全文HTML
--> --> --> 作为新兴药类污染物的抗生素,被大量用作兽药投加到禽畜养殖中,这些抗生素在动物肠道中无法被完全吸收,多达30%~90%的母体化合物会通过粪便或尿液排出[1]。四环素类抗生素的广泛使用使得动物粪便中残留大量的四环素[2]。动物粪便中的抗生素可利用高温堆肥处理技术来降解,堆肥温度可达70 ℃[3],甚至90 ℃[4]。土霉素(OTC)经过堆肥后的降解产物仍是大分子物质EOTC (m/z=461)、α-apo-OTC (m/z=443)和β-apo-OTC (m/z=443)[5]。堆肥中混合的抗生素、ARGs及菌体可被释放到大气环境中,人体长期暴露在这种环境中会导致传染病、毒性影响、过敏和癌症等风险[6],并且ARGs会降低抗生素治疗的功效[4]。近年来,处理OTC的方法多种多样,包括高级氧化、吸收法、化学氧化、电化学和生物法等[7]。KARPOV等[8]用Fe(Ⅲ)和Mn(Ⅳ)来降解OTC;?ELIK等[9]在膜生物反应器中用硝化菌降解OTC;HARRABI等[10]用河口沉积物中原生的细菌来降解OTC;LI等[11]和USLU等[12]用臭氧氧化来降解OTC。臭氧能快速与有害有机物反应并将其分解,将有机物降解成更简单的产物[11, 13]。为提高O3对有机污染物的降解效率,常将O3氧化与其他氧化过程结合起来,如UV/O3预处理PAHs、VOCs、CPs、APs和DBPs[14-15],O3/微波降解活性污泥[16],臭氧-过硫酸钠(O3/PS)降解布洛芬[17]、硝基苯[18]、渗滤液[19]和氯苯甲酸[20]。在这些与臭氧结合的方法中,PS具有强氧化性、宽的酸碱度范围、高溶解性[21],且PS产生的
