 ,1,2,3,*, 李强
,1,2,3,*, 李强 ,1,2,3,*
,1,2,3,*Late Miocene micromammalian assemblage of Tuchengzi and its biochronological position in Neogene faunal sequence in central Nei Mongol, China
ZHANG Li-Min1,2,3, DONG Wei1,2, NI Xi-Jun ,1,2,3,*, LI Qiang
,1,2,3,*, LI Qiang ,1,2,3,*
,1,2,3,*通讯作者: *nixijun@ivpp.ac.cn;liqiang@ivpp.ac.cn
收稿日期:2020-07-2网络出版日期:2021-01-20
| 基金资助: |
Received:2020-07-2Online:2021-01-20

摘要
近百年来,在内蒙古中部地区发现了丰富的新近纪哺乳动物化石,命名和描述了10余个哺乳动物群,其时代跨越早中新世至早上新世。它们已经成为建立中国北方新近纪哺乳动物生物年代地层框架的重要依据。早在1959年中苏古生物联合考察过程中就已经发现土城子地区有丰富的哺乳动物化石,但这些化石以大-中型哺乳动物为主,缺乏小哺乳动物。土城子动物群的构成和其在中国北方新近纪哺乳动物年代地层框架中的位置长期以来不是特别明确。报道了在土城子新发现的6种小哺乳动物化石。根据Lophocricetus grabaui-Sinocricetus zdanskyi-Prosiphneus licenti-Hansdebruijnia pusilla-Moschus grandaevus的组合特点,认为土城子动物群的年代应该可以很好地约束在晚中新世(或者保德期)的晚期。土城子动物群明显比内蒙古的宝格达乌拉动物群要进步一些,与二登图动物群非常相似,时代上晚于前者,略早于后者。依据动物群整体面貌,推测在晚中新世晚期土城子地区存在森林和草原混合的环境。
关键词:
Abstract
Neogene strata rich in fossil mammals are well exposed in central Nei Mongol, China. Over a dozen mammalian faunas in chronological succession from Early Miocene to Early Pliocene were discerned in this region, and they built a fundamental part of the Neogene land mammal biochronological system in northern China. Tuchengzi was first recognized for producing abundant mammalian fossils during the Sino-Soviet Paleontological Expedition (SSPE) initiated in 1959. However, all unearthed fossils from the SSPE were either large- or middle-sized mammals, and small mammal fossils in the Tuchengzi Fauna were deficient for a long time. The composition and biochronological position of the Tuchengzi Fauna in the Neogene mammalian biochronological system in northern China was not particularly clear. The new fossils here reported are represented by 6 taxa of small mammals. Based on the co-occurrence of Lophocricetus grabaui, Sinocricetus zdanskyi, Prosiphneus licenti, Hansdebruijnia pusilla, and Moschus grandaevus, the age of the Tuchengzi Fauna is constrained to late Late Miocene or Baodean Chinese Land Mammal Age, slighter younger than the Baogeda Ula Fauna and older than the Ertemte Fauna. Judging from the fossil composition, the Tuchengzi Fauna possibly inhabited a forest-steppe mixed habitat during the late Late Miocene.
Keywords:
PDF (2353KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
张立民, 董为, 倪喜军, 李强. 晚中新世晚期土城子小哺乳动物组合及土城子动物群在内蒙古中部地区新近纪哺乳动物群序列中的位置. 古脊椎动物学报[J], 2021, 59(1): 45-63 DOI:10.19615/j.cnki.1000-3118.200821
ZHANG Li-Min, DONG Wei, NI Xi-Jun, LI Qiang.
Central Nei Mongol (Inner Mongolia) has a large exposed area of the late Cenozoic sediments containing plentiful mammal remains, which has been a significant lure for paleontologists in China and abroad. Almost a century has passed since the pioneering paleontological survey at Ertemte, Huade County and Gaotege, Abaga Banner in the 1920s (Andersson, 1923; Teilhard de Chardin, 1926). After the introduction of micromammalian screen-washing technology in the 1980s (Fahlbusch et al., 1983), the principle paleontological aims were focused on collecting small mammals and establishing a precise Neogene mammalian biochronological framework (Qiu and Wang, 1999; Qiu et al., 2006, 2013). Through arduous efforts by generations of Chinese paleontological workers and their international cooperation partners, now a total of 18 fossiliferous faunas have been distinguished, and their ages range from Early Miocene to Early Pliocene (Qiu and Li, 2016). The Neogene mammalian faunal sequence in central Nei Mongol forms an important part of the Chinese land mammal biochronological system (Deng, 2006; Qiu et al., 2006, 2013; Qiu Z X et al., 2013). The Tuchengzi Fauna was discovered by the SSPE in 1959 (Chow and Rozhdestvensky, 1960), and yielded a great number of large- and middle-sized mammalian fossils (Zhai, 1963; Qiu, 1979). Previous Tuchengzi Fauna lacks small mammalian remains, so makes it difficult to directly compare to other Late Miocene or Baodean faunas in central Nei Mongol or northern China. In recent near ten years, the Institute of Vertebrate Paleontology and Paleoanthropology, CAS (IVPP) has intermittently undertaken fieldworks in Tuchengzi area and successfully collected a number of small mammalian fossils through screen-washing method (Dong et al., 2014, 2016). To clarify the composition of the Tuchengzi Fauna and its precise biochronological position, and further improve the Neogene mammalian biochronological system in central Nei Mongol and northern China, here we report the new small mammalian materials.
