全文HTML
--> --> --> 我国铝型材行业发展快速,随之产生的铝型材污泥亦逐年增多。经调查,2017年,全国新增铝型材污泥583.20×104 t·a?1,其中含铬的污泥占总量的10%~15%。2016年,《国家危险废物名录》将含铬的铝型材污泥归类为HW17危险废物。目前,含铬铝型材污泥尚未得到妥当处理处置,一般采用就地堆放、填埋等方式[1]。这给企业带来极大负担,也给环境带来了巨大风险[2-4]。含铬铝型材污泥干基中含铝量均在50%(以Al2O3计)以上[5],污泥中Cr(Ⅲ)严重阻碍其铝资源开发利用。ZHANG等[6]和BAO等[7]以含铬铝泥为原料,通过酸(碱)溶再沉淀制备纳米材料,纳米材料掺杂较多Cr(Ⅲ)。污泥中Al(OH)3和Cr(OH)3化学性质相似,故难以利用酸碱溶解性差异进行分离。GEZER等[8]和陈巍等[9]利用氧化剂将污泥或废渣中Cr(Ⅲ)转化为Cr(Ⅵ)离子,可在不影响污泥含铝量的前提下,采用氧化方法分离出污泥中的铬。污泥中的Al(OH)3胶体物质会对分离Cr(Ⅵ)过程造成阻碍。在已有含Cr(Ⅵ)铝泥除铬的研究中,发现利用水洗或解胶剂可对铝泥中Cr(Ⅵ)进行有效分离[10-12]。因此,可利用氧化-解胶的方法有效地将Cr(Ⅲ)氧化成Cr(Ⅵ),并将其去除,达到分离污泥中铬金属的目的。
本研究以某铝型材企业产生的含Cr(Ⅲ)铝型材污泥为研究对象,采用次氯酸钠氧化-硫酸钠解胶联合法有效地氧化Cr(Ⅲ)并去除Cr(Ⅵ);通过分析污泥的基本性质,同时研究氧化条件、浸出规律以及分离机理,为含铬铝型材污泥的处理处置提供新的途径与参考。
1.1. 实验材料
