. 2016. Visible-light-induced photocatalysis and peroxymonosulfate activation over ZnFe2O4 fine nanoparticles for degradation of Orange Ⅱ[J]. Catalysis Science & Technology, 6(7): 2296-2304. |
| Zhang J, Zhao X, Wang Y, et al. 2018. Peroxymonosulfate-enhanced visible light photocatalytic degradation of bisphenol A by perylene imide-modified g-C3N4[J]. Applied Catalysis B: Environmental, 237: 976-985. DOI:10.1016/j.apcatb.2018.06.049 |
| Zada A, Qu Y, Ali S, et al. 2017. Improved visible light activities for degrading pollutants on TiO2/g-C3N4 nanocomposites by decorating SPR Au nanoparticles and 2, 4-dichlorophenol decomposition path[J]. Journal of Hazardous Materials, 342: 715-723. |
