全文HTML
--> --> --> 苯酚对环境和人体的危害不容忽视[1]。美国环保署(EPA)制定标准指出,3.5 mg·L?1苯酚即可对人体造成危害[2]。因此,必须建立一种有效的方法处理含酚废水。Fenton氧化法有着较为广泛的适用范围、反应条件不苛刻、设备简单且可与多种方法联用,在水处理领域已得到了广泛的应用。传统的均相芬顿工艺中使用Fe2+(Fe3+)作为催化剂,但为了防止溶液中的Fe2+(Fe3+)沉淀,溶液必须为酸性(pH<3),对设备造成一定的腐蚀,而且随着反应的进行,会产生大量铁泥。此外,催化剂在反应过程中浓度不断降低且无法回收,双氧水利用率低,废水处理成本高,这些不利因素限制了芬顿氧化法的实际应用。为克服以上缺点,非均相类Fenton催化剂的研发成为了研究热点[3-7]。高聪等[8]利用Cu掺杂MIL-88B-Fe制备非均相类Fenton催化剂降解苯酚,从而提高了MIL-88B-Fe的催化效率。LUO等[9]以介孔ZSM-5分子筛为支撑体制备FeCu双金属催化剂,其催化活性优于单组分催化剂,且Cu和Fe之间的相互作用得到了加强。ZUBIR等[10]采用共沉淀法制备Fe3O4-GO复合材料催化双氧水降解偶氮染料,由于GO与Fe3O4之间的协同作用,偶氮染料的降解率可达到99%。羟基氧化铁(FeOOH)有着较大的比表面积,其表面含有大量的羟基基团,具有很强的吸附和催化性能[11]。常洪铭等[12]采用水热法合成羟基氧化铁,在不调节pH的情况下,可以直接吸附矿山废水中的铜、铁等重金属,且吸附性能优于普通吸附剂。张丽清等[13]采用自制的FeOOH催化H2O2降解甲基橙,降解率达到97%,且发现在降解过程中羟基自由基起到了重要的作用。但羟基氧化铁为粉末状,不易沉降,使用后难以回收。