1 Geologic settings
Tuchengzi fossil site is not far from other well-known mammalian fossil sites in the Huade area, such as Ertemte, Harr Obo, Olan Chorea and Bilike. It is located at the “Dragon bone” hill near the Tuchengzi (=Tuchetse, Tuchenzi) village, Chaoyang town, about 20 km southeast to Huade county seat, Nei Mongol (see Dong, 2014). In the 1980s, the villagers had massively dug the “Dragon bone” hill for the traditional Chinese dragon bone trade. Their gains were concentrated in a horizon about 3 m below the surface. From 2009 to 2015, a team from IVPP surveyed the Tuchengzi village and its surroundings, and did excavations and screen-washing (Dong, 2014; Dong et al., 2014, 2016). They dug four pits in the “Dragon bone” hill for exposing fresh profiles and collecting new fossils. Only one pit produced abundant fossils, including some cervid fragments, a well-preserved Chilotherium skull and small mammals.The total thickness of the section in Chilotherium pit is about 8 m. From top to bottom, the section can be divided into 6 layers. Layer zero is an artificial disturbed deposit in about half meter thickness. Layer 1 is yellowish sandy mudstone about 1 m thick. Layer 2 is a thin layer of calcareous concretions without fossils with a thickness of about 30 cm. Layer 3 is yellowish sandy mudstone about 40 cm thick. Layer 4 is lower layer of calcareous concretions and produces the skull of Chilotherium. Layer 5 is a series of reddish sandy mudstones in about 5 m thickness, and its upper part yielded small mammalian remains (Fig. 1).
Fig. 1
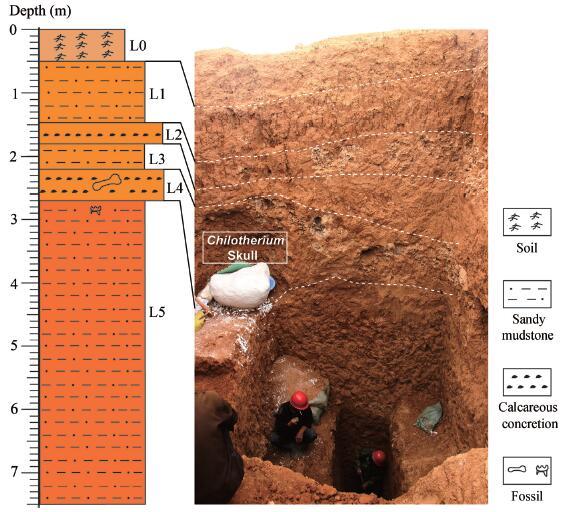 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 1Fresh profile of Chilotherium pit in “Dragon bone” hill near Tuchengzi village, Huade Photo taken by LIU Wenhui in 2015; L. abbreviation of layer
2 Material and methods
During the fieldwork seasons of 2013 and 2015, about three tons of matrixes were collected from the Chilotherium pit. Through screen-washing technology, hundreds of small mammal remains were gathered. The majority of them are fragmentary bones. Mandibles and isolated cheek teeth were picked out for taxonomical identification. All fossils are housed in IVPP.The specimens were CT-scanned using the 100 kV Micro-CT at the Key Laboratory of Vertebrate Evolution and Human Origins, Chinese Academy of Sciences. Segmentations and 3D virtual reconstructions were made following the standard procedure introduced by Ni et al. (2012). We reconstructed the 3D virtual models of specimens by VGStudio Max (version 2.0) installed in the laboratory. Specimens were measured using an Olympus SZ61 microscope with a precision of 0.1 mm. For the molar, the length is defined as the mesiodistal chord. The width is defined along the chord perpendicular to the length. The Talpidae humeral measurements are following Storch and Qiu (1983:text-fig. 5). Item 2 represents the length between the pectroral crest/teres tubercle notch and the distal most point of the humerus. Item 3 is the length between the distal apex of the teres tubercle and the distal most of the humerus. Item 5 is the width of the middle of the humeral shaft. Item 6 is the width between the lateral margin of the capitulum and the medial margin of the entepicondylar foramen. The humeral and teeth terminology for the family Talpidae is cited from Hutchison (1974) and Storch and Qiu (1983), the molar terminologies for the Cricetidae and the Lophocricetinae are following Qiu and Li (2016), the Ochotonidae following López-Martínez (1989), and the Myospalacinae and Murinae following Zheng et al. (2004) and Storch (1987), respectively.
Abbreviations CAS, Chinese Academy of Sciences; CLMA, Chinese Land Mammal Age; IVPP, the Institute of Vertebrate Paleontology and Paleoanthropology, CAS; V, prefix to the fossil vertebrate collections stored in the IVPP.
3 Systematic paleontology
Family Talpidae Fischer von Waldheim, 1817Subfamily Talphinae Fischer von Waldheim, 1817
Genus Yanshuella Storch & Qiu, 1983
?Yanshuella primaeva (Schlosser, 1924)
(Fig. 2A-C)
Age Late Late Miocene, or Baodean of CLMA.
Referred specimens A broken right mandible preserved p3 and m1-3, IVPP V 26813.1; a left humerus (proximal broken), V 26813.2; a right distal of humerus, V 26813.3.
Measurements Maximum length×maximum width (in mm): V 26813.1, p3=0.9×0.7, m1=2.15×1.65, m2=2.30×1.80, m3=2.0×1.55. The V 26813.2 humeral measurement items 2, 3, 5 and 6 used in Storch and Qiu (1983:text-fig. 5) are ~8.5, 6.0, 2.5, and 6.0 mm, respectively; in V 26813.3, the item 6 is 5.7 mm.
