全文HTML
--> --> --> 焦化废水是焦化、煤气化和焦化副产物回收过程中产生的一类典型工业废水,主要来自于炼焦过程中产生的剩余氨水、冷却水及其他污水[1],组分复杂,污染物浓度高、毒性大、易发生诱变和致癌[2-3]。焦化废水一般经氨汽提和溶剂萃取预处理后进一步进行生物处理[4-5],然而生化出水COD仍为200~300 mg·L?1[6],难于达到相关排放标准[7],需要进一步深度处理[8]。常见的深度处理技术有臭氧氧化、光催化氧化和Fenton氧化等。Fenton氧化方法在实际的废水深度处理中最为常用,主要通过H2O2/Fe2+在酸性条件下产生 · OH,以降解污染物[9]。然而H2O2利用率低,存在投药量大、反应效率差等缺点[10]。为此,近年来探索了许多类芬顿技术,如光芬顿法[11-12]利用与亚铁离子协同催化H2O2的方式提高处理效果,但反应太阳能利用率低,能耗大;电芬顿法[11, 13]利用O2在电解池阴极产生的H2O2与亚铁离子反应,生成 · OH和Fe3+,但阴极材料电流效率低,H2O2产量不高。为此,以Fenton反应为基础的相关处理技术仍在研究中,如微波与Fenton、Fe-EDTA的联合等[12, 14],在研究中发现,这些类芬顿技术可以针对性地克服常规Fenton存在的反应效率低等问题,以达到更有效的处理效果。
微波是一种超高频电磁波(0.3~300 GHz),为避免干扰雷达和电信频率,用于工业和民用的频率分别为915 MHz和2 450 MHz,其中915 MHz的微波穿透深度要高于2 450 MHz近3倍[15]。当微波辐射与Fenton联用时,由于微波穿透性强,能直接加热反应物分子,降低反应的活化能和分子的化学键强度,因此,可显著提高Fenton反应活性,从而降低Fenton反应时间、增强反应效率[16]。