聚铁硅盐(PFSC)是一种新型复合絮凝剂[14]。将聚铁硅盐和羟基氧化铁结合可以制备聚铁硅盐掺杂羟基氧化铁(PFSC-FeOOH),由于羟基氧化铁紧紧得结合在硅酸盐上,故大大的提高了催化剂的沉降性能。聚铁硅盐掺杂羟基氧化铁催化臭氧去除有机污染物性能良好[15],但作为类芬顿催化剂催化H2O2氧化有机物的研究还未见报道。
因此,本文采用共沉淀法制备了聚铁硅盐掺杂羟基氧化铁,用作类芬顿催化剂降解苯酚,利用X射线衍射(XRD)、扫描电子显微镜(SEM)以及傅里叶变换红外光谱(FT-IR)等技术手段对晶相结构、样品形貌及表面基团等物化性质进行了表征,并考察了反应时间、投加量、pH、初始浓度等因素对苯酚去除率的影响,探讨了可能的反应机理。本研究可为类芬顿催化剂的制备及其实际应用提供参考。
1.1. 药剂
硝酸铁(Fe(NO3)3)、硅酸钠(Na2SiO3)、氢氧化钠(NaOH)、苯酚(C6H5OH)、盐酸(HCl)、甲醇(CH3OH)及叔丁醇(TBA)均为分析纯,购自国药集团化学试剂有限公司。过氧化氢(H2O2)纯度为30%。实验使用的去离子水电阻为18.0 MΩ。1.2. 催化剂制备
将0.60 mol?L?1的Na2SiO3溶液缓慢加入至300 mL 0.20 mol·L?1 Fe(NO3)3溶液中,并以200 r?min?1的速度不断搅拌,将pH调节为7,再将1 mol·L?1 NaOH溶液逐滴加入混合液中,调节至pH>12;静沉15 min后,在60 ℃烘箱活化24 h,用去离子水反复洗涤直至上清液的pH和电导率不再变化,置于80 ℃的烘箱中干燥48 h,所得固体经研磨后得到PFSC-FeOOH。对照为不加Na2SiO3,其余步骤同上,最后制得FeOOH。1.3. 催化剂的表征分析