本实验所用污泥样品为广东省某铝型材厂铝材表面处理时产生的含铬铝型材污泥。将原始污泥分为2个部分:一部分保存于4 ℃中,作为原始污泥,用于表观特征、含水率、pH等的分析;另一部分用于制备干基污泥,即对原始污泥烘干与均质化预处理,用于分析其化学成分、晶体结构、官能团结构及后续除铬实验等。取适量干基污泥进行XRF分析,污泥主要成分(以氧化物计)分析结果如表1所示。1.2. 次氯酸钠氧化除铬实验
称取15.00 g含铬铝型材污泥(干基污泥)置于250 mL的烧杯中,量取预先配置的不同浓度的次氯酸钠(AR,有效氯>8%)溶液100 mL并加入到烧杯中,在磁力恒温搅拌器上,60 ℃水浴并搅拌一定时间,反应结束后,抽滤处理,收集并计量滤液体积,测定滤液中含铬量与有效氯含量,滤渣保留待用。1.3. 水洗-解胶除铬实验
水洗实验:称取不同质量氧化后的滤渣放于250 mL烧杯中,加入100 mL去离子水,置于恒温磁力搅拌器上,70 ℃水浴搅拌,水洗30 min后,抽滤分离,取少量滤饼,干燥后,测定其中的含铬量,剩余大量滤饼按照前述水洗条件,洗涤3次,记为V水洗-1、V水洗-2、V水洗-3。解胶实验:称取不同质量水洗后的滤渣,放于250 mL烧杯中,并加入100 mL去离子水,添加一定量的解胶剂(AR,硫酸钠),70 ℃下搅拌解胶一定时间,抽滤分离,取少量滤饼,干燥后,测定其中的含铬量;剩余大量滤饼按照前述解胶条件,解胶3次,记为V解胶-1、V解胶-2、V解胶-3。
1.4. 分析方法
污泥含水率采用重量法测定。pH采用上海奥豪斯STARTER 2100酸度计进行测定。溶液中有效氯含量采用碘量法[13]进行测量。污泥中金属元素总量采用日本岛津的能量色散型X射线荧光光谱分析仪(Shimadzu EDX-7000)测定。实验中溶液的金属浓度利用电感耦合等离子体发射光谱仪(Agilent 713)进行测定。污泥矿物组成和晶体结构采用X-射线衍射光谱(Rigaku UItima IV)分析。污泥光能团特征使用傅里叶红外光谱(Thermo Scientific Nicolet iS5)进行表征。2.1. 含铬铝型材污泥表征