Description On the V 26813.2 (Fig. 2B), the proximal humeral head, the greater tuberosity and lesser tuberosity, the distal ectepicondyle, and the pectoral ridge and tubercle on shaft anterior face are not preserved, so the “scalopine-ridge” is unobserved. The proximal pectoral process is well-developed, the notch between pectroral crest and teres tubercle is shallow. The teres tubercle is short and hook-like. The distal entepicondyle is also hook-like; the capitulum is ovoid and directed mediodistally, the trochlea area is separated by the prominent medial edge of trochlea into a deep and wide outer part and a shallow and narrow inner part, the entepicondylar foramen is large and elliptical, the fossa for ligament of musculus flexoris digitroum is cup-shaped, the olecranon fossa is triangular and deep. The V 26813.3 (Fig. 2A) preserves only its distal part, which size and morphology are identical to those of the V 26813.2. In gross, two hurmeri show a moderate torsion of shaft. On the V 26813.1 (Fig. 2C), the mandible is slender with curved ventral margin. Because its ascending ramus is broken, the angle between the ascending and the horizontal rami is unidentified. The mental foramen is located beneath the distal root of the p3. Both the p3 and p4 have two roots. The p3 has only main cusp protoconid, whereas the parastylid and the entoconid are not present; its distal cingulid is present. Lower molars’ metastylids are present, the talonid basin is always wider than the trigonid basin, the precingulid on the m1 is weaker than that of m2-3, the crista oblique of m2-3 extends to the middle part of the protocristid, the entostylid is well-developed on m1-2 but absent on the m3.
Fig. 2
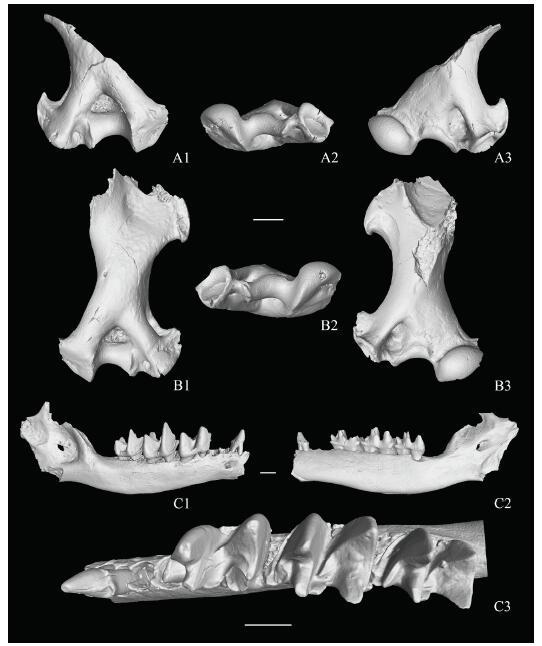 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 2Humeri and mandible of ?Yanshuella primaeva from Tuchengzi, Huade
A. IVPP V 26813.3, right distal of humerus; B. V 26813.2, left humerus; C. V 26813.1, right mandible with p3 and m1-3. A1 and B1, A2 and B2, A3 and B3 are in posterior, distal and anterior views, respectively; C1, C2, C3 are in buccal, lingual and occlusal views, respectively. Scale bars equal 1 mm
Remarks These three specimens represent the appearance of a mole in the Tuchengzi Fauna. Their teeth and humeral sizes are distinctly larger than those of Quyania chowi, but very close to Yanshuella primaeva from Ertemte 2 (Storch and Qiu, 1983). In dental morphology, the Tuchengzi sample differs from Q. chowi in having higher crowned cheek teeth, a more lingually situated end of the crista oblique, and the crista oblique not separated from protocristid on the lower molars. In humeral morphology, the Tuchengzi sample is different from Q. chowi in having a relatively more distorted shaft and greatly asymmetric trochlear area, whereas it is identical to those of Y. primaeva from Ertemte 2 (Storch and Qiu, 1983). However, there are three dental morphological differences between the Tuchengzi sample and Y. primaeva from Ertemte 2. First, the Tuchengzi’s p3 has two roots, whereas the Ertemte 2’s p3 has a single root. Second, the lower molars from Tuchengzi have well-developed metastylids, whereas those of Ertemte 2 absolutely lack metastylids. Third, the end of the crista oblique on the lower molars from Tuchengzi is more lingually situated than those of Y. primaeva from Ertemte 2 (Qiu and Tong, 2015). The Tuchengzi sample may represent a new member of the Talpidae with a similar ecomorphological humerus in Neogene northern China. Here, considering paucity of material, we temporarily refer the Tuchengzi sample to Y. primaeva with further research recommended.
Family Cricetidae Fischer von Waldheim, 1817
Subfamily Cricetinae Fischer von Waldheim, 1817
Genus Sinocricetus Schaub, 1930
Sinocricetus zdanskyi Schaub, 1930
(Fig. 3A)
Cricetidae gen. et sp. indet., Dong et al., 2014, p.34
Age Late Late Miocene, or Baodean of CLMA.
Referred specimens A left maxillary fragment with M1-2, IVPP V 26815.1; a right maxillary fragment with M1, V 26815.2.
Measurements Maximum length×maximum width (in mm): V 26815.1, M1=2.0×1.4, M2=1.55×1.3; V 26815.2, M1=1.9×1.35.