采用丹东浩元仪器有限公司XRD-2000型X射线衍射仪(XRD)分析样品的晶相结构,Cu Kα射线、管电压为36 kV、管电流为20 mA、步进角度为0.02 °;采用日本JEOL公司的JSM-6700F型电子扫描电镜(SEM)分析样品的表面形貌;采用德国布鲁克公司的Tensor27型傅里叶变换红外光谱仪(FT-IR)分析样品表面基团。1.4. 降解实验
取100 mL苯酚模拟废水于锥形瓶中,加入一定量的催化剂与30%质量浓度的H2O2,在25 ℃下恒温振荡,反应一定时间后,加入0.50 mL甲醇终止氧化反应,沉淀,取0.50 mL上清液置于50 mL比色管中,用超纯水定容至50 mL,采用4-氨基安替比林比色法测定苯酚浓度[16]。2.1. 催化剂的表征
1) XRD分析。图1为FeOOH及PFSC-FeOOH的XRD图谱。由图1可见,FeOOH分别在2θ=21.2°、33.2°、36.6°、53.2°、59.1°和61.4°出现衍射峰,分别对应FeOOH的 (110)、(130)、(111)、(221)、(160)和(002)晶面,其中强度最强的为(110)晶面,与标准JCPDS文件(29-0713)[17-18]一致,衍射峰尖锐清晰,这说明晶体结晶度较好、无杂质。PFSC-FeOOH中出现FeOOH的衍射峰,由于聚铁硅盐(PFSC)为共聚物,衍射峰强度减弱,这说明成功合成了PFSC-FeOOH。2) SEM分析。FeOOH和PFSC-FeOOH的SEM表征结果如图2所示。由图2(a)可见,FeOOH呈现针状晶体结构且形貌分布均一,直径细小,排列无规则,不易沉降;由图2(b)可见,聚铁硅盐将FeOOH包裹,形成的PFSC-FeOOH呈紧密的块状,该聚集体的主干部分是聚硅酸,铁及其水解产物被吸附、螯合在聚硅酸颗粒表面,与聚硅酸之间产生键合作用成枝杈部分,生成聚合态较大的颗粒,易于沉降。