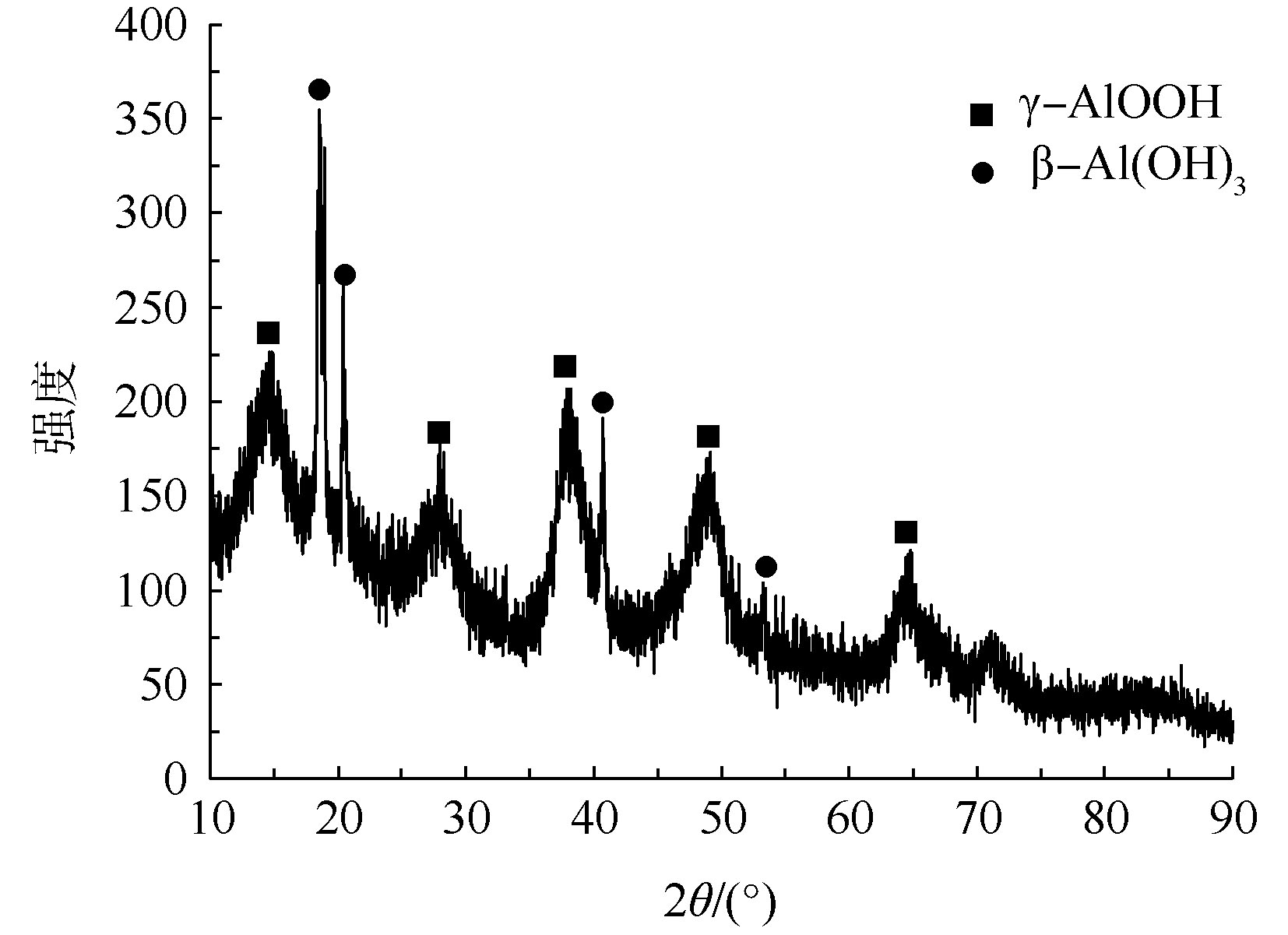
在铝型材表面喷涂过程中,为了提高涂层的附着力和耐蚀性,须在喷涂工序前进行铬化处理。铬化后的铝型材在水洗阶段产生大量含Cr(Ⅵ)废水,废水经还原加碱处理后形成了含铬铝型材污泥。对原始污泥进行表观特征分析发现,污泥颜色呈灰白色,含水率为84%,pH为7.20,是高水分中性偏碱性污泥。由表1可知,铝型材污泥主要成分为Al2O3(以氧化物计算,下同),其含量为64.03%以及0.60%的Cr2O3和少量Fe、Ca、Si等常见化合物。污泥在900 ℃的烧失量为12.07%,烧失的主要物质是污泥颗粒间的结晶水、吸附水,故污泥中的有机物含量极少[14]。为了解污泥的物相组成与晶体结构,须对污泥进行XRD分析,结果如图1所示。由图1可知,污泥XRD图主要是宽峰较多的非晶区,说明污泥的成分以结晶度差的非晶体为主。经分析,污泥中的非晶体为γ-AlOOH,晶体为γ-AlOOH、β-Al(OH)3[6]。污泥存在2种不同形态的γ-AlOOH,其主要原因是污泥具有胶体物质的特点,而胶体相互联结形成的空间网状结构可使污泥颗粒表面晶格缺陷、有序度下降,造成晶形结构不清晰,形成非晶体;其次是污泥部分颗粒粒径稍大,出现晶体[15]。污泥含铬量相对较少,容易被吸附或夹带在Al(OH)3胶体结构中,造成特征峰被掩盖,故谱线中未发现铬的特征峰。
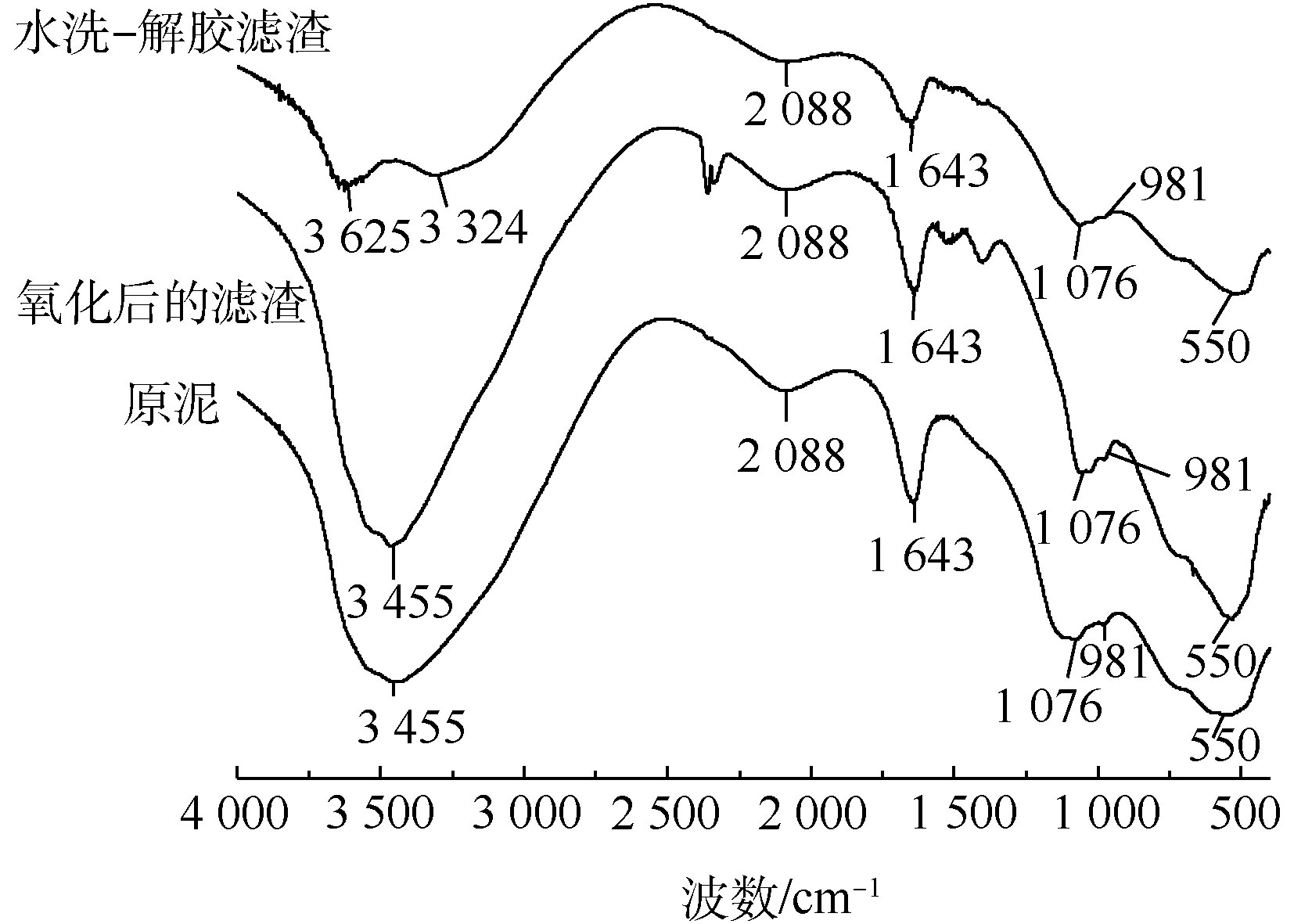
为确定污泥中主要物质的官能团,在400~4 000 cm?1内对污泥进行FT-IR测定,如图2所示。经分析,位于3 455 cm?1处的宽峰是水分子中—OH的伸缩振动峰,在1 643 cm?1处的宽峰为吸附水的弯曲振动峰[16],说明污泥颗粒存在表面吸附水的现象。