全文HTML
--> --> --> 近年来,我国自然水体中砷和磷复合污染案例屡见不鲜。砷(As)及其化合物主要通过人类工农业生产及地球化学循环而进入地表及地下水中。长期饮用高砷水会对人类的生理健康造成严重的危害,故地表水砷污染导致的饮用水健康风险问题受到人们的广泛关注[1-2]。因此,世界卫生组织(WHO)、美国环境保护署(EPA)等众多组织将饮用水中的砷含量限定为10 μg·L?1以下[3-5]。此外,水体富营养化也对我国各类水体(主要是湖泊、水库及河流城市河段)造成了严重威胁,而磷是引起水体富营养化的主要因子之一[6-8]。有研究表明,环境中磷和砷存在着竞争关系,砷容易置换出磷,从而被细胞吸收导致中毒[9-10]。因此,从环境治理、资源回收等角度考虑,有必要对污染水体中的砷和磷进行控制。在现有砷、磷去除技术中,吸附法作为较成熟的除砷及除磷方法之一,具有处理效果好、经济安全、操作简单等特点,在小城镇及农村等分散供水地区水处理技术中具有明显优势[11-12]。近年来,新型吸附剂的开发成为国内外****的研究重点。其中,介孔材料具有均一的孔径、较大的比表面积、稳定的骨架结构和易于修饰等优点,在环境治理领域已得到广泛的运用[13-15]。目前,已有研究报道吸附法单独去除水中的磷或砷时均有良好的效果[16-17],但是,对于砷、磷共存情况下对吸附剂性能的研究还较少。因此,本研究通过镧金属掺杂对介孔材料进行改性,合成了La-MCM-41吸附剂,考察了其同步去除砷、磷复合污染的性能,以期为同步去除环境水体中砷和磷提供参考。