3) FTIR分析。FeOOH和PFSC-FeOOH的FTIR表征结果如图3所示。在FeOOH和PFSC-FeOOH的FTIR谱图中,3000~3300 cm?1和3000~3600 cm?1处有强宽峰,为表面羟基(—OH)的伸缩振动吸收峰,1350 cm?1处的峰为羟基的面外弯曲吸收峰[19-21],且PFSC-FeOOH的峰较FeOOH宽,说明PFSC-FeOOH的表面—OH密度更大。此外,由图3可见,886、790和623 cm?1处为Fe—O和Fe—OH—Fe的特征峰,峰型尖锐且对称[22-23]。在PFSC-FeOOH的FTIR谱图中,940 cm?1处为Si—O—Fe弯曲振动吸收峰,这说明PFSC和FeOOH通过Si—O—Fe键结合。因此,FTIR 表征结果从结构上证实了PFSC-FeOOH中部分铁离子和水解络合铁离子可与共存的聚硅酸发生螯合反应,进而生成高分子聚合物。
2.2. 催化剂的活性评价
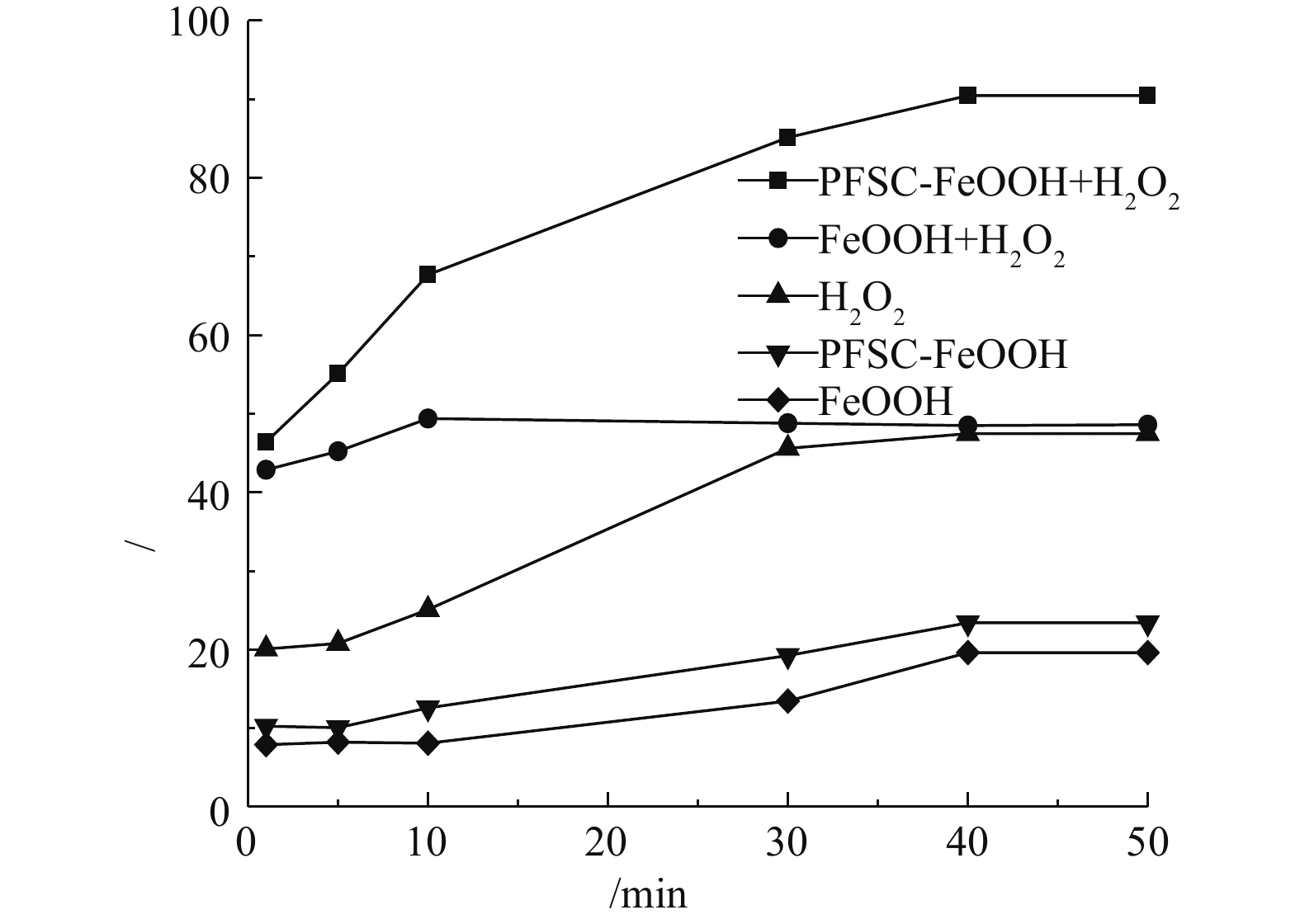
在催化剂投加量为3 g·L?1、H2O2投加量为297 mmol·L?1、苯酚浓度为100 mg·L?1、pH=4.0、温度为25 °C的条件下,分别考察了单独 H2O2氧化、单独催化剂和催化剂+H2O2对苯酚的去除效果,结果如图4所示。在仅加入H2O2时,苯酚去除率在40 min时达到最高,为47.47%。反应符合准一级反应动力学(R2=0.9667),速率常数为0.012 1 min?1。在仅加催化剂时,由于FeOOH和PFSC-FeOOH具有一定的吸附性能,吸附后苯酚的去除率分别为19.62%和23.42%,PFSC-FeOOH吸附性能好于FeOOH。