1.1. 实验装置与步骤
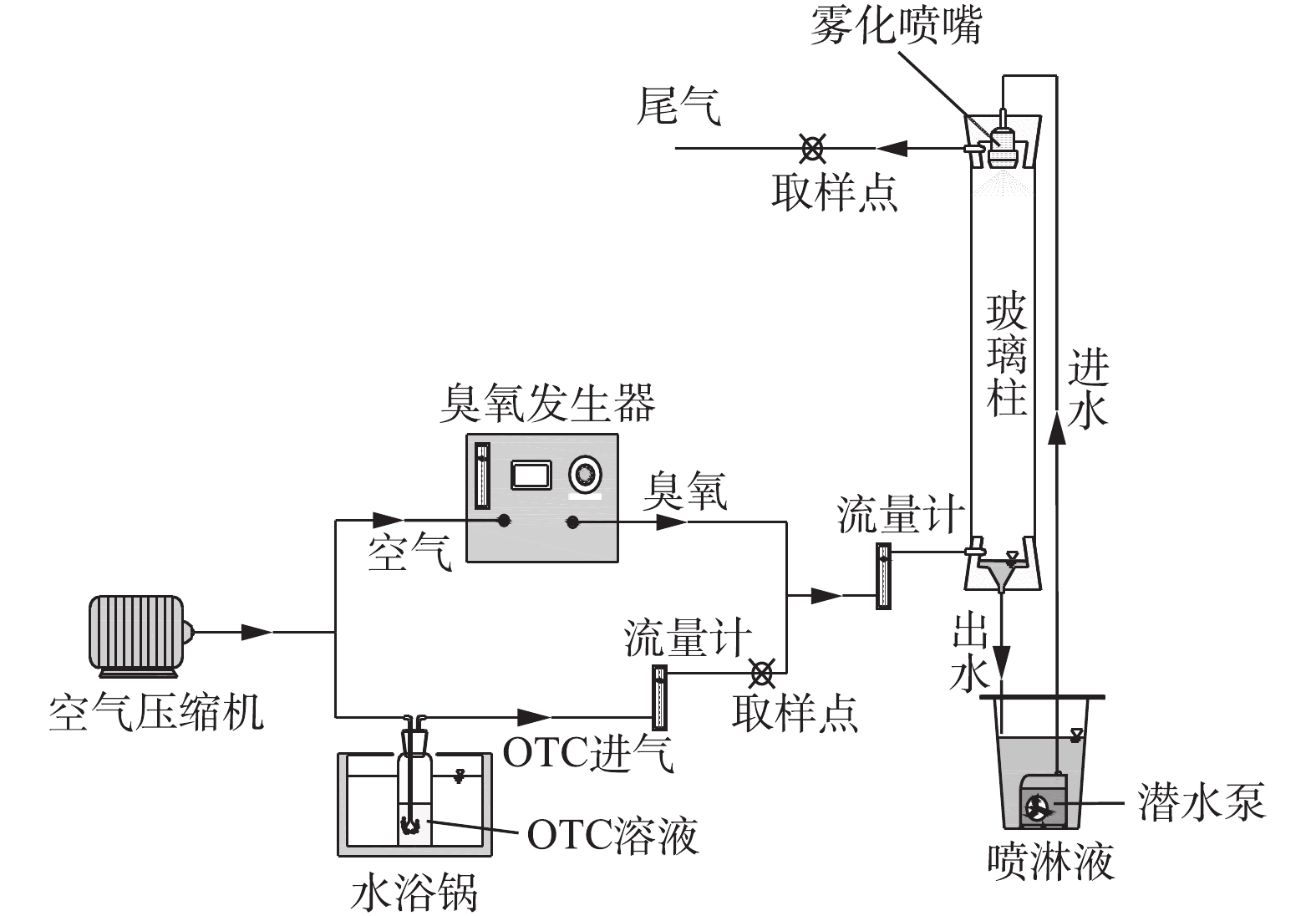
O3/PS协同降解OTC废气的实验装置如图1所示。喷淋塔由D×H=3 cm×50 cm的透明玻璃柱构成,玻璃柱外包覆锡纸避光。喷淋液由雾化喷嘴(孔径0.8 mm)形成喷雾后,均匀喷入。模拟OTC废气采用动态法配制,通过加热和鼓吹OTC浓溶液制得,将装有100 mg·L?1 OTC溶液的孟氏洗瓶(500 mL)放入恒温水浴锅(BHS-2)中加热(100 ℃),同时用空气压缩机泵入空气,鼓泡吹出含OTC的混合气体。由台式臭氧发生器(CH-ZTW6G)产生的臭氧和OTC废气混合后,一起从底部进入喷淋塔,混合气在上升过程中与喷淋液接触、反应,经过O3/PS降解后的废气从喷淋塔顶部排出;反应后的喷淋液流入循环槽中,在潜水泵的作用下重新进入喷淋塔,每运行0.5 h,更换新鲜喷淋液。实验所涉及的溶液均由超纯水配制。O3/PS喷淋塔对OTC的去除性能实验在OTC进气流量为2.4 L·min?1,O3进气流量为0.9 L·min?1,雾状喷淋液流速为30 mL·min?1的条件下进行。实验包括4个步骤。
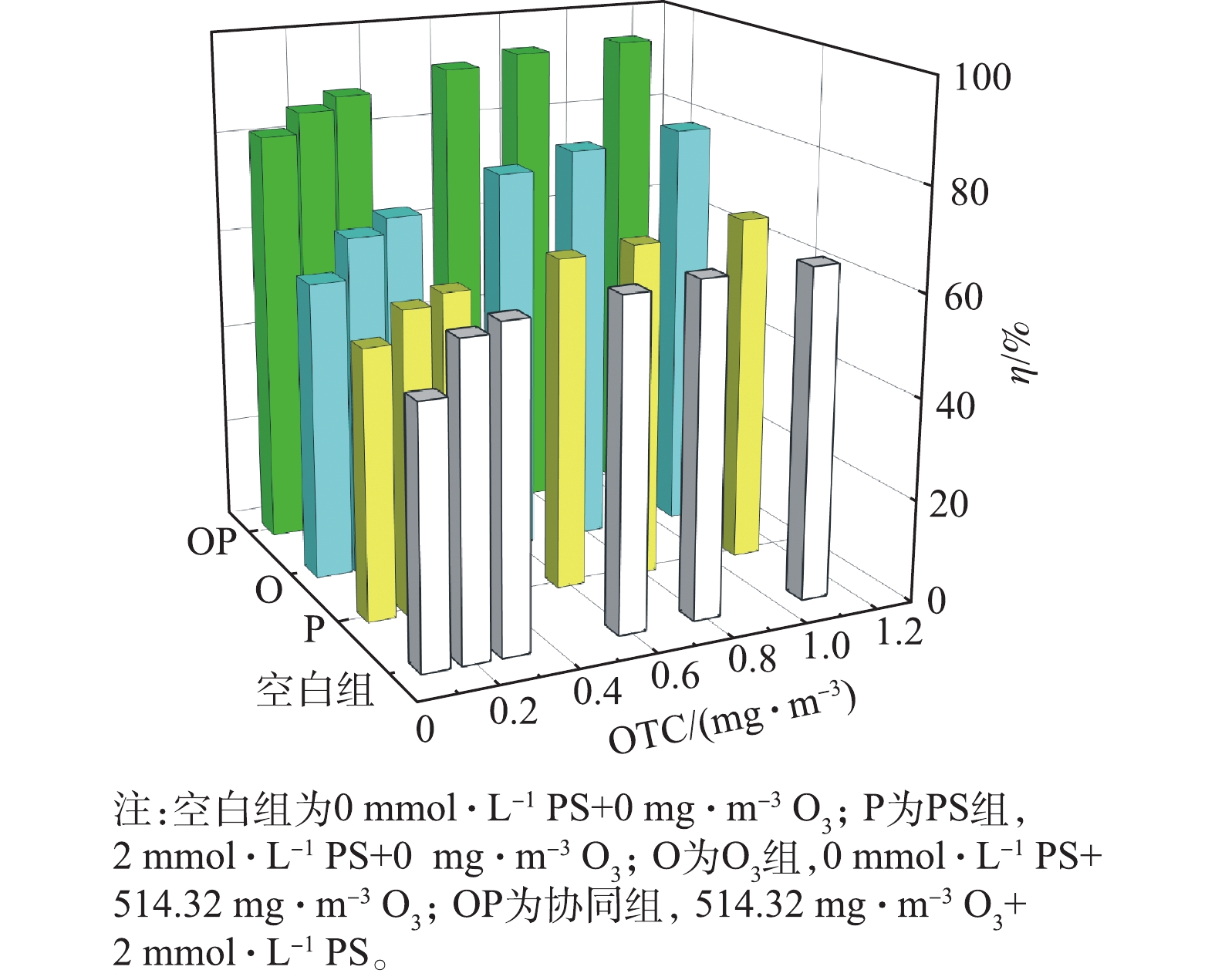