而在2 088 cm?1和550 cm?1处吸收峰属于Al—OH的伸缩振动吸收峰,1 076 cm?1处吸收峰为Al—OH弯曲振动吸收峰[8]。在981 cm?1处出现1个弱吸收峰,属于Cr—OH的特征峰[17]。由图1和图2可知,污泥中铝、铬以Al(OH)3、Cr(OH)3的形式存在,其中Al(OH)3具有胶体特性,可以吸附Cr(OH)3。
2.2. 次氯酸钠氧化除铬实验研究
在碱性环境下,污泥中的Cr(Ⅲ)可与次氯酸钠发生氧化还原反应,其标准电极电势?θ(ClO?/Cl?)高于?θ(Cr(OH)3的氧化还原反应主要受以下因素的影响:pH、次氯酸钠浓度、固液比、温度、时间等[21]。次氯酸钠是强碱弱酸盐,能满足反应所需OH?,反应后pH约为8.70,污泥中Al(OH)3得到较好保留,故反应无须添加碱。提高温度有利于增铬浸出效果,但过高的温度会造成次氯酸根分解,故选取最适温度为60 ℃[22]。由于污泥具有一定的吸附作用,过高固液比会阻碍次氯酸根与Cr(Ⅲ)的接触,经过实验分析,选取适宜固液比为1∶7[23]。
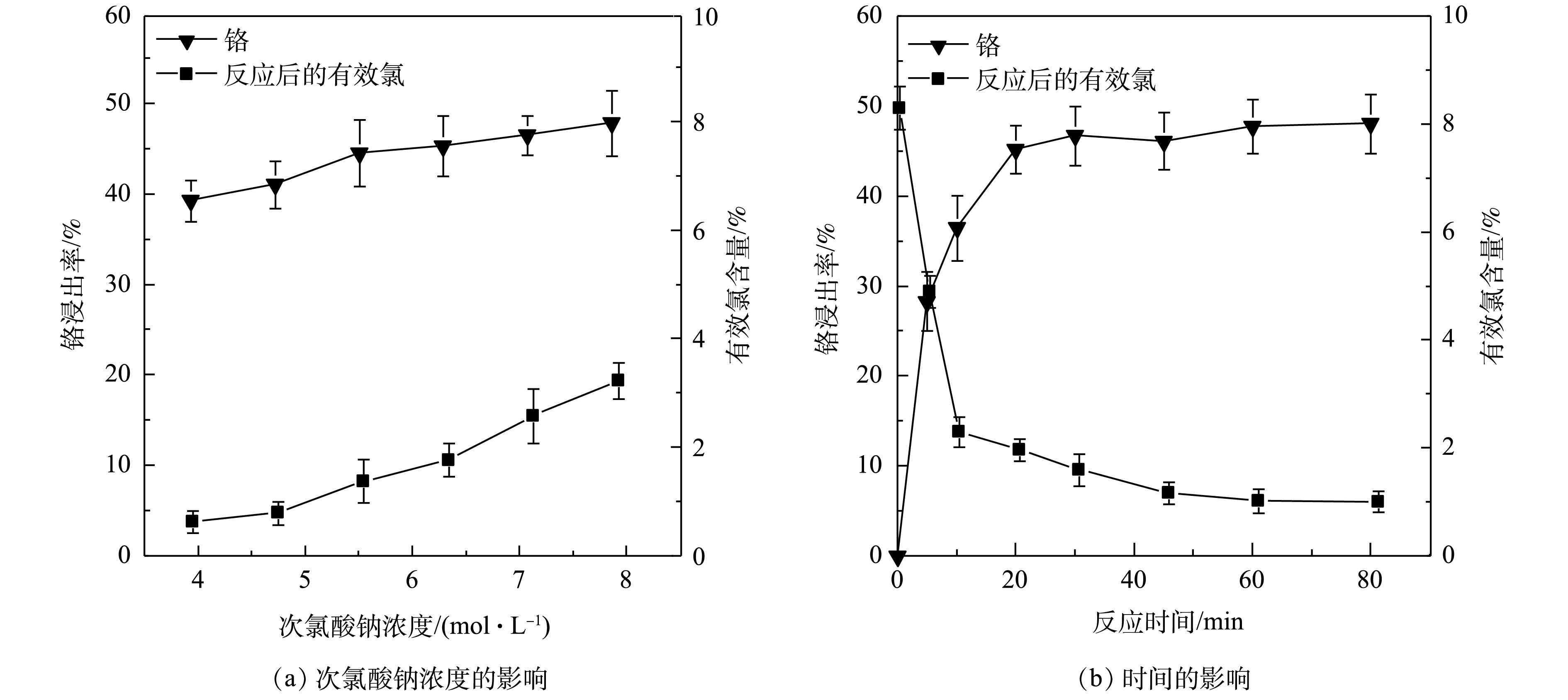
改变次氯酸钠浓度为3.93、4.72、5.50、6.29、7.07、7.86 mol·L?1,反应60 min后,研究次氯酸钠浓度对铬浸出率及有效氯含量的影响,结果如图3(a)所示。由此可知,随着次氯酸钠浓度的增加,铬浸出率与有效氯含量逐渐增加;在次氯酸钠浓度为5.50 mol·L?1时,铬浸出率基本稳定在44.52%左右,浸出液有效氯含量为1.38%。由于污泥的含氧基团及疏松多孔的胶体团粒结构易与