FeOOH催化H2O2氧化苯酚,苯酚的去除率随着反应的进行先增大后减小,在10 min时去除率达到最大,为49.4%,这表明FeOOH可以催化H2O2氧化苯酚,但在10 min以后,苯酚的去除率略有下降。这是因为,随着反应的进行,中间产物吸附于FeOOH表面,降低了FeOOH的比表面积,且中间产物在转化过程中与苯酚会存在竞争关系,从而降低了去除率,此外,还可能与FeOOH表面的复杂配合反应有关。反应符合准一级反应动力学(R2=0.9874),速率常数为0.013 6 min?1。PFSC-FeOOH催化H2O2降解苯酚,苯酚的去除率随着反应的进行不断增大,在40 min时去除率达到了90.48%,反应符合准一级反应动力学(R2=0.991 6),速率常数为0.041 5 min?1。与催化剂FeOOH相比,该反应体系反应速率快,苯酚降解效果好。PFSC-FeOOH催化H2O2降解苯酚效果较好,原因可能要是硅酸聚合是由相邻分子上羟基间的脱水聚合形成具有硅氧键的聚合物,硅原子模型是四面体,硅酸分子可以向各个方向进行聚合,形成带支链的、环状的、网状的三维立体结构聚合物,最终形成硅酸凝胶,在其聚合过程中的某一时间引入铁离子后,由于其可与聚硅酸的链状、环状分子端的氢氧根进行络合和吸附,这阻断了聚硅酸的凝胶化,从而制得大分子聚合物,在掺杂羟基氧化铁后,增加了催化剂的比表面积,负载了更多的羟基,增加了羟基密度,强化了催化效果[24]。刘玥等[25]制得的聚铁硅盐掺杂羟基氧化铁比表面积为309.01 m2·g?1,相当于市售羟基氧化铁表面积的3倍,表面羟基密度为4.58 mmol·L?1,约为市售羟基氧化铁表面羟基密度的4倍。同时,在本实验中观察到PFSC-FeOOH的沉降性能明显好于FeOOH,沉降速度为0.1 m·min?1,这便于催化剂的回收利用。