1)控制进气OTC浓度为(0.7±0.1) mg·m?3,通过改变进气中O3的浓度和喷淋液中PS的浓度,研究O3和PS剂量对OTC去除率的影响。喷淋液中PS的投加量分别为0、0.5、1、2 mmol·L?1,以超纯水作为空白组(投加量为0)。O3浓度梯度设为0、128.58、385.74、514.32、771.48 mg·m?3,以空气作为空白组(浓度为0)。
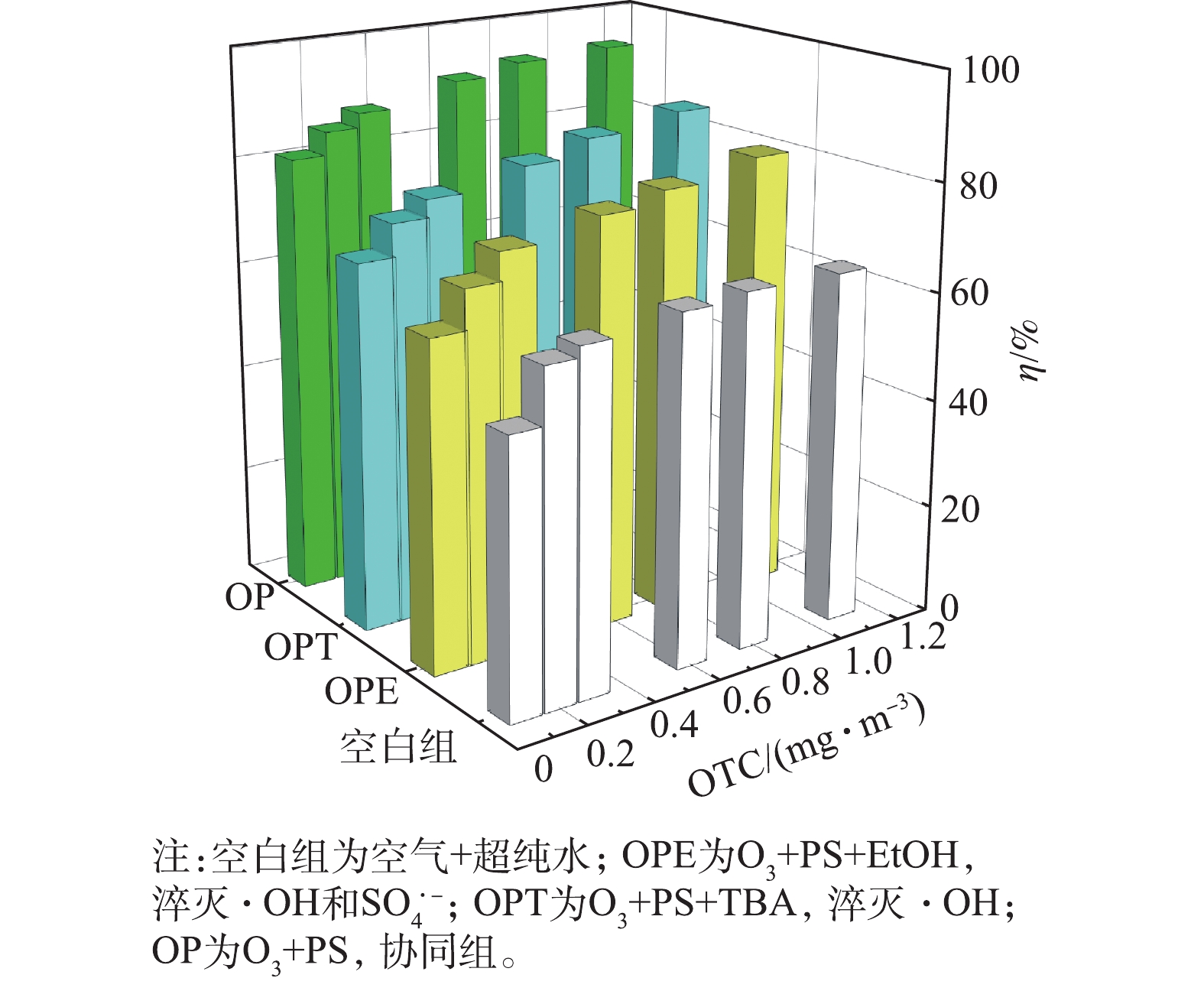
2)通过控制O3和PS来探究O3和PS对OTC的去除作用及贡献率。实验分为4组:空白组(超纯水和空气)、单独O3、单独PS和O3/PS协同,其中PS的浓度均为2 mmol·L?1,O3的浓度均为514.32 mg·m?3。
3)通过控制
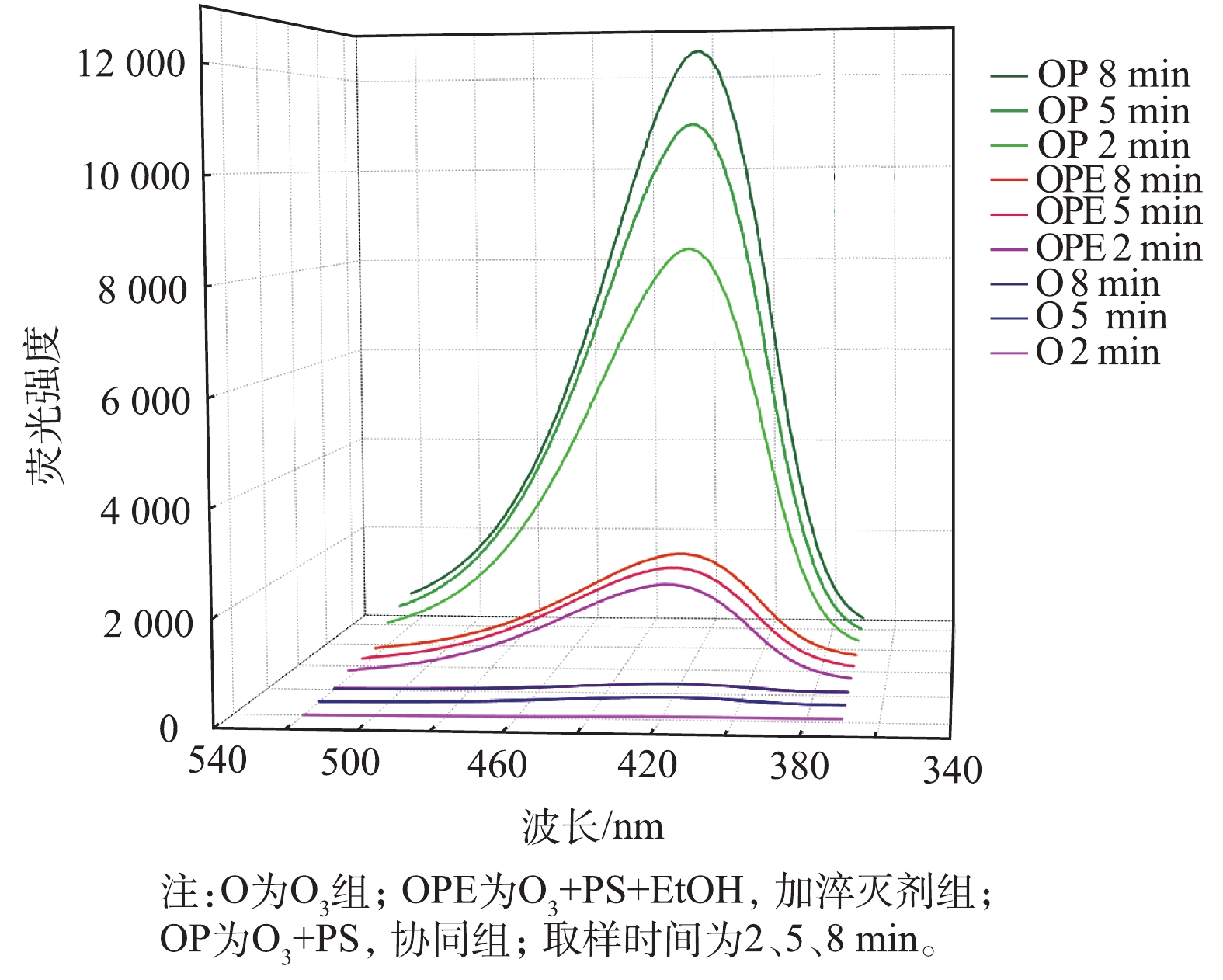
4)验证自由基的存在,并定量观察自由基的产生量。OTC废气发生装置中的OTC溶液换成超纯水,在PS喷淋液中加50 mL对苯二甲酸(0.5 mmol·L?1)。实验分为3组:514.32 mg·m?3 O3,514.32 mg·m?3 O3+2 mmol·L?1 PS,514.32 mg·m?3 O3+2 mmol·L?1 PS+EtOH。在装置运行2、5、8 min后,取反应后的喷淋液2 mL,再用超纯水稀释4倍后,检测对苯二甲酸被·OH氧化后的荧光产物强度。
1.2. 分析与计算方法