Description The M1 has a kidney shape. The anterocone is wide and well anteriorly bifid. The protolophule I (mesial protolophule) and metalophule I (mesial metalophule) are both absent. The mesoloph is well developed and long on the V 26815.1, but weak and short on the V 26815.2, and touches anterior wall of the metacone. The M1 has 4 roots. The M2 is slightly posteriorly elongated. The protolophule I is as strong as the protolophule II (distal protolophule). The mesoloph is strong and long, extending in half way to the tooth buccal edge. The metalophule I is absent, whereas the meatalophule II (distal metalophule) is present. The M2 has 4 roots.
RemarksSinocricetus often occurs in the late Neogene strata of northern China and has three species. S. zdanskyi was reported from the latest Miocene Ertemte, Nei Mongol and other Late Miocene localities in northern China (Schaub, 1930; Wu, 1991; Zheng and Zhang, 2001). S. progressus was found from the Early Pliocene Bilike and Gaotege, Nei Mongol and Nihewan Basin, Hebei (Qiu and Storch, 2000; Li et al., 2008; Li, 2010), and the large-sized S. major was described by Li (2010) from the Early Pliocene Gaotege, Nei Mongol. The Tuchengzi sample is similar to S. progressus from Bilike and Gaotege in size but differs from the latter in having a higher tooth crown with robust cusps. The Tuchengzi sample is distinctly smaller than S. major from Gaotege and has a lower tooth crown. In both size and morphology, the Tuchengzi sample falls well within the variable range of those of S. zdanskyi from Ertemte 2 (Wu, 1991).
Family Muridae Illiger, 1811
Subfamily Murinae Illiger, 1811
Genus Hansdebruijnia Storch & Dahlmann, 1995
Hansdebruijnia pusilla (Schaub, 1938)
(Fig. 3B)
Age Late Late Miocene, or Baodean of CLMA.
Referred specimen A left fragmentary maxilla with M1, IVPP V 21817.
Measurements Maximum length×maximum width of M1 (in mm) = 2.20×1.60.
Description The M1 occlusal face is moderately worn; the ridges are thin relative to the main cusps; the t1bis (=t0 in Storch, 1987) and the t2bis (=prestyle in Storch, 1987) are absent; the t1 is weakly connected with the t5, but is well connected with the t2; the t3 is not connected posteriorly with the t5 or the t6; the cusps t4, t5, t6, t9, and t8 form a continuous garland; the t6 is well connected with the t9; there is no t7; the t12 is present and ridge-like. The M1 has 3 roots.
Remarks This M1 has a ridge-like t12, but lacks t1bis, t2bis and t7. The t4 and t8 are connected, and t6 and t9 are also connected. The t3 are separated from both t5 and t6. These features are identical to those of Hansdebruijnia pusilla (=Occitanomys pusillus Schaub, 1938) from Ertemte 2, Nei Mongol (Storch, 1987). This specimen differs from primitive H. perpusilla from Baogeda Ula, Nei Mongol in having a strong t6-t9 connection (Storch and Ni, 2002). As a result, it is appropriate to assign this specimen to derived H. pusilla.
Family Zapodidae Coues, 1875
Subfamily Lophocricetinae Savinov, 1970
Genus Lophocricetus Schlosser, 1924
Lophocricetus grabaui Schlosser, 1924
(Fig. 3C)
Age Late Late Miocene, or Baodean of CLMA.
Referred specimens A right maxillary fragment with M1-2 and alveoli of P4 and M3, IVPP V 26814.1; a right maxillary fragment with M1-2, V 26814.2.
Measurements Maximum length×maximum width (in mm): V 26814.1, M1=1.7×1.35, M2=1.25×1.20; V 26814.2, M1=1.9×1.35, M2=1.55×1.25.
Description The M1 and M2 mesolophs and hypostyles all are absent, protostyles are strong, the anteroloph is single, the entoloph is mesiobuccally connected with the paracone, the posteroloph is mesiobuccally connected with the metacone. The M1 mesocone is strong, whereas the M2 mesocone is weak. Both the M1 and M2 have 4 roots.
Remarks The two dentitions display the characteristic morphology of Lophocricetus: the M1 and M2 have a strong protostyle but no hypostyle, and have a mesocone but no mesoloph. These features exclude their attribution of Paralophocricetus Zazhigin et al., 2002. In China, Lophocricetus has only two species, L. grabaui from Ertemte 2, Bilutu in Nei Mongol and the Mahui Formation in Yushe Basin, and L. xianensis from Bahe, Shaanxi Province and several localities of central Nei Mongol (Qiu, 1985, 2017; Qiu et al., 2008; Qiu and Li, 2016). Based on their lager size, distinct absence of a mesoloph and hypostyle on M1-2, the Tuchengzi sample should be assigned to L. grabaui.
Family Myospalacidae Lilljeborg, 1866
Subfamily Myospalacinae Lilljeborg, 1866
Genus Prosipheus Teilhard de Chardin, 1926
Prosiphneus licenti Teilhard de Chardin, 1926
(Fig. 3D-G)
Prosiphneus sp., Dong et al., 2014, p.34
Age Late Late Miocene, or Baodean of CLMA.