2.3. 反应条件的影响
1) pH的影响。在催化剂投加量为3 g·L?1、H2O2投加量为297 mmol·L?1、苯酚浓度为100 mg·L?1、温度为25 °C的条件下,pH对苯酚的去除率的影响如图5所示。由图5可知:随着苯酚模拟废水pH的增大,苯酚的去除率呈先增大后减小的变化趋势,在pH为4时,苯酚的去除率达到最高(90.91%);当pH<4时,去除效率较低,这是由于强酸性条件下,H2O2获得一个质子生成2) H2O2投加量的影响。在催化剂投加量为3 g·L?1、苯酚浓度为100 mg·L?1、pH为4.0、温度为25 °C的条件下,H2O2的投加量对苯酚去除率的影响如图6所示。由图6可知,随着H2O2用量的增大,苯酚的去除率先增大再略有降低。当H2O2用量为297 mmol·L?1时,苯酚的去除率高达90.63%。这是由于随着H2O2用量的增加,会产生更多的·OH,但是,过量的H2O2发生的清除反应,如式(1)所示,可生成过氧化氢自由基(·HO2),该自由基氧化能力很低,导致苯酚去除率降低[29]。因此,确定最佳H2O2投加量为297 mmol·L?1。
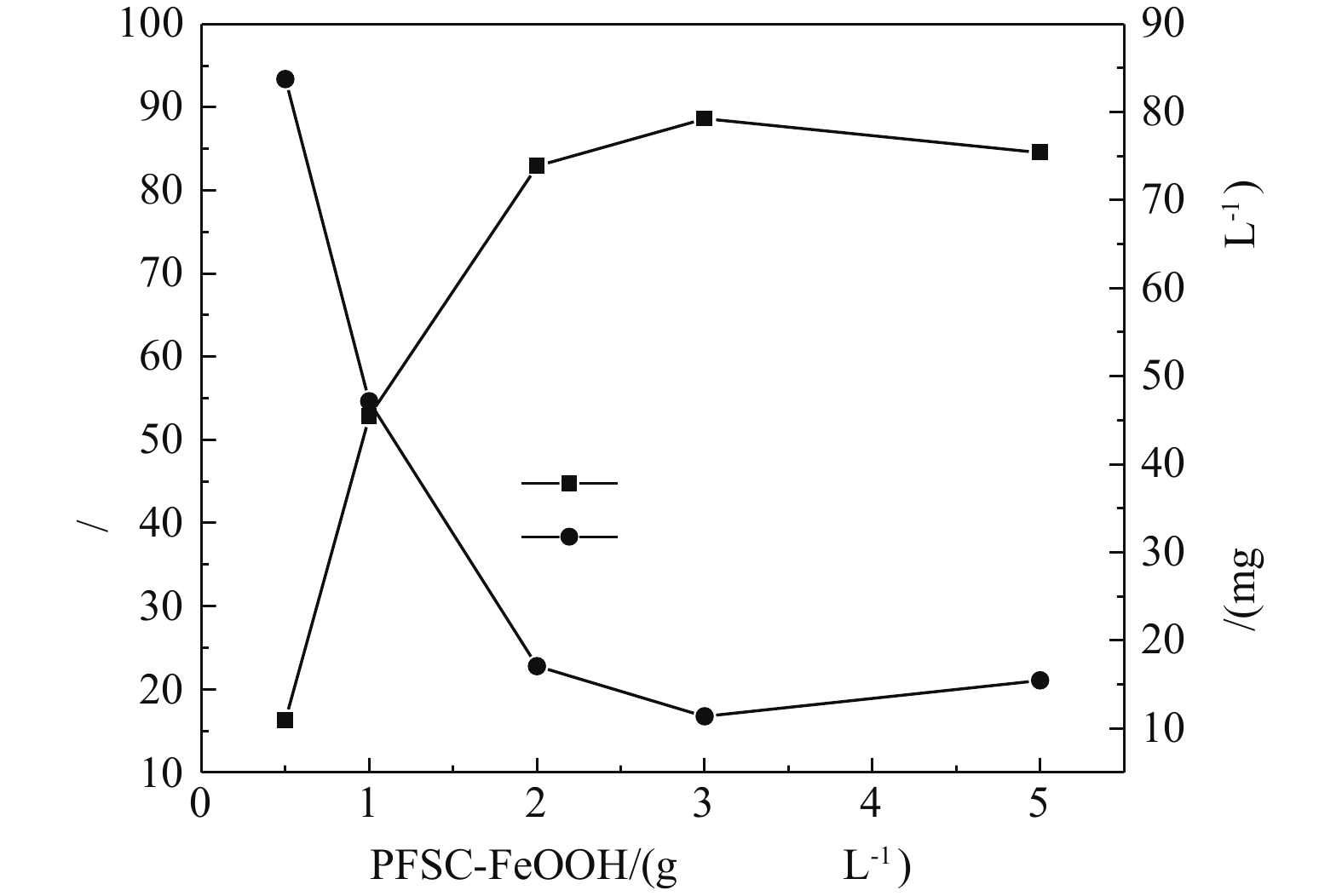
3)催化剂投加量的影响。在H2O2投加量为297 mmol·L?1、苯酚浓度为100 mg·L?1、pH为4.0、温度为25 °C的条件下,PFSC-FeOOH的投加量对苯酚去除率的影响如图7所示。由图7可知,随着催化剂投加量的增加,苯酚的去除率随之增加,当PFSC-FeOOH的投加量为3 g·L?1时,苯酚去除效果最好,去除率达到88.62%,之后逐渐下降。这是因为,在H2O2的量确定时,催化剂的投加量的增加会使·OH增加。但过量的Fe2+与·OH之间会发生清除反应[30](式(2)),形成Fe3+和OH?,导致生成Fe(OH)3沉淀。因此,确定最佳催化剂投加量为3 g·L?1。