由图3(b)可知,在30 min内,反应中有效氯消耗与铬浸出率增加较快,说明氧化反应迅速,所需时间短。30 min后,氧化反应基本结束,铬浸出率达到最大值46.47%,浸出液有效氯含量为1.60%。反应之所以能迅速完成,是因为固液间的传质速度较大,促进了体系的反应速度,表现为Cr(OH)3易被氧化进入液相,铬浸出率迅速增加。在反应结束后,有效氯稍有下降,其原因是次氯酸根受热会分解。故最佳反应时间为30 min。综上所述,在固液比1∶7、次氯酸钠浓度为5.50 mol·L?1、水浴温度60 ℃、反应30 min条件下,次氯酸钠有效地氧化污泥中Cr(Ⅲ)并浸出Cr(Ⅵ),浸出率为46.47%,滤渣含铬量为2.20 mg·g?1。
2.3. 水洗-解胶除铬实验
污泥粒度和胶体性质的限制会使滤渣残留2.3.1. 水洗对污泥除铬效果的影响
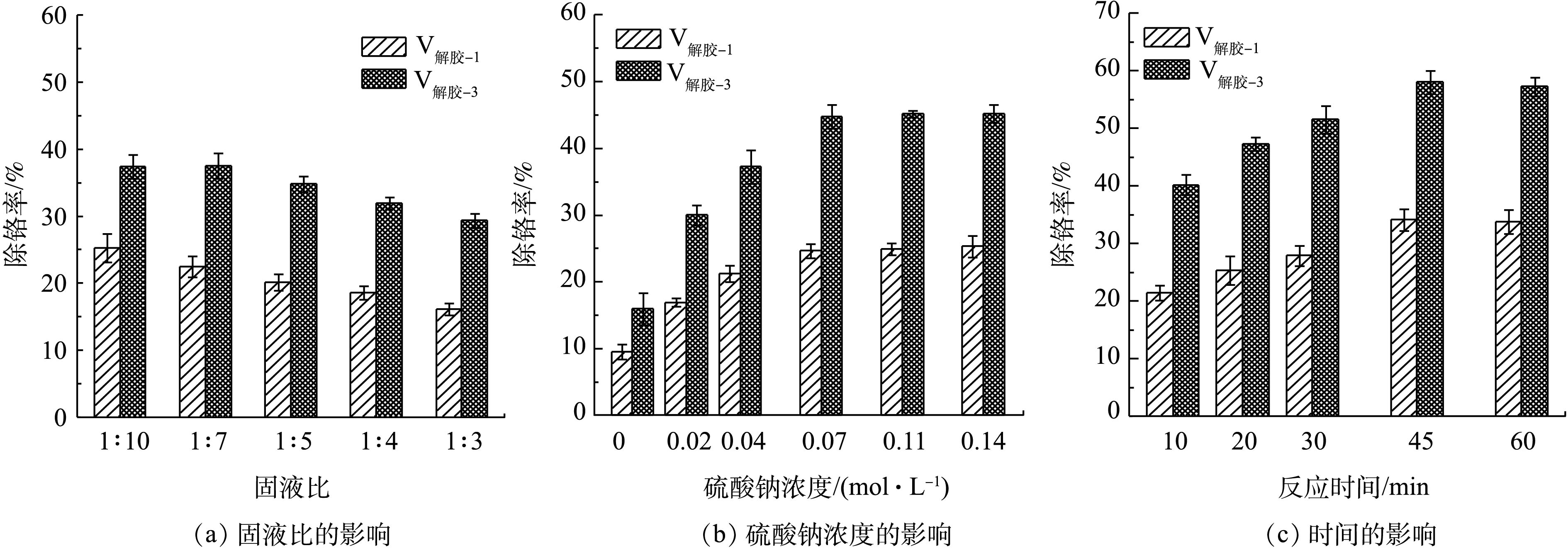
氧化后的滤渣含铝量为338.82 mg·g?1,含铬量为2.20 mg·g?1,含水率为80%。由于滤渣含水分过高,会影响固液比实验探究范围。因此,在水洗实验前,滤渣须室内风干5 d,风干后的滤渣含水率为29%。适宜的温度可以抑制污泥胶体物质的稳定性,降低吸附作用,提高除铬率。根据铬酸钠溶解度曲线的特征,在温度为70 ℃时,Cr(Ⅵ)溶解度最大[26]。改变固液比为1∶10、2∶10、3∶10、4∶10、5∶10 (kg∶L),并对滤渣重复水洗3次(记为V水洗-1、V水洗-2、V水洗-3),研究固液比对水洗效果的影响,结果如图4所示(水洗除铬率以含铬量为2.20 mg·g?1计算)。由图4可知,随着固液比增加,体系中黏稠性提高,不利于Cr(Ⅵ)转移至液相。液固比在(1∶10)~(3∶10)时,V水洗-3除铬率基本一致,证明水洗3次后,水溶性Cr(VI)基本去除。考虑产生的含铬滤液量,故确定最佳水洗固液比为3∶10。在滤渣初始含Cr(Ⅵ)量较高时,水洗1次效果显著,除铬率为15.45%。而当Cr(Ⅵ)含量相对较低时(<1.86 mg·g?1),每增加1次水洗处理,除铬率仅提高5.90%。综合考虑产生的废水量与经济效益,对滤渣进行水洗1次处理即可。