1.1. 实验原料
十六烷基三甲基溴化铵(C19H42BrN, CTAB)、正硅酸乙酯(C8H20O4Si, TEOS)、硝酸镧(La(NO3)3·xH2O)、磷酸二氢钾(KH2PO4)、钼酸铵((NH4)6Mo7O24)、L-抗坏血酸(C6H8O6)、酒石酸氧锑钾(C4H4KO7Sb·0.5H2O)、乙二胺四乙酸二钠(C10H14N2O8Na2)、甲酸(CH2O2)、硫脲(CH4N2S)、氢氧化钠(NaOH)均为分析纯。1.2. MCM-41的制备
本研究模板剂采用十六烷基三甲基溴化铵(CTAB),硅源采用正硅酸乙酯(TEOS),并按照TEOS∶CTAB∶NaOH∶H2O = 1∶0.1∶0.24∶100的摩尔配比进行实验。具体步骤:将NaOH溶于去离子水中,加入相应量的CTAB,36 ℃下磁力搅拌至溶液澄清,剧烈搅拌下逐滴加入TEOS;调节溶液pH = 9~11,混合体系剧烈搅拌2 h,将所得乳白色凝胶转入聚四氟乙烯晶化反应釜中,120 ℃下晶化24 h;取出冷却至室温,过滤、洗涤、干燥,得MCM-41原粉;将其置于马弗炉中,升温至550 ℃,焙烧6 h,制得MCM-41介孔材料。1.3. La-MCM-41的制备
本研究模板剂采用十六烷基三甲基溴化铵(CTAB),硅源采用正硅酸乙酯(TEOS),硝酸镧为镧源,以La/Si = 0.03的量掺杂金属离子。具体步骤同MCM-41的制备。1.4. 样品表征