臭氧浓度用臭氧检测仪(ZCP-O3)检测,测量范围为0~2 143 mg·m?3。羟基自由基(·OH)强度用荧光分光光度计(SHIMADZU RF-6000)间接检测,激发光波长为315 nm,发射波长为370~520 nm。气体和液体流量采用玻璃转子流量计(LZB-3)控制,量程分别为0~1.5 L·min?1和0~100 mL·min?1。OTC进出气浓度检测方法:用1 mL甲醇在采样点吸收含OTC的混合气5 min后,再用紫外可见分光光度计在354 nm波长处测量吸收液的吸光度,根据标准曲线换算出吸收液中OTC浓度,再以式(1)换算出OTC在进气和出气中的浓度。
式中:Cg为空气中OTC的浓度,mg·m?3;Cl为吸收液中OTC的浓度,mg·L?1;Vl为吸收液体积,mL;t为吸收时间,min;Qg为取样口的气体流量,mL·min?1(进气口为OTC进气流量,出气口为进气OTC流量和O3进气流量之和)。由于气体发生温度为100 ℃,高温OTC废气携带了水蒸气,1 mL的甲醇吸收液同时起到冷凝和吸收的作用,因此,吸收后的溶液为甲醇、水和OTC的混合液,即Vl为混合液的总体积。
OTC中间产物的鉴定采用高分辨液质联用仪(Ultimate3000-timsTOF)和Hypersil GOLD C18柱(1.9 μm,2.1 mm×100 mm),样品注射体积为3 μL,柱温为40 ℃,流速为0.3 mL·min?1。流动相为0.1%甲酸(A)和乙腈(B),洗脱过程为梯度洗脱:0~2 min,5%~60% B;2~8 min,60%~100% B;8~10 min,100% B;10~12 min,5% B。
2.1. O3/PS喷淋塔对OTC的去除性能