2.3.2. 解胶剂(硫酸钠)对污泥除铬效果的影响
氧化后的滤渣经过1次水洗后,残留含铬量为1.86 mg·g?1,主要成分为酸溶性Cr(Ⅵ),可以通过解胶剂进行脱除。铝型材污泥主要为无机化合物,为了不引入杂质成分,选用无机解胶剂优于有机解胶剂[27]。无机解胶剂(如硫酸钠、碳酸钠、三聚磷酸钠、六偏磷酸钠)均可促进Cr(Ⅵ)的浸出[11, 24]。污泥样品属于中性偏碱性污泥,且为了方便过滤,选用硫酸钠更为适合。硫酸钠解胶实验受到以下因素的影响:固液比、解胶剂浓度、温度、解胶时间等。陈胜娴[11]的研究表明,在解胶时,适当的温度不但加快胶粒布朗运动,使其更易碰撞而聚结沉淀,而且有利于Cr(Ⅵ)离子的扩散,提高除铬率;同时,该研究发现70 ℃下解胶除铬效果较好。解胶剂在不同条件下对滤渣除铬率的影响结果见图5。在硫酸钠浓度为0.04 mol·L?1、温度为70 ℃条件下,解胶15 min,不同的固液比(1∶10,1∶7,1∶5,1∶4,1∶3)对硫酸钠解胶的除铬效果如图5(a)所示(解胶除铬率以含铬量为1.86 mg·g?1计算)。由图5(a)可知,随着固液比的增加,解胶除铬率逐渐下降,在解胶3次、固液比为1∶7时,除铬效果最佳。硫酸钠作为解胶剂,不同用量会对滤渣解胶的除铬效果产生不同的影响。在固液比为1∶7、温度为70 ℃下,解胶15 min,硫酸钠添加量(0、0.02、0.04、0.07、0.11、0.14 mol·L?1)对滤渣除铬率的影响如图5(b)所示。由图5(b)可知,随着硫酸钠浓度的增加,除铬率逐渐增大,在浓度为0.07 mol·L?1时,除铬率达到最大值44.78%,且远大于单纯水洗的除铬效果。硫酸钠解胶浸出Cr(Ⅵ)属于交换-解吸规律,并与