采用X射线衍射(德国布鲁克公司D8ADVANCE型,Cu靶,扫描角度为1°~10°)对介孔材料晶体结构进行测定;采用比表面积与孔隙度吸附仪测定介孔材料改性前后比表面积、孔容及孔径分布(美国麦克公司ASAP2460型);采用扫描电子显微镜观察介孔材料改性前后微观形貌(FEI公司NANO SEM430型)。1.5. 单独吸附体系
分别配制As(V)、1.6. 同步吸附体系
配制As(V)、P浓度均为20 mg·L?1的混合溶液[18-19],取50 mL混合溶液倒入具塞锥形瓶中,吸附剂投加量为0.01 g,调节溶液pH为5.0。将锥形瓶置于恒温振荡箱中,调节温度为15、25、35、45 ℃,在转速为150 r·min?1条件下,振荡24 h,反应过程中保持pH稳定。反应结束后,取水样,过0.45 μm滤膜,采用日本岛津公司ICPS-7510 PLUS电感耦合等离子体原子发射光谱仪分别测定As(V)和P的浓度。2.1. 结构表征
2.1.1. X射线衍射
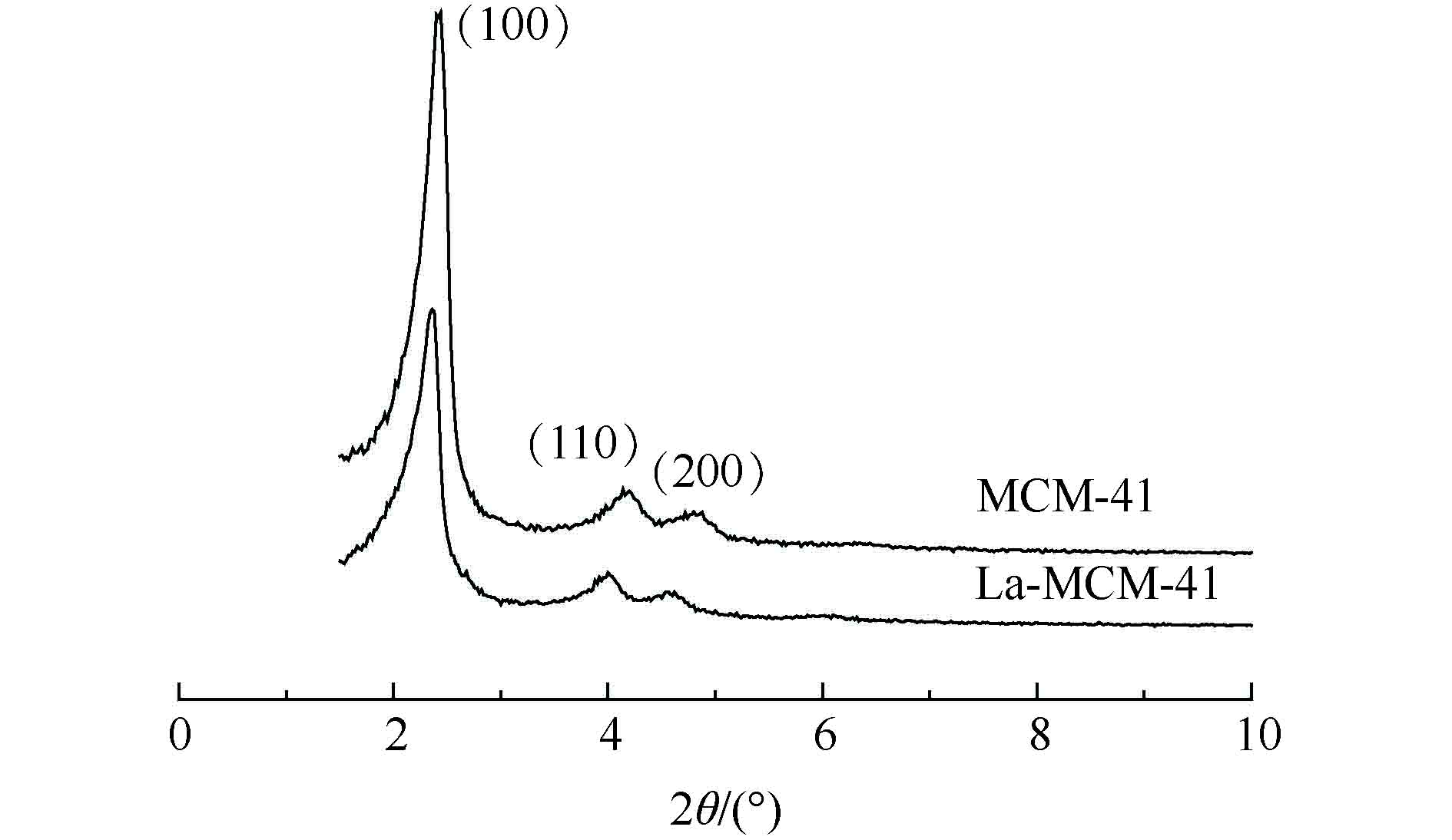
图1是MCM-41及La-MCM-41的XRD衍射图。由图1可见,2组材料在小角范围内都有3个明显的衍射峰。其中,在2θ=2.2°附近有1个较强的衍射峰,对应于介孔分子筛(100)晶面;在2θ=4.0°、4.3°附近出现2个强度相对较弱的衍射峰,分别对应于介孔分子筛(110)、(200)晶面。这与已有报道[20-21]中关于介孔材料的结构特征峰位置的结果一致,说明本研究制备的MCM-41和La-MCM-41都具有长程有序的六方相介孔结构特征,掺杂镧之后的介孔材料和MCM-41具有相似的结构特性。