陈艳芳等[17]采用微波诱导Fenton降解水中对硝基氯苯,对硝基氯苯和COD的去除率分别可达98.9%和90.8%;李硕等[18]采用微波强化Fenton方法降解了水中的BPA,发现可减少H2O2和Fe2+的投加量,促进 · OH的生成。但微波强化Fenton反应对于不同实际废水的处理效果及其对应的反应机理有待于进一步的研究。
本研究以焦化废水的生化出水作为研究对象,探讨了利用微波强化Fenton处理的效果和应用的可行性。在优化且确定Fenton反应条件的基础上,对同等实验条件下微波强化Fenton反应与Fenton反应的处理效果进行了比较,并初步探讨了在微波引入后Fenton处理效果显著提升的可能机理,可为目前我国焦化废水处理和达标排放处理技术的选择提供参考。
1.1. 材料与试剂
实验水样取自内蒙古乌海某焦化厂生化出水(原水)。该厂采用A/O二级处理方法,水样取回后置于4 ℃保存。水样pH为8.4±0.2,COD为(203±5) mg·L?1,TOC为(34.0±2.0) mg·L?1,氨氮为(4.45±0.3) mg·L?1,总氮为(83.6±2.3) mg·L?1,色度为836±43。实验所使用的FeSO4·7H2O,30% H2O2,NaOH,98% H2SO4等均为分析纯,购自国药集团化学试剂有限公司。
1.2. 实验方法
首先对Fenton氧化的处理效果进行评价,相关药剂投量的比例参考文献中的方法[19]进行优化。采用批量实验的方式进行,分别取200 mL生化出水水样于5个烧杯中,用3 mol·L?1的H2SO4溶液调节pH至3,加入Fe2+1.8 mmol·L?1,分别按照摩尔比1∶2,1∶4,1∶6,1∶9,1∶10投加量加入H2O2。