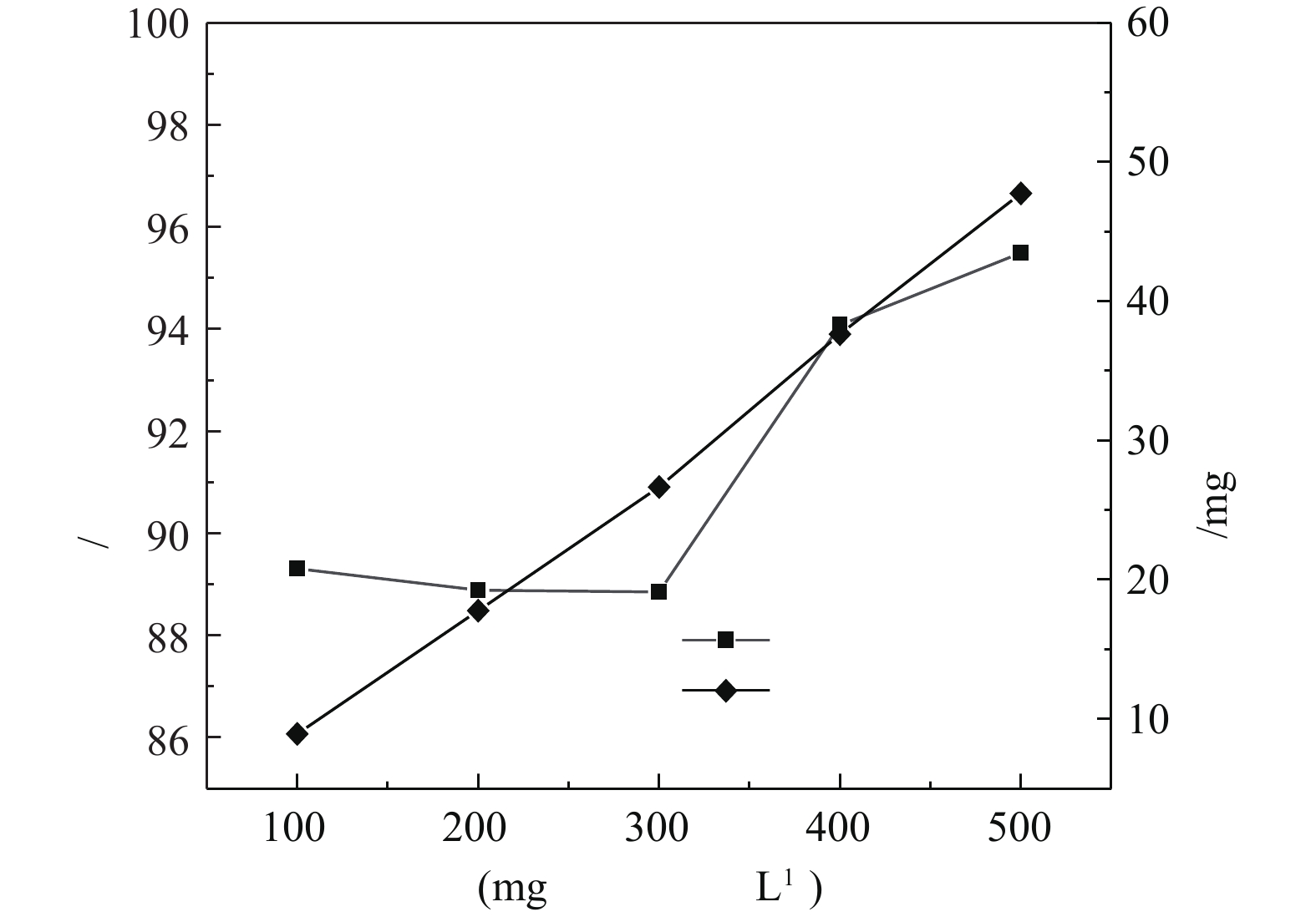
4)苯酚初始浓度的影响。在催化剂投加量为3 g·L?1、H2O2投加量为297 mmol·L?1、pH为4.0、温度为25 °C的条件下,苯酚初始浓度对苯酚的去除效果的影响如图8所示。由图8可知,随着苯酚浓度的增加,苯酚的去除量呈线性增长,而去除率呈不规则变化。在苯酚初始浓度为100~300 mg·L?1时,去除率基本不变,约为85%。而随着苯酚浓度继续增加,去除率呈增长趋势。将该方法运用到高浓度苯酚废水的处理中效果更明显。
5)温度的影响。在催化剂投加量为3 g·L?1、H2O2投加量为297 mmol·L?1、苯酚浓度为100 mg·L?1、pH为4.0 的条件下,反应温度对苯酚去除效果的影响如图9所示。由图9可知,随着反应温度的升高,苯酚的去除率先升高再降低,25 ℃时去除效果最好。这表明随着反应温度的升高,·OH的活性增大,导致分子剧烈碰撞,有利于反应正向进行;当温度达到25 ℃后继续升温,会导致H2O2分解成O2和H2O2,丧失氧化性能,且该反应为放热反应,温度过高会减缓反应速率,从而降低苯酚的去除率,此结论与TONY等[31]的研究结果相符。
2.4. 机理分析
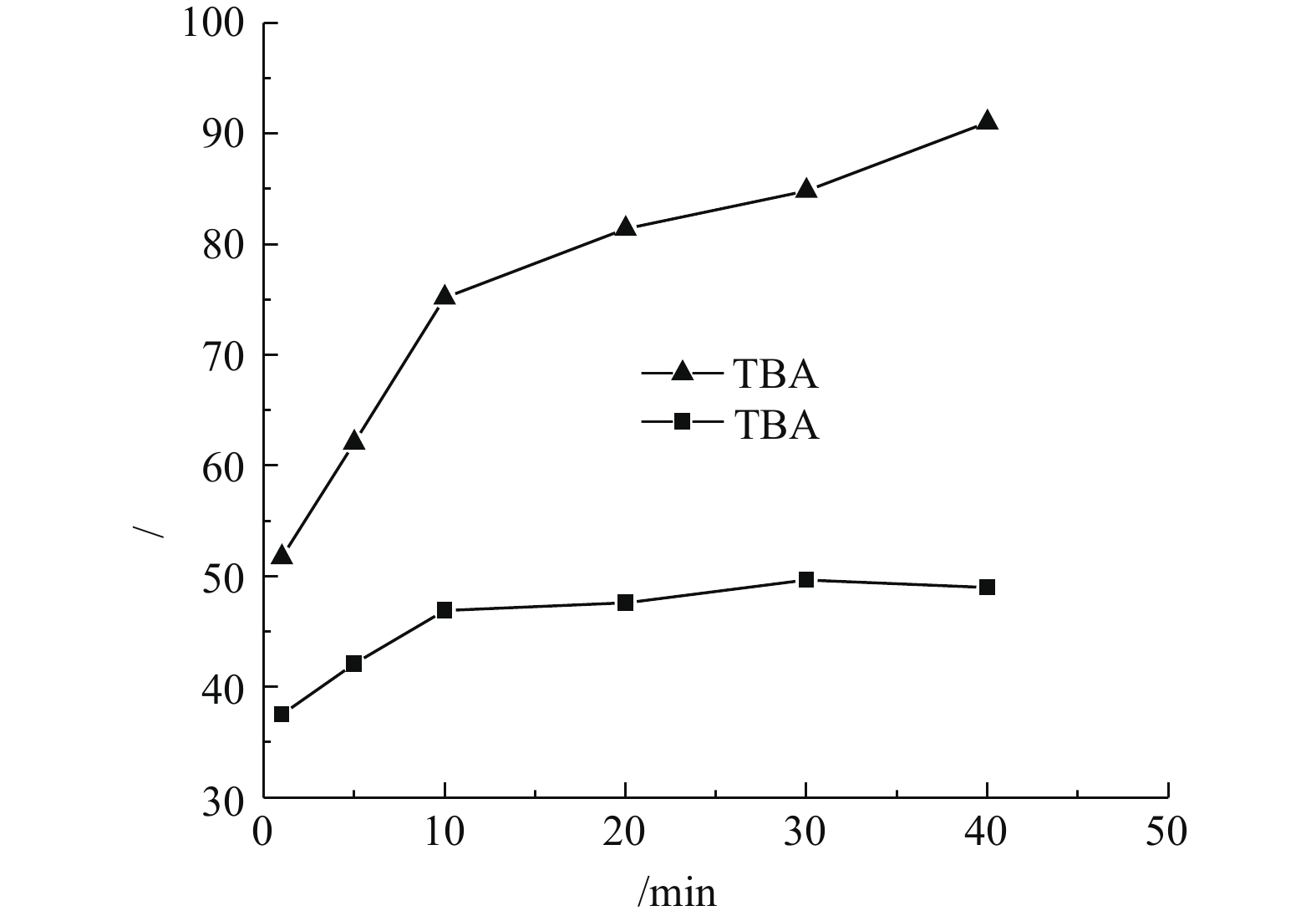