经La改性后的MCM-41的特征峰强度有明显的减弱,而且相应衍射峰2θ角向低角度偏移,这表明,骨架中镧的引入会改变分子筛的表面结构,从而降低了材料的有序性。2.1.2. 比表面积及孔结构分析
通过N2-吸脱附测试,可以表征多孔材料孔道结构等信息。MCM-41和La-MCM-41的N2吸脱附曲线和孔径分布如图2所示。2种材料的N2吸脱附等温线均为Ⅳ型[22],属于典型的介孔材料吸附曲线,镧掺杂后也没有明显改变介孔材料的结构特征,但La-MCM-41的比表面积和孔容积都有所减小,而孔径有所增加。其中比表面积从1 193.68 m2·g?1减小到812.27 m2·g?1,孔容从0.71 cm3·g?1减小到0.64 cm3·g?1,孔径从2.38 nm增加到3.18 nm,这是因为镧掺杂后占据了部分孔道,影响了原有孔道结构[23]。2.1.3. 扫描电镜
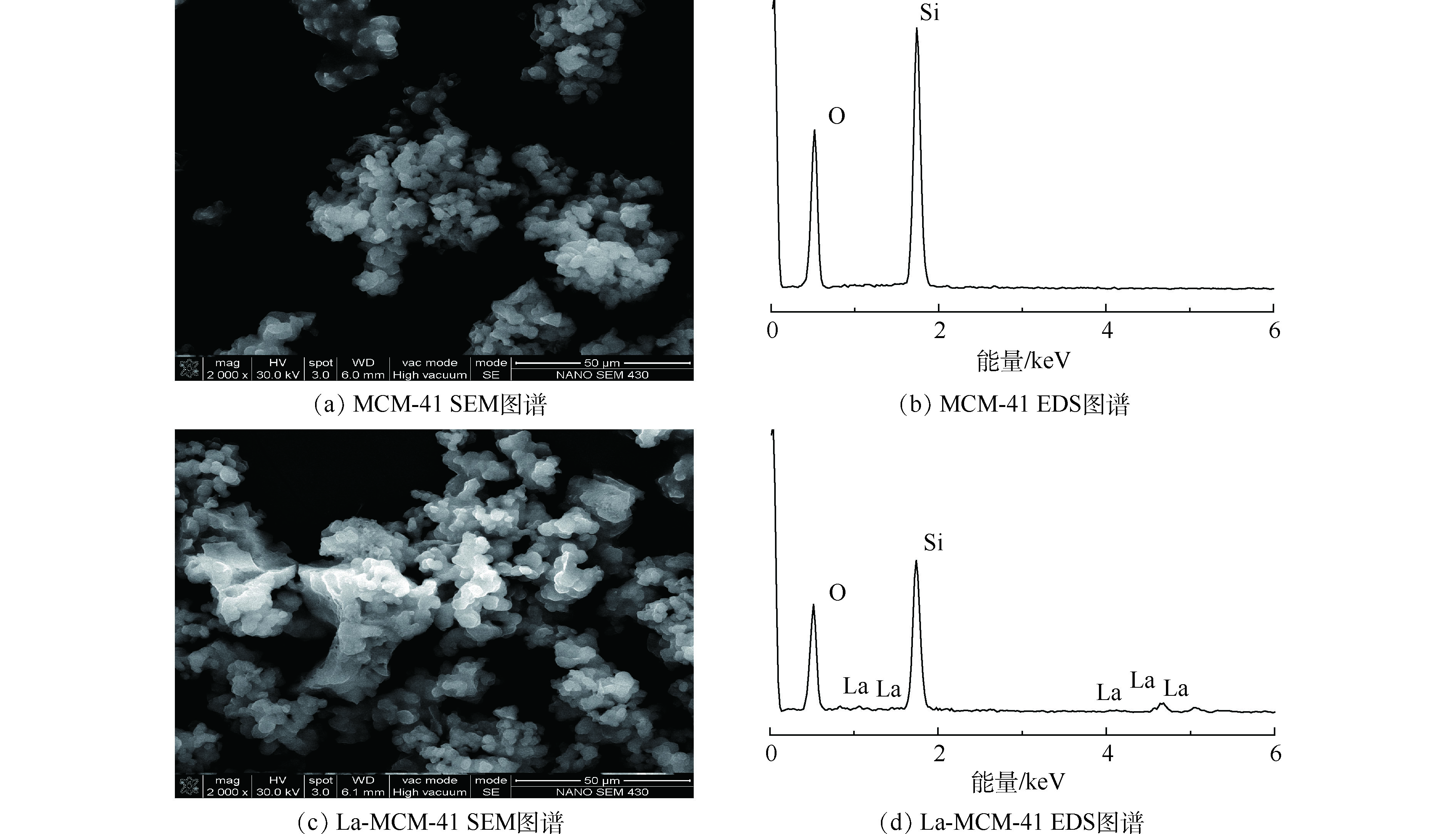
图3为MCM-41和La-MCM-41的扫描电镜(SEM)和X射线能谱分析图(EDS)。从图3中可以看出,MCM-41和La-MCM-41的颗粒外形均呈现颗粒状,改性并未改变介孔材料的形貌特征。对比二者发现,MCM-41介孔吸附材料颗粒粒径较小,团聚后呈现一些不规则的形状结构。La-MCM-41介孔分子筛外形轮廓粗糙,并出现了团聚后的大颗粒。改性前后EDS图也呈现出差别,改性后的吸附剂硅含量减少,同时出现镧元素,说明镧成功取代硅,掺杂到介孔材料中。2.2. 吸附剂的筛选