Referred specimens Ten specimens: a left maxillary fragment with M1-3, IVPP V 26816.1; a right maxillary fragment with M1-3, V 26816.2; a right fragmentary mandible with m1-2 and mesial alveolus of the m3, V 26816.3; a right mandibular fragment preserved broken incisor and m1’s alveolus, V 26816.4; a left M1, mesial lobe broken, V 26816.5; 2 m1s (left m1, V 26816.6; right m1, V 26816.7); 2 m2s (left m2, V 26816.8; right m2, V 21816.9); a left m3, V 21816.10.
Measurements See Table 1.
Table 1
Table 1Molar measurements of Prosiphneus licenti from Tuchengzi, Huade, Nei Mongol (mm)
| Tooth | Number | Length | Width | |||
|---|---|---|---|---|---|---|
| Range | Mean | Range | Mean | |||
| M1 | 2 | 3.10-3.20 | 3.15 | 2.26-2.30 | 2.28 | |
| M2 | 2 | 2.60-2.65 | 2.63 | 2.15-2.20 | 2.18 | |
| M3 | 2 | 2.10-2.15 | 2.13 | 1.95-1.95 | 1.95 | |
| m1 | 3 | 2.90-3.35 | 3.10 | 1.65-2.15 | 1.92 | |
| m2 | 3 | 2.75-3.10 | 2.88 | 2.20-2.45 | 2.35 | |
| m3 | 1 | - | 2.55 | - | 2.15 | |
新窗口打开|下载CSV
Description On the mandibles, the mental foramen is situated below the mesial root of the m1 and is mesioventral to the apex of the masseteric ridges. The molar roots are well separated. The M1’s lingual salient angle 1 (LSA1) is deep, the LSA2 is slightly distobuccally extending. The M2’s occlusal pattern is ω-like. The M3 has a fossette enclosed by the posteroloph and entocone. The m1’s anterior cap (ac) is wide and short, the lingual reentrant angle 3 (lra3) and the buccal reentrant angle 2 (bra2) are opposite. The m3 is as long as the m2 but with a narrower posterior part. The dentine tract of the upper and lower molars is strongly undulating. The peaks of the dentine tract are higher than the bottoms of the main valleys. The enamel parameters of A, B, C, D of upper molars and a, b, c, d, e of the lower molars are moderate (see Table 2), and are close to those of Prosiphneus licenti from Qin’an and Qingyang, Gansu Province (Zheng et al., 2004).
Table 2
Table 2Molars’ enamel parameters of Prosiphneus licenti from Tuchengzi, Huade, Nei Mongol (mm)
| Tooth | Inventory number | Enamel parameters of upper molars | ||||
|---|---|---|---|---|---|---|
| (IVPP) | A | B | C | D | ||
| M1 | V 26816.1 | 0.1 | 0.3 | 0.6 | 0.6 | |
| M1 | V 26816.2 | 0 | 0.55 | 0.55 | 0.6 | |
| M2 | V 26816.1 | ? | ? | 0.2 | 0 | |
| M2 | V 26816.2 | 0 | 0.25 | 0.25 | 0 | |
| M3 | V 26816.1 | 0 | 0.05 | 0.15 | 0 | |
| M3 | V 26816.2 | 0.1 | 0.05 | 0.2 | 0 | |
| Enamel parameters of lower molars | ||||||
| a | b | c | d | e | ||
| m1 | V 26816.6 | 0 | 0.25 | 0.2 | 0.3 | 0 |
| m1 | V 26816.7 | 0 | 0.45 | 0.25 | 0.4 | 0 |
| m2 | V 26816.8 | 0.15 | 0.5 | 0.3 | 0.25 | 0 |
| m3 | V 26816.10 | 0.05 | 0.15 | 0.25 | 0.1 | 0 |
新窗口打开|下载CSV
Remarks These ten specimens represent the appearance of a zokor in the Tuchengzi Fauna. Based on the molar features of having roots, ω-like occlusal pattern on the M2, opposite lra3 and bra2 on the m1, and the moderate enamel parameters of the molars, these zokor specimens should be attributed to Prosiphneus licenti. P. licenti was distributed in the late Late Miocene of northwestern China (Teilhard de Chardin, 1926; Zheng et al., 2004).
Order Lagomorpha
Family Ochotonidae Thomas, 1897
Genus Ochotona Link, 1795
Ochotona lagreli (Schlosser, 1924)
(Fig. 3H-Q)
Ochotona sp., Dong et al., 2014, p.34
Age Late Late Miocene, or Baodean of CLMA.
Referred specimens Twenty-two specimens: a broken left mandible with p3-m1, IVPP V 21818.1; 1 P2, V 21818.2; 5 P3s, V 21818.3-7; 7 P4/M1s, V 21818.8-14; 3 M2s, V 21818.15-17; 3 p3s, V 21818.18-20; 2 lower middle cheek teeth, V 21818.21-22.
Measurements See Table 3.
Description On the mandible V 21818.1 (Fig. 3H), the diastema is long (~8 mm) but shallow (~2.5 mm, height between the alveoli and ventral margin of the diastema). The incisor terminates at the lingual side of the p4 and forms a bulge. The mental foramina are double, the mesial one is below the p3 and the distal one is below the m2. Several nutrient foramina are developed between and below these two mental foramina. The P2 has a simple occlusal pattern with only one anterior fold; the lingual fold is absent (Fig. 3I). The P3 has a short lingual hypostria and a U-shape crescentic fold filled with cements; its distobuccal corner is prominent; the mesial wall is rounded, whereas the buccal wall is oblique, and the distal wall is straight (Fig. 3J-K). The upper middle cheek teeth (P4/M1) all have two lobes, which connect to each other through a buccal dentine bridge; the mesial lobe (trigon) is higher and wider than the distal lobe (talon) (Fig. 3L). The M2 is similar to P4/M1 but has an additional distolingual salient angle in the distal lobe (Fig. 3M). The p3 has a subtriangular occlusal outline; it comprises one inner fold and two outer folds, of which the distobuccal fold is the deepest, the two mesial folds are opposite and separated by a short enamel bridge; two very shallow grooves are present on the mesial wall of the mesial lobe; the lingual wall is straight, whereas the distal wall is curved (Fig. 3N-P). The lower middle cheek teeth (p4/m1/m2) have two lobes, which connect to each other through a short and wide dentine bridge in middle of the tooth; the mesial lobe (trigonid) is higher and longer than the distal one (talonid) (Fig. 3Q).