置于多位磁力搅拌器(WH-410D,德国Wiggens公司)上搅拌反应78 min,调节pH至7,终止Fenton反应。将反应后的焦化废水置于数显恒温水浴锅(LUX-12,北京陆希科技有限公司)内,在50 ℃下加热30 min,以去除残留的H2O2[20]。实验中采用的微波反应体发射频率为915 MHz,装机功率为5 kW,实用功率为0.9~1.1 kW,采用半批量运行的方式进行实验。将实验用水用3 mol·L?1 H2SO4溶液调节pH至3,同时加入一定浓度的Fe2+和H2O2 (分别为1.8 mmol·L?1和15.6 mmol·L?1),在微波反应体内开启电磁辐照,反应18 min,之后进入深度反应池慢速搅拌60 min,采集相关样品进行分析。
1.3. 分析方法
测定TOC和色度所用水样均过0.45 μm滤膜。分别对样品COD (NOVA 60,德国MERCK)、TOC (TOC-Vcph,日本岛津)、色度(LICO 620,美国HACH)和pH (AS600,日本ASONE)等指标进行测定;使用荧光分光光度计(F-7000,日本日立)测定三维荧光光谱。用滤纸过滤100 mL混匀的Fenton反应出水及微波-Fenton反应出水,将滤纸放入电热恒温鼓风干燥箱(DGG-9070B,上海森信),在105 ℃条件下烘干1 h,置于干燥皿中,冷却后测定反应产生的污泥量。
取混匀的Fenton反应出水及微波-Fenton反应出水静置沉淀30 min,在转速为1 200 r·min?1的条件下离心6 min,将上清液倒出,用冷冻干燥机(FD-1A-50,北京博医康实验仪器有限公司)于?80 ℃进行冷冻干燥[21],然后用红外光谱仪(Nicolet iZ10,美国赛默飞世尔)测定铁泥的红外光谱。