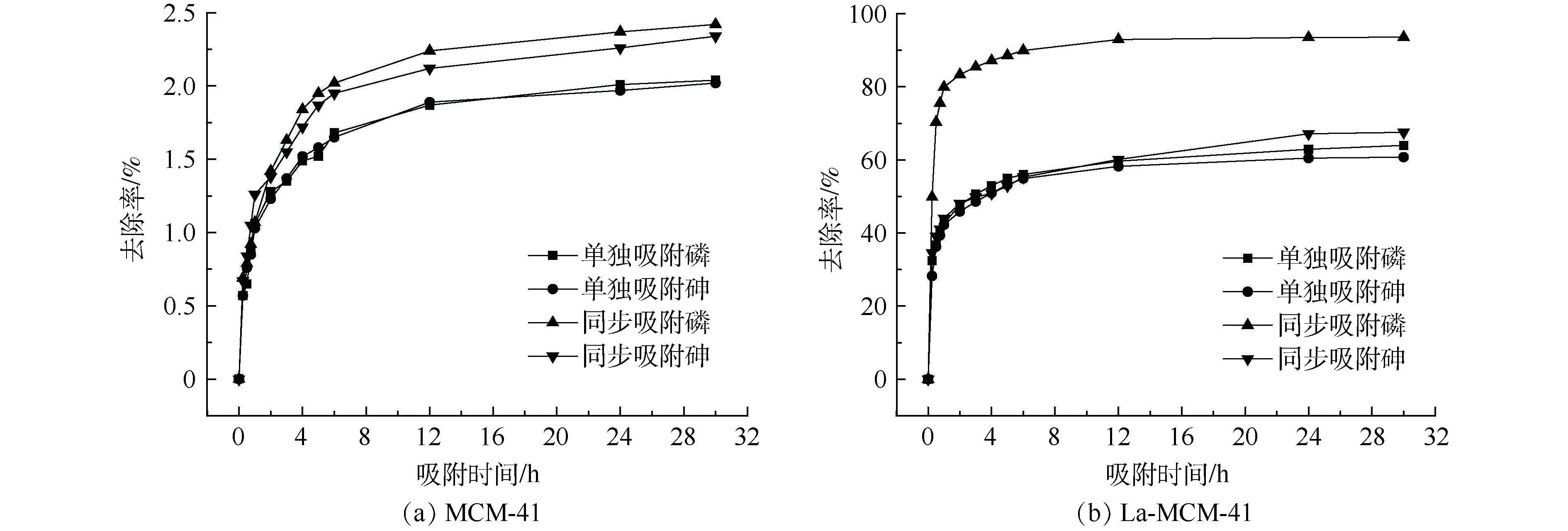
本研究考察了吸附剂改性前后对砷、磷吸附效果的影响,结果如图4所示。未经过改性的介孔吸附剂MCM-41对溶液中的砷、磷几乎没有吸附作用,单一吸附和同步吸附体系中砷、磷的去除率均低于2.5%。这主要是因为,未经过改性的MCM-41本身几乎不含活性位点;而经过改性的La-MCM-41吸附剂对砷、磷具有良好的吸附效果,吸附去除率大大提高。由此可见,介孔吸附材料对砷、磷吸附效果的提升是由于La元素的加入,La的加入使得吸附剂上活性吸附位点增加,故对砷、磷的去除率也提高。且与其他常用吸附剂相比(见表1),本研究的La-MCM-41对砷、磷的吸附效果更突出[24-28],表明La-MCM-41是一种能够同步去除砷、磷的良好吸附剂。2.3. 吸附动力学研究
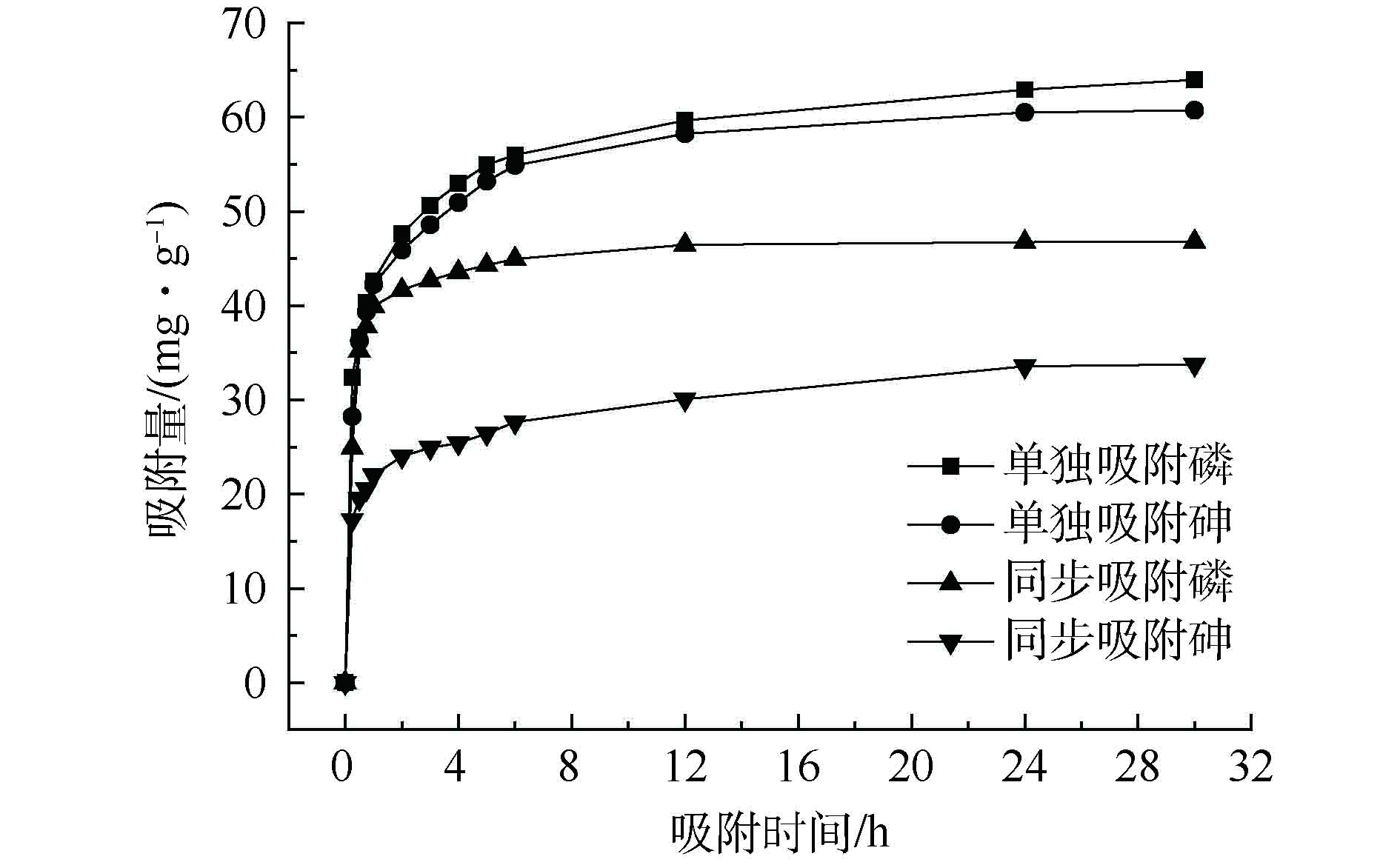
图5是在25 ℃条件下La-MCM-41对目标污染物的动力学吸附结果。La-MCM-41对目标污染物的吸附过程可分为2个阶段:快速反应阶段,由于溶液与吸附剂表面的浓度梯度较大,吸附剂对目标污染物的吸附量快速上升;慢速反应阶段,由于吸附剂表面位点被大量占据,目标污染物吸附量升高速度缓慢,到24 h基本达到平衡,随着平衡随着吸附时间的增加,吸附剂上的吸附点位被不断占用,吸附速率减慢从而达到平衡。从图5中还可发现,单一吸附体系中的吸附量均要高于同步吸附体系,其中,P的吸附量高于As(V)。采用准一级和准二级动力学吸附模型对实验数据进行了拟合[29]。准一级和准二级动力学模型的数学方程如式(1)和式(2)所示。