Fig. 3
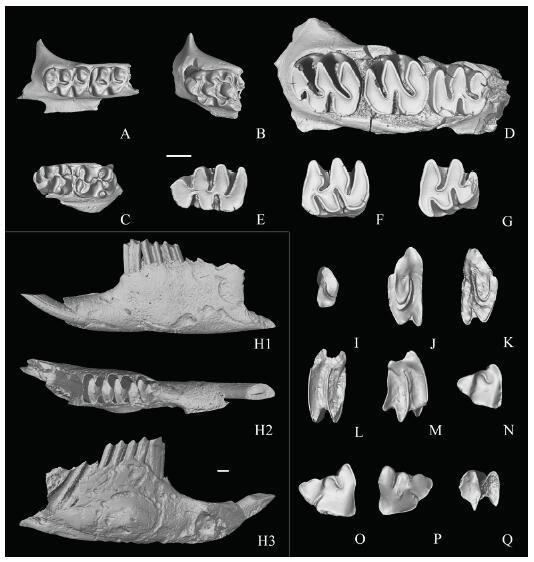 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 3Rodent and lagomorph remains from Tuchengzi, Huade
A. Sinocricetus zdanskyi, left maxilla with M1-2, IVPP V 26815.1; B. Hansdebruijnia pusilla, left maxilla with M1, V 21817; C. Lophocricetus grabaui, right maxilla with M1-2, V 26814.2; D-G. Prosiphneus licenti: D. left maxilla with M1-3, V 26816.1, E. left m1, V 26816.6, F. left m2, V 26816.8; G. left m3, V 26816.10; H-Q. Ochotona lagreli: H. left mandible with p3-m1, V 21818.1, I. right P2, V 21818.2, J. left P3, V 21818.3, K. right P3, V 21818.6, L. left P4/M1, V 21818.8, M. right M2, V 21818.17, N. right p3, V 21818.19, O. right p3, V 21818.20, P. left p3, V 21818.18, Q. left p4/m1/m2, V 21818.21 A-G, H2, I-Q in occlusal views, H1 in buccal view, H3 in lingual view. Scale bars equal 1 mm
Remarks Based on the occlusal patterns of the P2, P3 and p3, it is no doubt that the Tuchengzi specimens should be referred to the genus Ochotona. The P2 has only an anterior fold. The p3 occlusal outline is near subtriangular with one inner and two outer folds filled with cements, and its outer distal fold is distolingually extending and its length is about half of the tooth width, its inner mesial fold and outer mesial fold are deep and separated by a short enamel bridge. The distolingual wall of the p3 is straight. All these features are identical to the diagnosis of Ochotona lagreli. Furthermore, the size of the specimens from the Tuchengzi falls within the variable range of O. lagreli from Ertemte 2 and Harr Obo, but it is distinctly larger than O. minor from Ertemte 2 (Qiu, 1987). O. lagreli is common in the Late Miocene to Early Pliocene localities in northern China, such as Ertemte, Harr Obo, Olan Chorea, and Yushe etc. (Schlosser, 1924; Bohlin, 1942; Wu and Flynn, 2017).