对除铬前后的污泥进行FT-IR谱图分析,结果如图6所示。在原泥与氧化后的滤渣FT-IR谱线中,3 455 cm?1和1 643 cm?1处吸收峰的吸附水,2 088、1 076、550 cm?1处吸收峰的Al—OH的基团结构和峰强皆无较大变化,滤渣仍在981 cm?1处出现Cr—OH特征峰,未有明显变化,说明氧化过程对污泥的胶体结构与吸附能力没有影响,污泥的胶体特性依然存在,从而导致铬残留较多。在水洗-解胶滤渣的谱线中发现,滤渣经水洗-解胶后,在3 455 cm?1处的吸附水转变为3 625 cm?1与3 324 cm?1处的结构水、结晶水。又因为1 643 cm?1处吸附水的振动吸收峰,2 088 cm?1和1 076 cm?1处Al—OH的振动吸收峰在水洗-解胶后峰强降低,说明硫酸钠对污泥胶体物质具有一定的解胶能力,能够破坏胶体相互联结的空间网状结构,降低污泥的吸附作用。又在981 cm?1处未发现Cr—OH特征吸收峰,进一步说明硫酸钠将被胶体包裹的Cr(Ⅵ)解析出来,从而提高污泥的除铬效果,因此,污泥中Cr(Ⅲ)经过氧化-解胶联合法处理后基本完成分离。
由表2可知,污泥中富铝组成特性表明其具有很高的资源化潜力,但其中Cr(Ⅲ)严重阻碍其综合利用途径。含铬铝型材污泥经次氯酸钠氧化-硫酸钠解胶联合法处理后,可有效地单独分离Cr(Ⅲ),除铬率为80.54%,最终污泥含铬量为0.80 mg·g?1,且含铝量损失率仅为1.08%,并且实验条件温和,为污泥的综合利用(如制备莫来石、堇青石等[28]、制备活性氧化铝[29]、陶瓷[30]以及絮凝剂等)提供一定的参考。
2.4. 氧化后的Cr(Ⅵ)废水处理
氧化-解胶联合法处理含铬铝型材污泥,在分离Cr(Ⅲ)过程中会产生含Cr(Ⅵ)废水,废水pH约为8.70,若处理不当,会引起二次污染。由方程式(1)可知,废水中的Cr(Ⅵ)以2.5. 工艺地处理成本