式中:k1为准一级动力学速率常数,min?1;k2为准二级动力学速率常数,g·(mg·min)?1;t为反应时间,min;Qe为反应平衡时吸附剂对吸附质的吸附量,mg·g?1;Qt为反应时间t时吸附剂对吸附质的吸附量,mg·g?1。
结合表2及图5可看出,准一级动力学方程R2 > 0.9,准二级动力学方程R2 > 0.99,准二级动力学方程的拟合性更好,并且准二级动力学方程模拟得到的平衡吸附量更加接近于实际测定值,综合来看,La-MCM-41对As(V)、P的吸附更加符合准二级动力学模型。
采用阿累尼乌斯方程计算反应活化能[30],阿累尼乌斯方程如式(3)所示。
两边取对数改写为式(4)。
式中:K为温度为T时的反应速度常数,min?1;Ea为反应活化能,kJ·mol?1;T为热力学温度,K;R为摩尔气体常数,8.314 J·(mol·K)?1;A为指前因子。
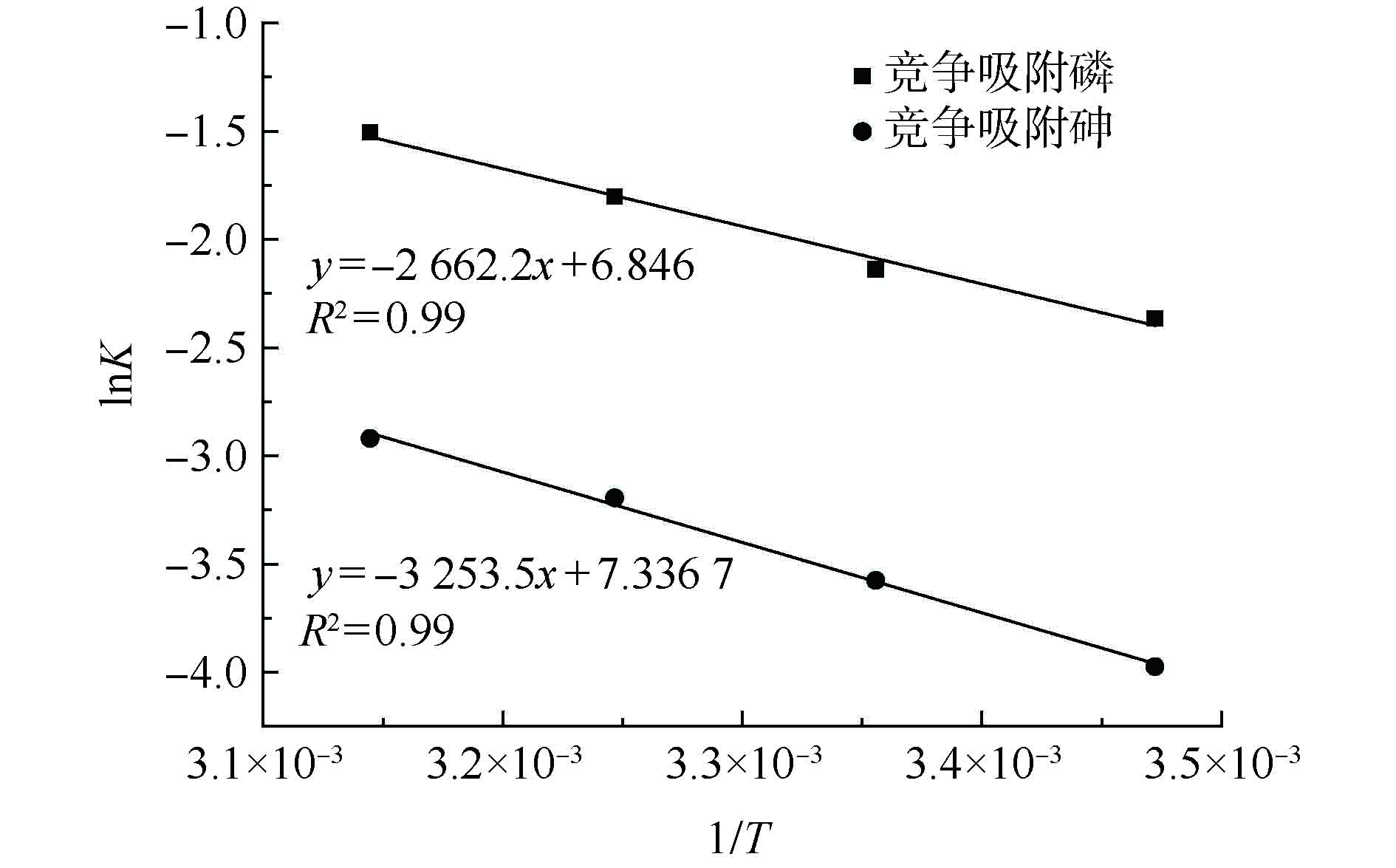
图6是同步吸附条件下La-MCM-41对P、As(V)吸附的阿累尼乌斯方程的拟合情况。根据图6中拟合曲线的直线方程,得出活化能Ea,La-MCM-41对砷和磷的同步吸附活化能分别为27.05 kJ·mol?1和22.68 kJ·mol?1。对比二者可发现,在同步吸附条件下,La-MCM-41对As(V)的吸附活化能稍高于对P的吸附活化能。