Table 3
Table 3Tooth measurements of Ochotona lagreli from Tuchengzi, Huade, Nei Mongol (mm)
| Tooth | Number | Length | Width | |||
|---|---|---|---|---|---|---|
| Range | Mean | Range | Mean | |||
| P2 | 1 | - | 1.0 | - | 2.0 | |
| P3 | 4 | 1.35-1.50 | 1.41 | 3.05-3.40 | 3.21 | |
| P4/M1 | 7 | 1.40-1.75 | 1.50 | 2.85-3.70 | 3.13 | |
| M2 | 3 | 1.75-1.90 | 1.83 | 2.70-3.15 | 3.0 | |
| p3 | 4 | 2.0-2.30 | 2.21 | 1.80-2.50 | 2.19 | |
| p4/m1/m2 | 4 | 1.35-2.30 | 1.84 | 1.15-2.50 | 1.95 | |
新窗口打开|下载CSV
4 Composition of the Tuchengzi Fauna
In 1959, the SSPE team had excavated “Hipparion red clay” in Tuchengzi area and unearthed numerous mammalian remains. Anchitheriinae Sinohippus zitteli was the first reported species of the collection by the SSPE (Zhai, 1963). Later, Qiu (1979) described another 11 species of large mammals, which included Hipparion plocodus, Chilotherium gracile, Aceratherium huadeensis, Moschus grandaevus, Cervocerus (= Cervavitus) huadeensis, C. novorossiae, Cervinae gen. et sp. indet., Dorcadoryx sp., Plesiaddax sp., Palaeotragus sp., and Samotherium sp. Qiu et al. (1987) recognized Hipparion fossatum from H. plocodus previously identified by Qiu (1979) . Hou et al. (2007) referred the materials of Sinohippus zitteli described by Zhai (1963) to her new species S. robustus. In recent ten years, Dong’s group from the IVPP undertook surveys and excavations in Tuchengzi and the surrounding area. New fossils include Perissodactyla: Hipparion “plocodus”, Sinohippus “zitteli”, Aceratherium huadeensis, Artiodactyla: Eostyloceros blainvillei, E. triangularis, Euprox sp., Cervavitus huadeensis, C. shanxius and Moschus grandaevus, Rodentia: Cricetinae gen. et sp. indet., Prosiphneus sp. and Lagomorpha: Ochotona sp. (Dong, 2014; Dong et al., 2014, 2016 , 2018a, b; Pan, 2018). Our report adds three more taxa of small mammals, i.e. a mole ?Yanshuella primaeva, a jumping mouse Lophocricetus grabaui, and a murid Hansdebruijnia pusilla. Table 4 shows the updated mammalian fossil list of the Tuchengzi Fauna and a comparison with other related faunas.Table 4
Table 4Composition of the Tuchengzi Fauna and a comparison with other related faunas
| TCZ | ETT | BGD | MH | WLH | |
|---|---|---|---|---|---|
| Insectivora | |||||
| Talpidae | |||||
| Yanshuella | primaeva? | primaeva | |||
| Rodentia | |||||
| Zapodidae | |||||
| Lophocricetus | grabaui | grabaui | xianensis | cf. grabaui | |
| Cricetidae | |||||
| Sinocricetus | zdanskyi | zdanskyi | zdanskyi | ||
| Myospalacidae | |||||
| Prosiphneus | licenti | eriksoni | eriksoni | murinus | |
| Muridae | |||||
| Hansdebruijnia | pusilla | pusilla | perpusilla & pusilla | ||
| Lagomorpha | |||||
| Ochotonidae | |||||
| Ochotona | lagreli | lagreli & minor | sp. | lagreli | |
| Perissodactyla | |||||
| Equidae | |||||
| Sinohippus | robustus | sp. | robustus | ||
| Hipparion | fossatum | richthofeni | tchikoicum | sp. | teilhardi & platyodus |
| Rhinocerotidae | |||||
| Chilotherium | gracile | anderssoni | |||
| Aceratherium | huadeensis | ||||
| Artiodactyla | |||||
| Cervidae | |||||
| Eostyloceros | blainvillei & triangularis | ||||
| Euprox | sp. | ||||
| Cervavitus | novorossiae & huadeensis & shanxius | sp. | novorossiae | ||
| Moschidae | |||||
| Moschus | grandaevus | grandaevus | sp. | ||
| Giraffidae | |||||
| Palaeotragus | sp. | sp. | sp. | microdon | |
| Samotherium | sp. | sinense | |||
| Bovidae | |||||
| Dorcadoryx | sp. | ||||
| Plesiaddax | sp. | depereti |
新窗口打开|下载CSV
5 Biochronology
Generally, the composition prosperity of the Tuchengzi Fauna, represented by the combination of Sinohippus robustus-Hipparion fossatum-Chilotherium gracile-Moschus grandaevus-Lophocricetus grabaui-Sinocricetus zdanskyi-Prosiphneus licenti-Hansdebruijnia pusilla, indicates its Badoean age (Qiu et al., 2013). Compared to other Baodean faunas in the faunal sequence in Neogene central Nei Mongol (Qiu et al., 2006; Qiu and Li, 2016), the Tuchengzi Fauna shows similarities with the Baogeda Ula Fauna (Qiu and Wang, 1999; Storch and Ni, 2002; Qiu et al., 2006; Tseng and Wang, 2007; Wang et al., 2012; Deng et al., 2016), the Ertemte Fauna (almost isochronous and homogeneous to Harr Obo Fauna) (Schlosser, 1924; Fahlbusch et al., 1983; Storch and Qiu, 1983; Qiu, 1985, 1987, 1991, 2003; Wu, 1985, 1991; Fahlbusch, 1987, 1992; Storch, 1987, 1995; Fahlbusch and M?ser, 2004), and the Wulanhua Fauna (Deng et al., 2011; Li, 2015). The Wulanhua Fauna lacks small mammals, and is similar to the Tuchengzi Fauna in generic level by sharing Hipparion, Sinohippus, Chilotherium, Cervavitus, Palaeotragus, and Samotherium, but differentiates in having different species, such as Hipparion teilhardi, H. platyodus and Chilotherium anderssoni (Deng et al., 2011). The Tuchengzi Fauna should be younger than the Baogeda Ula Fauna, because the former has some derived taxa such as Lophocricetus grabaui and Hansdebruijnia pusilla, which replace L. xianensis and H. perpusilla, respectively (Qiu and Li, 2016). The Tuchengzi Fauna is close to the Ertemte Fauna by sharing Lophocricetus grabaui, Sinocricetus zdanskyi, Hansdebruijnia pusilla, and Moschus grandaevus. However, the relatively primitive evolutionary stage of Prosiphneus licenti indicates that the Tuchengzi Fauna should be slightly older than the Ertemte Fauna (compared to P. eriksoni).The newly published paleomagnetic dating of the Baogeda Ula Formation (Sun et al., 2018) suggested that the Baogeda Ula Fauna could be placed