臭氧-过硫酸钠喷淋协同降解OTC的去除性能如图2所示。空气和超纯水代替O3和PS的空白组(0 mg·m?3 O3+0 mmol·L?1 PS)对OTC的去除率为64.5%。当空气(0 mg·m?3 O3)和2 mmol·L?1 PS喷淋液协同作用时,喷淋塔对OTC的去除率为65.5%,说明单独的PS达到的去除率和空白组相差甚微。当514.32 mg·m?3 O3和超纯水(0 mmol·L?1 PS)协同作用时,喷淋塔对OTC的去除率为79.9%,比空白组高了15.4%,说明单独的O3可以去除部分OTC。当514.32 mg·m?3 O3和2 mmol·L?1 PS喷淋液协同作用时,OTC去除率为94.7%,较单独的PS对OTC的去除率增加了29.1%,较单独的O3对OTC的去除率增加了14.9%。因此,在相同OTC进气浓度下,O3和PS协同降解OTC的去除率比单独的O3或单独的PS高。此外,OTC的去除率与O3和PS的剂量呈正相关。以2 mmol·L?1 PS作为喷淋液,通入的O3浓度从514.32 mg·m?3降到128.58 mg·m?3时,OTC去除率由94.7%降至89.1%;通入514.32 mg·m?3 O3时,在PS浓度由2 mmol·L?1降至0.5 mmol·L?1的过程中,OTC去除率由94.7%降至85.5%。2.2. O3和PS对OTC去除率的贡献