2.1. H2O2投加量对Fenton反应效果的影响
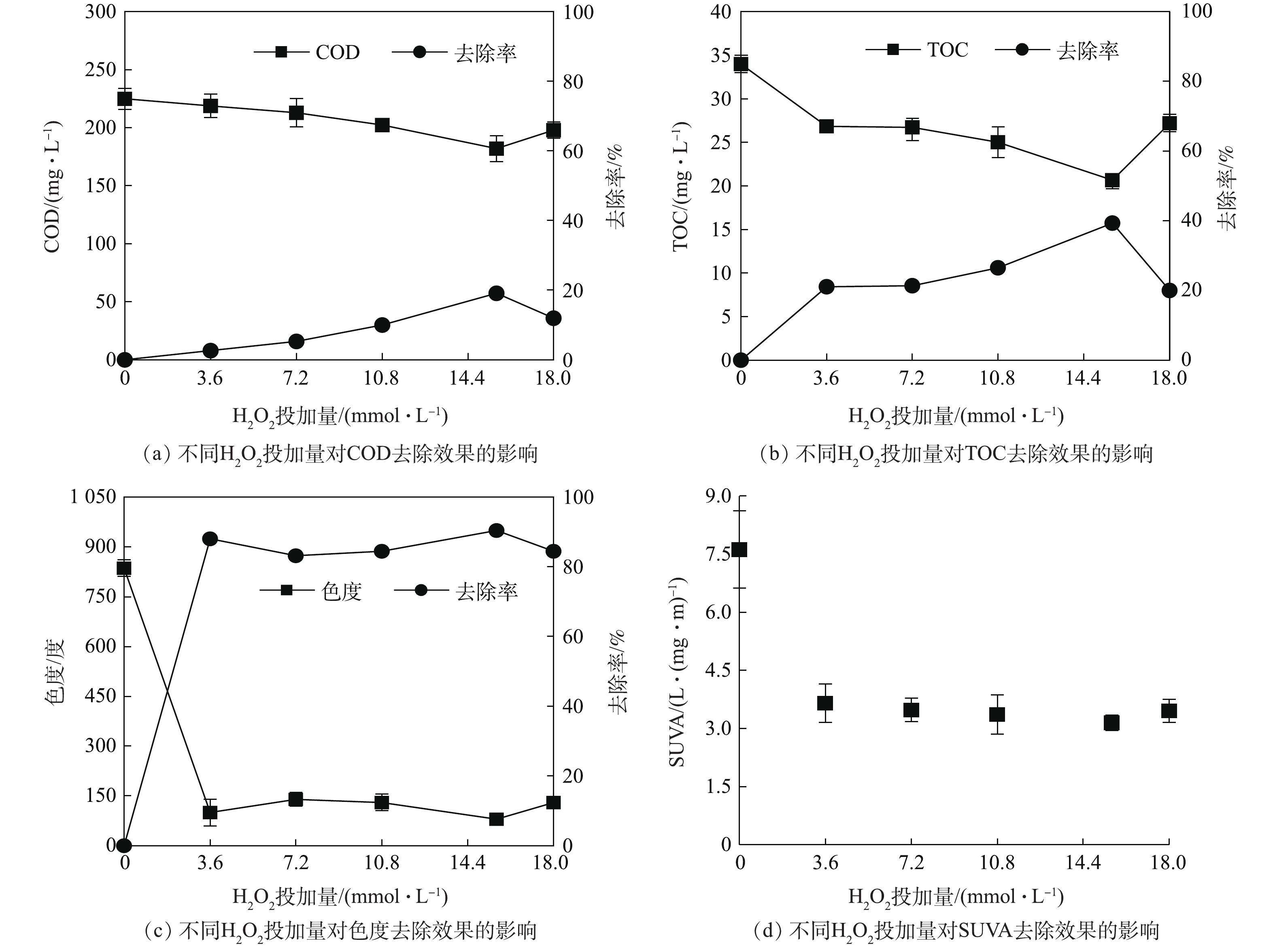
Fenton反应主要通过氧化作用和混凝作用来去除污染物,影响因素包括pH、Fe2+浓度和H2O2的浓度。为考察不同H2O2投加量对Fenton反应的影响效果,本实验在pH为3,Fe2+为1.8 mmol·L?1的条件下,分别按1∶2,1∶4,1∶6,1∶9,1∶10摩尔比投加H2O2,反应时间为78 min。图1为在不同H2O2投加量条件下,Fenton反应对废水的处理效果。由图1可知,随着H2O2投加量的增加,反应对有机物(以COD和TOC计)的去除呈现先增大后减小的趋势。 · OH是Fenton反应中氧化去除有机污染物的主要活性氧化物种,其生成量受Fe2+和H2O2的影响[22-23]。当H2O2投加过量时,H2O2会与生成的 · OH发生进一步的反应,从而降低其氧化作用[24];同时,H2O2能将Fe2+氧化为Fe3+,阻碍氧化反应的进行,降低了 · OH的生成量[25]。因此,在Fenton反应过程中,Fe2+和H2O2的投加比例对于处理效果有着重要影响。从色度处理效果来看,较低H2O2投加量(3.6 mmol·L?1)下,即可达到一定的处理效果,SUVA的变化趋势与色度相一致,说明导致色度的多为芳香类的化学物质[26],此类物质相对容易氧化去除。在Fe2+浓度为1.8 mmol·L?1、H2O2为15.6 mmol·L?1的条件下,可对COD和TOC达到最优的去除效果,其去除率分别为18%和39%。后续的相关实验均在此条件下开展。2.2. 微波强化Fenton处理效果