within chron C4n.1n (7.642-7.528 Ma). The age of the Tuchengzi Fauna should be younger than this. The paleomagnetical dating of Prosiphneus licenti layers in the Qin’an section is in range of 7.6-6.5 Ma (Guo et al., 2002). The Tuchengzi micrommalian assemblage can also be compared to those from the Mahui or Gaozhuang formations in Yushe Basin. In Yushe Basin, Ochotona lagreli occurs in the Late Miocene Mahui Formation and in the Early Pliocene Gaozhuang Formation (Wu and Flynn, 2017), Lophocricetus cf. L. grabaui only appears in the locality YS8 in the Mahui Formation (about 6.3 Ma) (Qiu, 2017). Dong et al. (2018a) pointed out that some taxa from the Tuchengzi Fauna, such as Eostyloceros blainvillei, E. triangularis, and Cervavitus shanxius are also common elements in the “Zone I” or the Mahui Formation in Yushe Basin (Tedford et al., 1991). The paleomagnetic dating of these large mammal fossil horizons in the Yushe Basin is in range of about 6.5-6.0 Ma (Flynn et al., 1997; Opdyke et al., 2013). The Ertemte Fauna has no precise magnetic dating, but a biochronological estimation constrained it in the latest Late Miocene (slightly earlier than 5.3 Ma), and its estimated age has been accepted by many researchers (Qiu Z D et al., 2013; Qiu Z X et al., 2013). Here we can roughly constrain the age of the Tuchengzi Fauna into a relatively long interval of about 6.5-5.3 Ma. In fact, colleagues from the Institute of Geology and Geophysics, Chinese Academy of Science carried out a paleomagnetic sampling project during the excavation in 2013, and the preliminary result is in consistence with our age estimation of mammalian biochronology (Deng C L, personal communication).
6 Paleoenvironment
Generally, the Tuchengzi Fauna is a part of the Late Miocene Chinese Hipparion faunas. The tooth enamel carbon isotopes (δ13C) analysis of herbivores demonstrated that during the Late Miocene, Hipparion faunas in northern China could inhabit the habitats dominated by steppes with C3 grasses (Wang and Deng, 2005; Hou et al., 2006). The rhinoceros Chilotherium gracile, Aceratherium huadeensis, three-toed horse Hipparion fossatum (pro. H. plocodus), zokor Prosiphneus licenti and pika Ochotona lagreli in the Tuchengzi Fauna are all representative dwellers in an open environment. However, based on the brachyodont cheek teeth of Sinohippus roubstus (pro. S. zitteli) and Cervavitus shanxius, they were regarded as browsers feeding on tender leaves (Hou et al., 2007; Pan, 2018). The highly diverse Cervoidea in the Tuchengzi Fauna strongly indicate a forest paleoenvironment. The murid Hansdebruijnia pusilla and jumping mouse Lophocricetus grabaui seem to be adapted to forests or brushes. In a brief, the paleoenvinoment in the Tuchengzi area during the late Late Miocene was possibly a mosaic landscape mixed with forest and steppe.Acknowledgments
The authors would like to express their gratitude to Liu Wenhui and Bai Weipeng from the IVPP, Wang Shengli from the Department of Land Resources of Huade and Cai Baoqun from Xiamen University for their participation during the fieldwork in Tuchengzi, as well as to Qiu Zhuding and Li Qian for their valuable discussion. Many thanks to Hou Yemao for his help with the CT-scans, and to Yinnie O’Connor for her critique of the manuscript.参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 3]
DOIURL [本文引用: 6]
[本文引用: 8]
[本文引用: 3]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
DOIURL [本文引用: 1]
[本文引用: 2]
DOIURL [本文引用: 2]
DOIURLPMID [本文引用: 1]

The initial desertification in the Asian interior is thought to be one of the most prominent climate changes in the Northern Hemisphere during the Cenozoic era. But the dating of this transition is uncertain, partly because desert sediments are usually scattered, discontinuous and difficult to date. Here we report nearly continuous aeolian deposits covering the interval from 22 to 6.2 million years ago, on the basis of palaeomagnetic measurements and fossil evidence. A total of 231 visually definable aeolian layers occur as brownish loesses interbedded with reddish soils. This new evidence indicates that large source areas of aeolian dust and energetic winter monsoon winds to transport the material must have existed in the interior of Asia by the early Miocene epoch, at least 14 million years earlier than previously thought. Regional tectonic changes and ongoing global cooling are probable causes of these changes in aridity and circulation in Asia.
[本文引用: 1]
[本文引用: 3]
DOIURL [本文引用: 1]
[本文引用: 2]
DOIURLPMID [本文引用: 2]

The Glass Flowers Collection, commissioned from Leopold and Rudolf Blaschka, was created in 1886 through 1936 to illustrate the plant kingdom for the Botanical Museum at Harvard University. The models are both examples of superb flameworking, and represent botanical sciences in the late nineteenth and early twentieth centuries. The Blaschkas' reference materials and the artistic license they sometimes incorporated are discussed. Emphasis is given to the early lifecycle series (1889, 1893) and the last models that depict blight on temperate fruits (1929, 1932, 1936).
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 3]
[本文引用: 4]
[本文引用: 2]
[本文引用: 2]
DOIURL
[本文引用: 6]
[本文引用: 7]
DOIURL [本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 4]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 5]
[本文引用: 1]
DOIURL [本文引用: 2]
[本文引用: 4]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 3]
[本文引用: 2]
[本文引用: 3]
[本文引用: 1]
[本文引用: 3]