这说明La-MCM-41对As(V)的吸附需要更多的能量来越过能垒,而对P的吸附相对更容易发生。因此,在同步吸附条件下,磷更容易被吸附在La-MCM-41的位点上,在同步吸附体系中占据优势地位。
2.4. La-MCM-41 对 As(V)、P 的吸附等温线
图7考察了25 ℃下La-MCM-41分别对不同初始浓度的As(V)、P的吸附效果。由图7可知,La-MCM-41对As(V)、P的等温吸附线呈现类似的变化过程,吸附量随着初始浓度的增加而增加,当初始浓度增大到一定程度时,吸附曲线增长速率减缓;达到平衡时,同步吸附时P的最大吸附容量可达到160.07 mg·g?1,As(V)的最大吸附容量为126.75 mg·g?1。两者吸附量的差异可能是因为砷酸根离子的分子大小比磷酸根离子大,砷酸根比磷酸根离子的直径大,因此,单位表面积的砷酸根离子的吸附量会低于磷酸根离子。上述结果与茹春云[30]的报道结果相似。分别采用Langmuir方程和Freundlich方程[31-32]对吸附等温线进行拟合,拟合所得参数如表3所示。
式中:Ce为平衡时溶液浓度,mg·L?1;qe为平衡吸附量,mg·g?1;qm为吸附剂最大吸附量,mg·g?1;KL为Langmuir平衡常数,L·mg?1;KF为Freundlich吸附常数;1/n为浓度常数,其值越接近1,说明吸附等温线的线性程度越高,1/n值小于1,说明吸附过程属于优惠吸附。