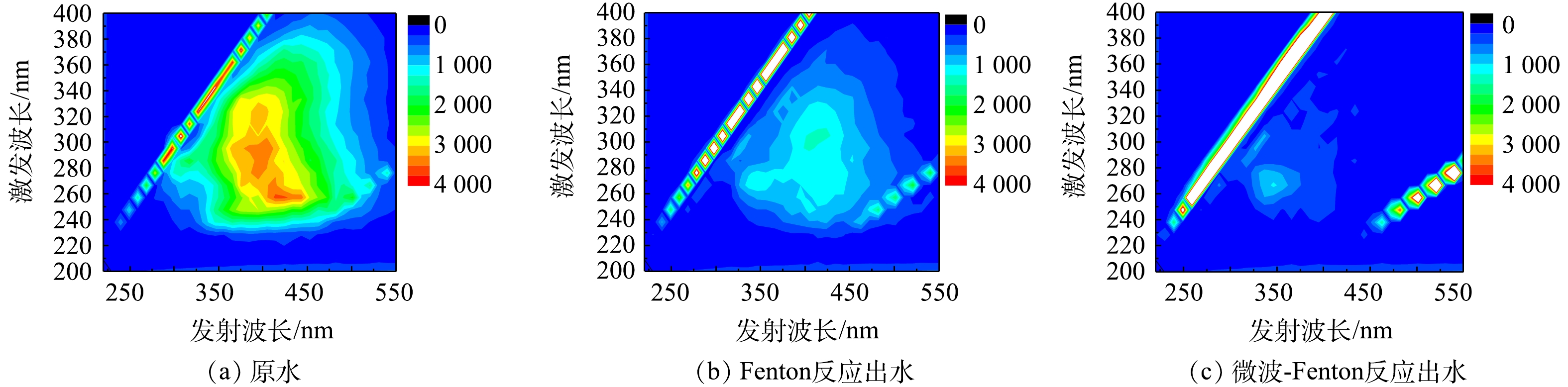
为探讨微波强化Fenton反应的处理效果和稳定性,在pH=3,Fe2+和H2O2的浓度分别为1.8 mmol·L?1和15.6 mmol·L?1的条件下,进行了连续7 d的实验,其中电磁辐照18 min,深度反应池慢速搅拌1 h进行反应。图2为连续运行7 d后微波强化Fenton反应前后TOC、COD以及色度的变化结果。与Fenton处理效果相比,加入微波处理后的效果显著提升,对于COD的去除率从18%左右提升至75%左右,TOC从40%左右提升至65%左右,且去除效果稳定,COD出水稳定在80 mg·L?1以下,达到了《炼焦化学工业污染物排放标准》 (GB 16171-2012)中直接排放的要求。色度的芳香类物质虽相对容易氧化去除,但与Fenton处理效果相比,仍有一定的提升。另外,在实验过程中,限于条件的限制,未对相关微波参数(辐射时间等)等进行优化,后续将进一步针对相关条件和影响因素进行研究确认。图3为原水及经过Fenton和微波强化Fenton处理后的三维荧光光谱图。溶解性有机物的荧光光谱可分为5类[27]:芳香类蛋白质(Ex为200~250 nm,Em为280~330 nm);芳香类蛋白质(Ex为220~250 nm,Em为330~380 nm);富里酸(Ex为220~250 nm,Em为380~480 nm);微生物渗滤液(Ex为250~280 nm,Em为280~380 nm);胡敏酸(Ex为250~400 nm,Em为380~480 nm)。由图3可知,与原水相比,Fenton反应出水中残留的溶解有机物主要为胡敏酸和微生物渗滤液类物质,而经微波-Fenton处理后出水的荧光强度极微弱,仅有较弱的微生物渗滤液类物质的荧光信号,这表明多数有机物已被氧化去除。