如图3所示,根据4种条件下OTC的去除率的差异,探究了PS和O3对OTC去除作用的贡献率。当OTC进气浓度为0~1.2 mg·m?3时,单独PS喷淋所达到的去除率和空白组相差无几,为50%~65%;单独O3作用时,OTC去除率为58%~80%,较空白组高6.2%~15.9%;同时存在O3和PS时,OTC去除率为81%~95%,较空白组高25.6%~31.5%,较单独O3时高13.9%~23.2%。PS需要O3、催化剂等活化剂活化后才能产生随着进气OTC浓度的增大,OTC的去除率随之升高。在O3和自由基氧化降解OTC的过程中,发生电子转移,产生以C为中心的自由基中间体。OTC在·OH自由基的攻击下,电子转移到—N(CH3)2的N原子上,产生胺基阳离子。α-C进一步去质子化,产生了以C为中心的自由基。另外,在OTC的仲醇氧化过程中,·OH自由基攻击OTC的C5后,转化为以碳为中心的碳自由基,反应中产生的有机物自由基可以继续参与·OH自由基生成的链式反应[26],增加了体系中·OH的量,并且这些有机物自由基也可能会对OTC有直接降解作用。随着进气OTC的增加,喷淋塔中的中间产物自由基也在增加,加速了OTC的降解进程。
2.3. ${\bf{SO}}_4^{ \cdot - }$![]()
![]()
和·OH 对OTC的去除作用
O3/PS较单独O3有更高效降解率的原因是,为了说明单独的O3是否存在间接氧化作用以及被EtOH淬灭后是否存在残余自由基,对·OH产量进行了测定实验。·OH会被对苯二甲酸捕获并生成强荧光物质2-羟基对苯二甲酸,产物的荧光强度和·OH浓度成正比[30]。