1)自由基淬灭实验。为了鉴定该反应体系中产生的自由基,采用叔丁醇(TBA)进行自由基的淬灭反应。在催化剂投加量为3 g·L?1、H2O2投加量为297 mmol·L?1、苯酚浓度为100 mg·L?1、pH为4.0、温度为25 °C的条件下,实验结果如图10所示。TBA作为一种自由基淬灭剂,可与·OH发生反应,通过生成惰性中间自由基以终止自由基链反应。由图10可知,加入TBA后苯酚的降解受到了明显的抑制,苯酚去除效果大幅减弱。这表明反应体系中起主要降解作用的物质确为·OH,此结果与高聪等[8]的研究结果一致。2)紫外谱图分析。PFSC-FeOOH催化H2O2氧化苯酚过程的紫外吸收光谱图如图11所示。由图11可知,苯酚原液的吸收峰在270 nm附近,但在加入H2O2和PFSC-FeOOH后,270 nm处的峰强逐渐降低,230 nm附近出峰,表明在苯酚降解过程中形成了新的中间体,且随着反应时间的增长,230 nm处的峰强逐渐降低,这表明中间体也在降解。相关研究[32]表明,在类芬顿体系中苯酚会生成环化合物(对苯二酚,邻苯二酚,苯醌等),进一步降解为短链酸,主要是马来酸,甲酸,乙酸和草酸,最终可以降解为CO2和H2O。由此可见,该反应通过破坏苯酚的分子结构进而对其进行降解。
2)在PFSC-FeOOH催化H2O2降解苯酚的体系中,最佳反应条件是:反应时间为40 min、pH为4、反应温度为25 ℃、H2O2投加量为297 mmol·L?1、PFSC-FeOOH投加量为3 g·L?1、苯酚的初始浓度为100 mg·L?1,在此条件下的苯酚去除率可达到90.48%。
3) PFSC-FeOOH催化H2O2反应符合准一级反应动力学,速率常数为 0.0415 min?1。在反应过程中产生的大量·OH,通过破坏苯酚的分子结构而对其进行降解。
参考文献


 下载:
下载: 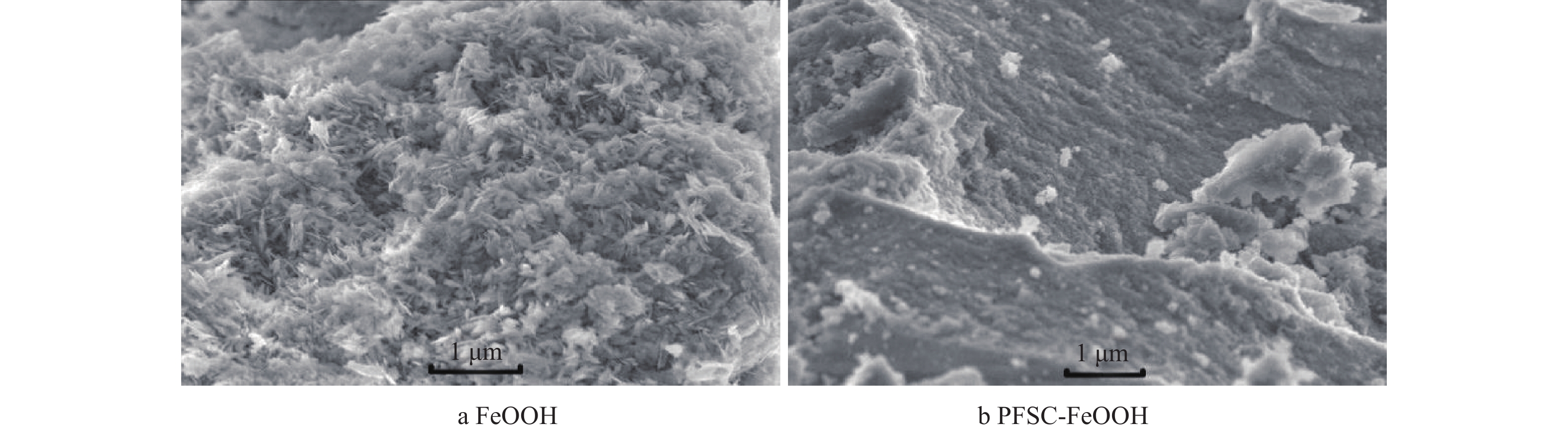





 点击查看大图
点击查看大图