2.3. 微波强化Fenton反应中的可能机理
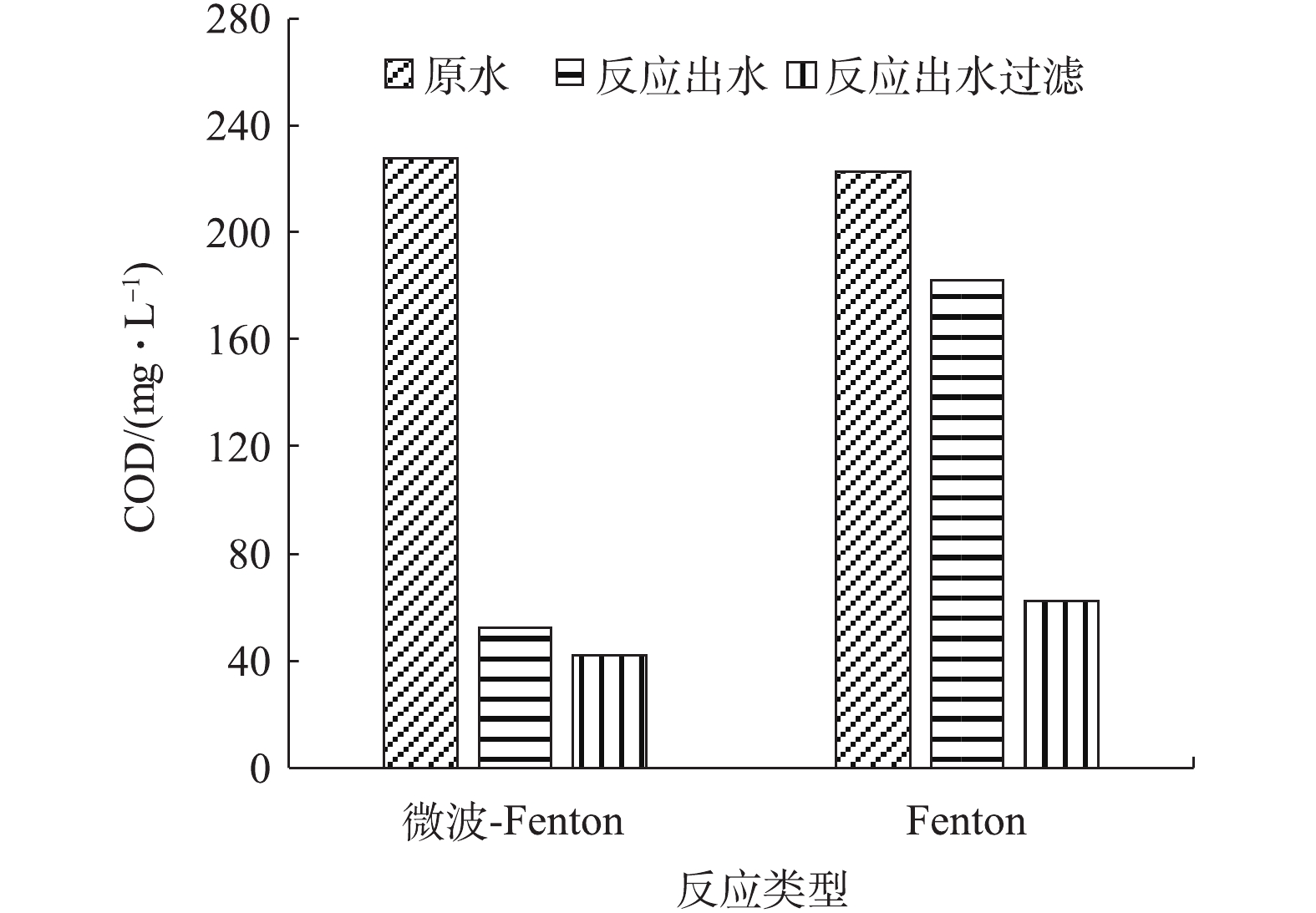
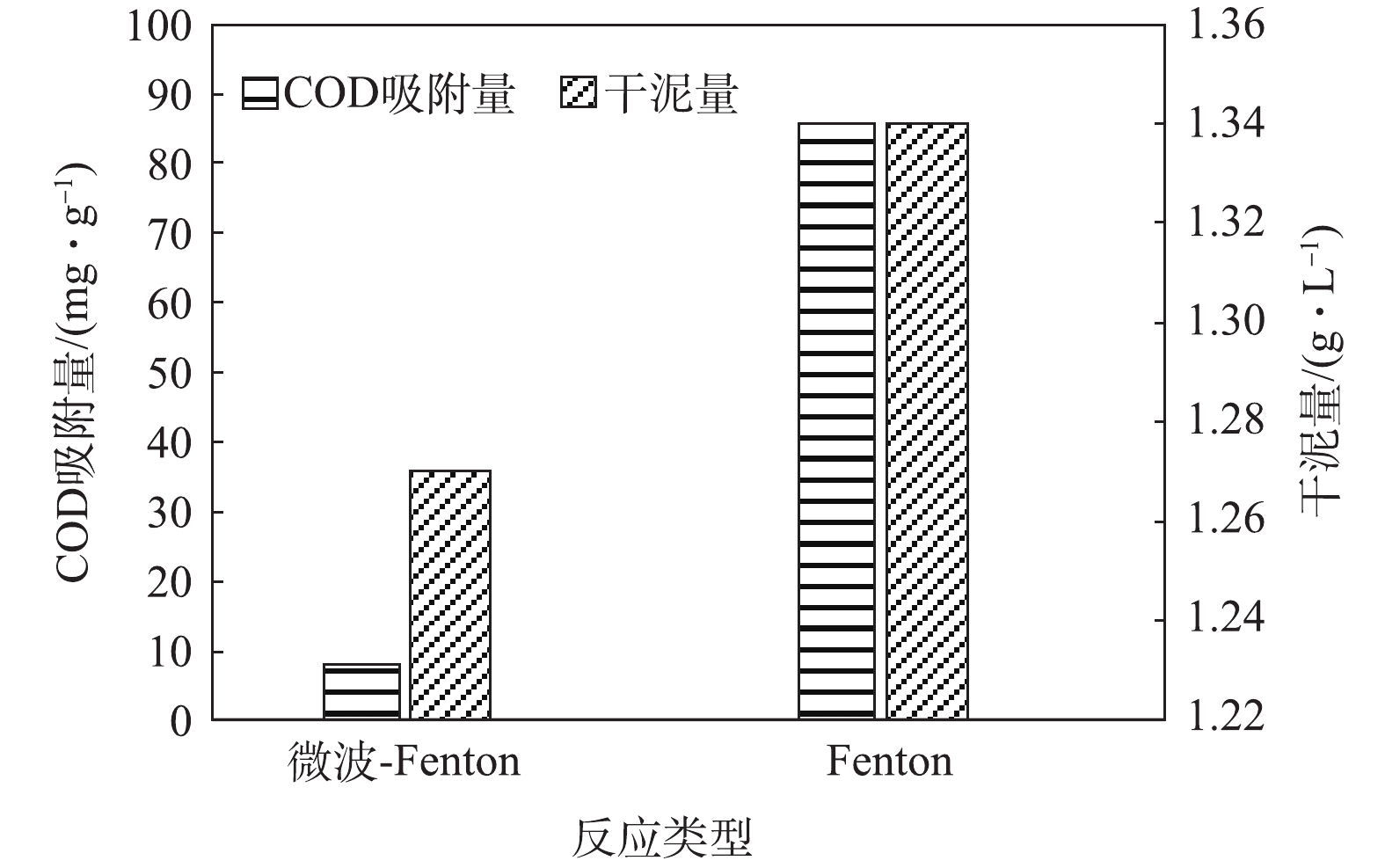
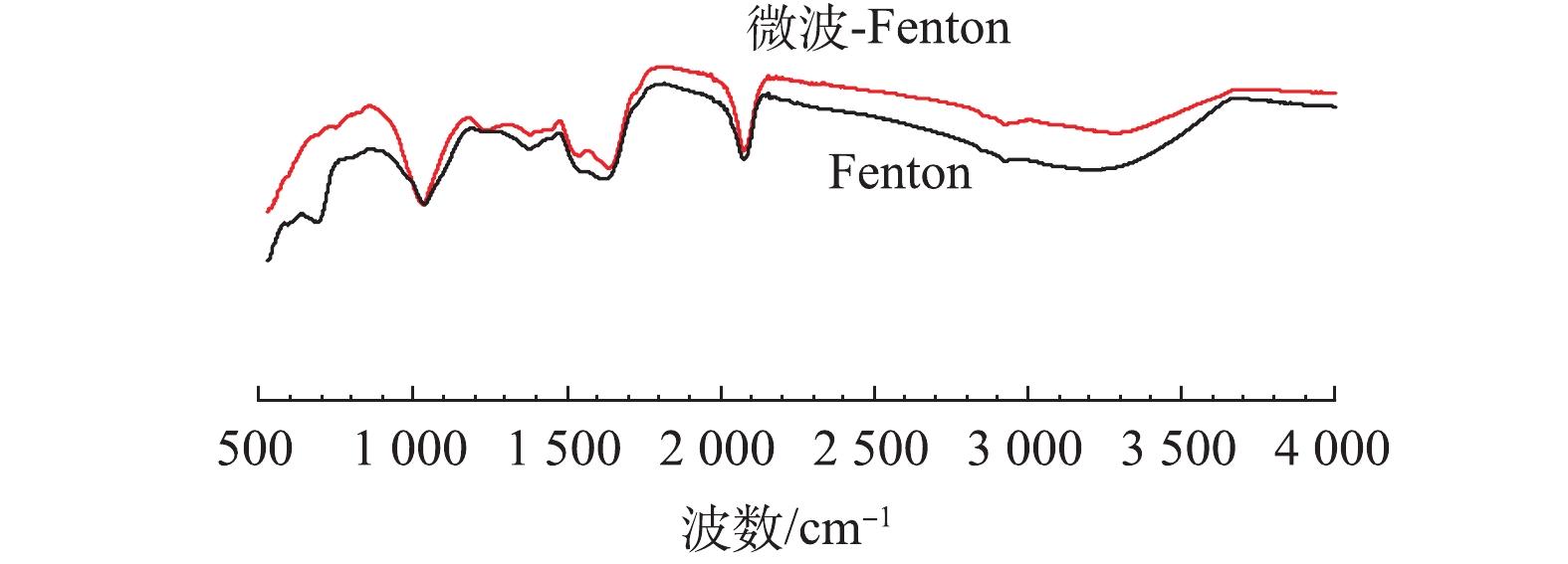
氧化及吸附是Fenton反应去除污染物的主要过程。为进一步比较Fenton及电磁强化Fenton处理的效果,对反应出水过滤(0.45 μm滤膜)前后的COD进行了比较测定,结果见图4。由图4可知,Fenton反应出水的COD为182 mg·L?1,去除率较低(18%),但过膜后的COD为61 mg·L?1,其去除率可达到72%,这表明大部分COD可吸附在泥相中;而微波强化Fenton反应后的出水COD为52 mg·L?1,对COD的去除效率达到77%,过滤后的出水COD为42 mg·L?1,这表明大部分有机物被氧化去除。图5反映了在2种处理方式下,过滤后所得泥相中吸附的COD量,微波强化Fenton和Fenton反应产生的干泥量分别为1.27 g·L?1和1.34 g·L?1,泥相中COD吸附量分别为8.07 mg·g?1和85.73 mg·g?1。由此可见,Fenton反应出水中的过滤物中吸附了大量COD,而对于微波强化Fenton处理来说,有机物的去除主要依靠氧化进行,降低了泥相中COD的残留量。由此可知,微波强化Fenton反应可显著提高Fenton反应的氧化能力。从反应机理上来说,微波具有的热效应能使极性分子产生高速旋转碰撞,从而改变体系的热力学函数,降低反应的活化能和分子的化学键强度[28]。