La-MCM-41对As(V)、P的等温吸附数据拟合结果均能较好地符合Langmuir方程和Freundlich方程,除单独吸附P,吸附对Freundlich方程的拟合性更好,故推测La-MCM-41对As(V)和P的吸附介于单分子层和多分子层吸附。同时根据公式(7)可知,RL值均介于0与1之间,说明此吸附过程为有利吸附,且RL值随着浓度的增加而减小,更加说明提高砷和磷的浓度有利于吸附的进行。
式中:C0为起始砷(磷)浓度,mg·L?1;KL为Langmuir平衡常数,L·mg?1。
从表3可以看出,单一吸附体系和同步吸附体系下P的最大吸附量和KF值均大于As(V)的最大吸附量和KF值,说明La-MCM-41对P的吸附能力优于对As(V)的吸附能力。
2)La-MCM-41对砷、磷的吸附能够在12 h内达到平衡。通过动力学研究分析可发现,在同步吸附体系中,La-MCM-41对2种污染物质的吸附能力具有一定的差距,其对磷的吸附能力比砷强,磷在同步吸附中占据优势,该过程符合准二级动力学模型。
3)采用Langmuir和Frreundlich方程对等温吸附数据进行拟合分析发现,La-MCM-41对磷、砷的吸附过程介于单分子层与多分子层之间,且在2种体系中n值均大于1,说明La-MCM-41对磷、砷的吸附均为优惠型吸附。相比单一吸附体系,同步吸附体系中La-MCM-41对2种离子的饱和吸附量均有不同程度的降低,其中As(V)吸附量下降较多。
参考文献

 下载:
下载: 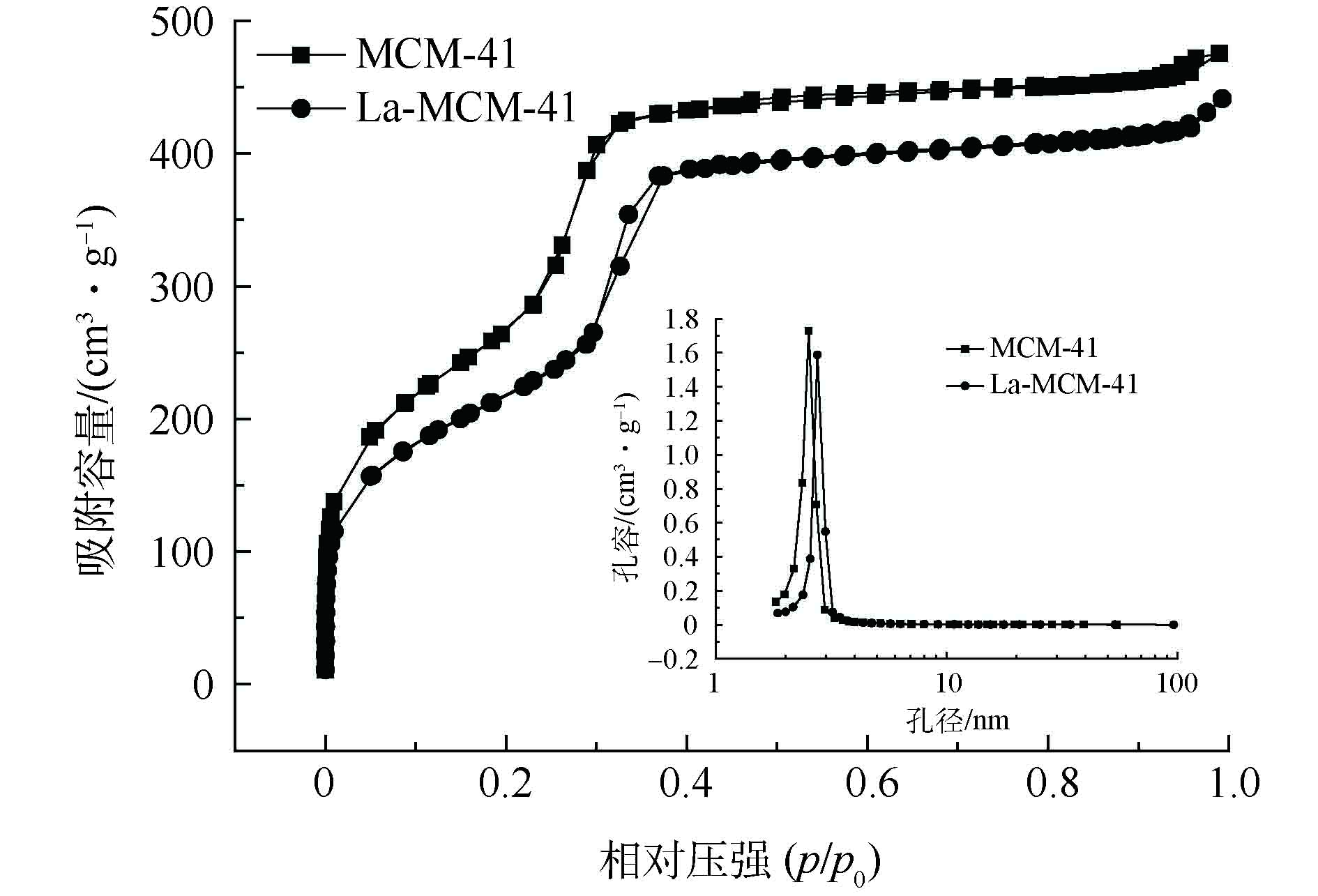

 点击查看大图
点击查看大图