由图5可知,2-羟基对苯二甲酸的荧光峰值出现在425 nm处,因此,本实验以425 nm处的荧光强度来表征·OH的最大生成量。荧光强度随时间的延长而增强,说明在反应器内·OH一直在生成,荧光产物得以累积。喷淋塔在只有O3的条件下,运行5 min后,喷淋液中2-羟基对苯二甲酸的荧光强度为173.3。这说明在O3单独存在时,2-羟基对苯二甲酸的荧光强度相对微弱,即·OH的产生量相对稀少。因此,O3对OTC废气6.2%~15.9%的去除率主要是由臭氧直接氧化产生的,靠臭氧产生·OH的间接氧化作用可以忽略。喷淋塔在O3和PS同时存在的条件下,运行5 min后,喷淋液中2-羟基对苯二甲酸的荧光强度为10 899。这说明有PS协同作用时,激发的
综上所述:OTC总去除率为81.5%~94.7%,喷淋液吸收的OTC占50.0%~65.1%,O3直接氧化作用占6.2%~15.9%;加入PS后,OTC的去除率增加了13.9%~23.2%,增加的这部分由8.6%~13.7%的·OH氧化作用和4.5%~7.5%的
2.4. 中间产物与反应过程机理探讨
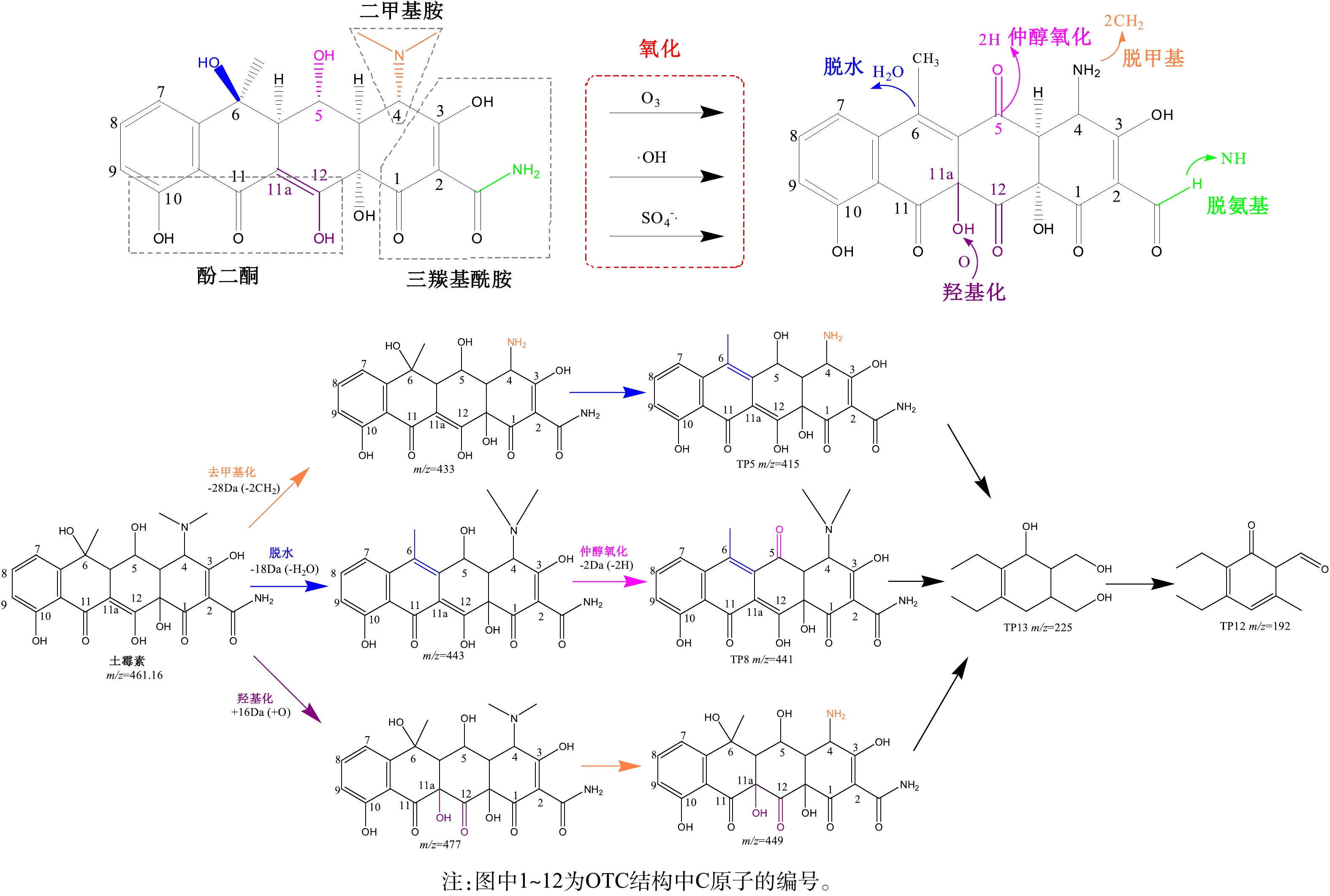
OTC经过O3/PS的氧化作用,主要生成了TP5C20H18N2O8(m/z=415.15)、TP8C22H20N2O8(m/z=441.13)、TP12C12H16O2(m/z=192.13)、TP13C13H21O3(m/z=225.19) 4种中间产物,结合OTC中间产物的分子离子和二级碎片的质谱信息,可推测出这4种主要中间产物的降解路径。O3/PS协同降解OTC的机理及途径如图6所示。