为经济和妥当地处置含铬铝型材污泥,需要考虑工艺成本是否具备实际应用价值。因此,对污泥的氧化-水洗-解胶过程和含Cr(Ⅵ)废水的处理过程进行了详细的成本估算,结果如表3所示。由表3可知,氧化-解胶联合法处理工艺的总成本为159.05元·t?1,其中主要成本来自次氯酸钠,占总成本的76.01%。目前,根据地区性危险废物收费标准,HW17危险废物处置费用为1 000元·t?1[32]。而本工艺是以干基污泥进行处理,相对直接交送湿泥于危险废物处置公司处理,成本价格更低,具有一定的实际应用意义。而且废水经处理后可以实现回用,可回收铬酸盐。在不影响污泥含铝量的前提下,氧化-解胶联合处理法不但分离了污泥中的铬金属,解决了处理处置困难,为后续铝的综合利用提供基础;而且无污染物排放并回收了铬资源,获得一定经济效益。因此,该处理方法绿色环保,具备良好的应用前景。2)根据阴离子交换-解析规律,水洗-解胶(硫酸钠)的方法可以去除滤渣中残留Cr(Ⅵ)。在固液比为3∶10、温度为70 ℃条件下,水洗1次,去除可溶性Cr(Ⅵ)。然后在固液比为1∶7、硫酸钠浓度为0.07 mol·L?1、温度为70 ℃、解胶45 min的条件下,解胶3次,可去除被滤渣吸附的酸溶性Cr(Ⅵ),水洗-解胶除铬率为63.64%。
3)在不影响污泥含铝量的前提下,污泥中Cr(Ⅲ)经过氧化-解胶联合法处理后,基本完成分离,滤渣含铬量为0.80 mg·g?1,总除铬率为80.54%,且含铝量损失率仅为1.08%。最终工艺总成本比HW17危险废物的处置费用低。除铬过程产生的废水,经氯化钡处理后可返回工艺中利用,并降低工艺用水量与废水排放量,避免产生二次污染问题。
参考文献

 下载:
下载: 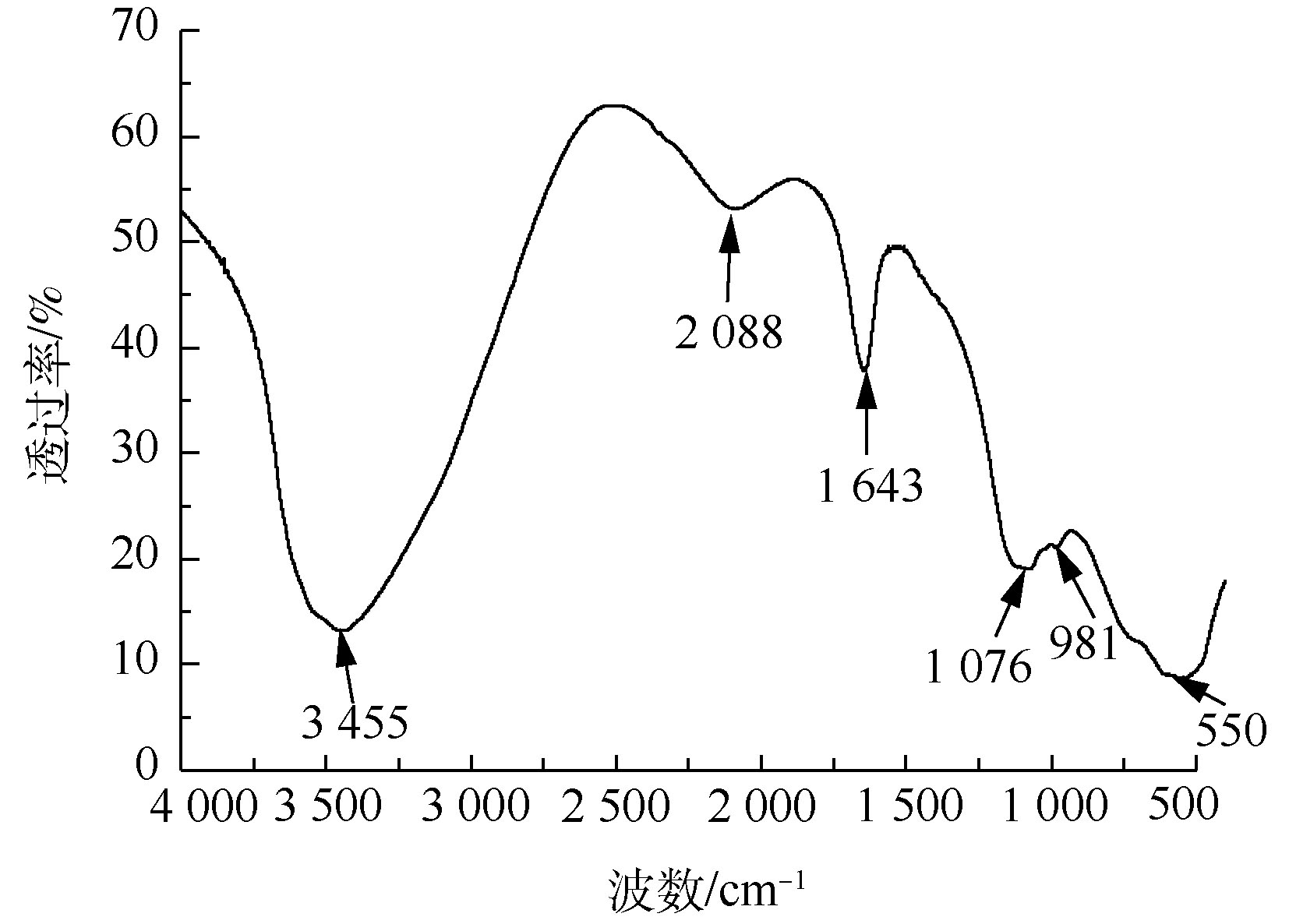

 点击查看大图
点击查看大图