另外,微波的非热效应能振荡微波场中的极性分子,使其化学键断裂[29-31],从而提高了Fenton反应的氧化能力。图6为过滤物(铁泥)的红外光谱图。由图6可见,2种反应体系中铁泥有明显较宽的O—H伸缩振动谱带,吸收峰为3 540~2 715 cm?1,表明含有COOH、醇或苯酚中O—H的吸收峰。在2 965~2 850 cm?1处,存在微弱的CH3反对称伸缩振动吸收峰与烷烃CH2反对称和对称伸缩振动吸收峰。在2 500~1 900 cm?1处,有一较强的吸收峰,这是C≡C和C≡N伸缩振动以及C=C=C、C=C=O等累积双键不对称伸缩振动。2种反应出水在(1 600±10) cm?1、1 500~1 450 cm?1处存在明显的芳环C=C伸缩振动,这说明芳香烃类化合物的存在。在1 135 cm?1附近出现较强的硫氢C=S伸缩振动吸收峰。2种反应中出水铁泥所含物质基本相同,大致是芳香族和硫氢化合物,不同之处在于,Fenton反应中铁泥在625 cm?1附近出现硫氢C—S伸缩振动吸收峰,这进一步说明微波-Fenton反应能直接氧化去除含C—S键的物质,而Fenton反应则是通过吸附在铁泥中将其去除[32-33]。
2)氧化是微波强化Fenton处理过程中有机物去除的主要机理,可能与微波辐射通过热效应或非热效应加快羟基自由基的生成、氧化反应效率的提高有关。Fenton处理后泥相中的吸附对于有机物的去除有重要贡献。红外分析结果表明,吸附作用对象主要是芳香族和硫氢化合物。
3)微波强化Fenton方法是焦化废水达标排放的一种可供选择的技术,后续将对相关反应条件、特征污染物的去除以及工艺适用性开展进一步的研究和优化。
参考文献

 下载:
下载: 
 点击查看大图
点击查看大图