第1种途径是OTC C4所连接的二甲基胺[—NCH3)2]在·OH自由基的攻击下,电子转移到—N(CH3)2的N原子上,产生胺基阳离子。α-C进一步去质子化,产生了以C为中心的自由基,该自由基随后可以与氧化剂快速反应,然后生成亚胺。亚胺不稳定,快速水解生成m/z=433的中间产物[33]。在此基础上,C6所连羟基脱水得到TP5(m/z=415),再降解为TP13(m/z=225)和TP12(m/z=192)。
第2种路径是OTC的C6先脱水生成m/z=443的中间产物,·OH自由基攻击C5后转化为以碳为中心的碳自由基[26],随后加入氧生成过氧化物基团,氢过氧基团被消除后,再产生产物TP8(m/z=441)[32],再进一步降解为TP13(m/z=225)和TP12(m/z=192)。
第3种路径是O3攻击OTC的C11a和C12之间的双键,发生1, 3-偶极环加成反应[34],加入氧原子,生成m/z=477的产物,通过去甲基作用得到m/z=449的中间产物,再进一步降解为TP13(m/z=225)和TP12(m/z=192)。
2) O3/PS协同降解OTC废气的机制为:O3和PS协同产生·OH和
3) LC-TOF-MS/MS分析结果表明,C20H18N2O8、C22H20N2O8、C12H16O2、C13H21O3为OTC降解的主要中间产物。
参考文献





 下载:
下载: 
 点击查看大图
点击查看大图