 ,1,2,*, 福雅曼3, 王敏1,2, 郑晓廷4,5
,1,2,*, 福雅曼3, 王敏1,2, 郑晓廷4,5First report of immature feathers in juvenile enantiornithines from the Early Cretaceous Jehol avifauna
Jingmai K. O’CONNOR ,1,2,*, Amanda FALK3, WANG Min1,2, ZHENG Xiao-Ting4,5
,1,2,*, Amanda FALK3, WANG Min1,2, ZHENG Xiao-Ting4,5收稿日期:2019-04-15网络出版日期:2020-01-20
| 基金资助: |
Corresponding authors: *jingmai@ivpp.ac.cn;jingmai.oconnor@gmail.com
Received:2019-04-15Online:2020-01-20

摘要
换羽——将一种羽毛替换成另一种的过程——对鸟类具有非常重要的生物学作用。这一过程能够每年将受损的羽毛替换掉,产生与个体发育相关的羽毛类型,或产生处在繁殖活跃期成年个体所具有的覆羽类型。处于萌发阶段的未成熟羽毛被角质鞘包裹,外形呈狭窄管状,没有明显特征。角质鞘完全脱落标志着羽毛的成熟。虽然热河生物群发现了大量与皮肤衍生物相关的化石,但是确切无疑的未成熟羽毛还未有报道,尽管九佛堂组(120 Ma)发现的一件窃蛋龙类——似尾羽龙,保存了疑似未成熟的羽毛。一件缅甸琥珀(9 Ma)中的反鸟幼崽保存了处于萌发阶段的羽毛,由于是三维保存,对外皮结构的解读更为直观。描述了发现于九佛堂组的4件反鸟类幼年个体上保存的疑似未成熟羽毛。与现生鸟类相似,上述疑似未成熟羽毛的近端窄,外形没有明显结构,仅在末端显示出分叉的羽支。认为此前报道的反鸟类多齿胫羽鸟(Cruralispennia multidonta)掌部和胫跗骨上的相似类型的羽毛可能也是未成熟羽毛。未成熟羽毛和与性双型相关的装饰性羽毛在反鸟类幼年个体上的同时出现,可能说明新鸟类具有的复杂的换羽模式——与性双型相关的装饰性羽毛在个体达到性成熟若干年后(经历了若干次换羽后)才会出现,并且只在更接近鸟类冠群的一些类群中出现。
关键词:
Abstract
Molting—the process replacing one plumage with another—is a critically important biological function in Aves. This process annually replaces the feather coat, damaged by normal wear and tear, produces ontogenetic changes in feathering, and produces alternate breeding plumages associated with reproductive activity in adults. Immature, growing feathers are encased in a keratinous sheath, giving them a narrow, tubular, and featureless appearance. The complete loss of the sheath indicates the feather is mature. Despite the wealth of integumentary data published from the Jehol Biota, immature feathers have never been definitively reported, although they may potentially be preserved in a juvenile specimen of the non-avian oviraptorosaur theropod dinosaur Similicaudipteryx from the 120 Ma Jiufotang Formation. A developing feather has been reported in a 99 Ma enantiornithine neonate preserved in Burmese amber, in which three-dimensional preservation makes interpretations of integumentary structures more straightforward. Here we report on probable immature feathers in four juvenile enantiornithines (Aves: Ornithothoraces) from the Jehol Group. As observed in developing feathers in extant birds, the purported immature fossil feathers appear proximally narrow and featureless with barbs protruding only distally. Based on our observations, we suggest that similar-appearing feather structures preserved on the manus and tibiotarsus in the holotype of the enantiornithine Cruralispennia multidonta may alternatively be interpreted as immature feathers. The presence of immature feathers in combination with sexually dimorphic ornamental feathers in juvenile enantiornithines suggests the complex molting patterns of Neornithes, in which such ornaments only appear after several years (following several molts) when reproductive activity is achieved, are limited to a subset of crownward avians.
Keywords:
PDF (13622KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
邹晶梅, 福雅曼, 王敏, 郑晓廷. 未成熟羽毛在早白垩世热河鸟类生物群幼年反鸟类中的首次报道. 古脊椎动物学报[J], 2020, 58(1): 24-44 DOI:10.19615/j.cnki.1000-3118.190823
Jingmai K. O’CONNOR, Amanda FALK, WANG Min, ZHENG Xiao-Ting.
1 Introduction
Most data concerning the integument of the non-neornithine Pennaraptora - the clade that includes all dinosaurs (including birds) with pennaceous feathers, comes from the Middle-Upper Jurassic Yanliao and Lower Cretaceous Jehol lagerstatten in northeastern China (Zhang et al., 2006; Sullivan et al., 2017). Thousands of specimens have been collected from these volcanolacustrine deposits, hundreds of which preserve traces of integument that are typically rare in the fossil record. These specimens have provided direct evidence of plumage patterns (Zheng et al., 2017; Li et al., 2018) and melanosome-based coloration (Zhang et al., 2010), revealed extinct feather morphotypes (Zhang et al., 2006; O’Connor et al., 2012), and shed light on the evolution of individual feather tracts (e.g., crus, tail) (Zheng et al., 2013; Wang et al., 2014; O’Connor and Chang, 2015). Despite this wealth of data, many gaps remain in our understanding. The preserved plumage cannot be considered complete in any specimen, and the two-dimensional preservation of most specimens makes preserved traces difficult to interpret with certainty. Ontogenetic changes in plumage, non-melanosome based coloration, the location of apteryia and much more remain largely unexplored.Most modern birds begin with a natal plumage that is replaced, through molting, with a series of plumages (juvenal, pre-basic) until the first basic plumage of the subadult is acquired, and then go through another series of plumages (second basic, third basic) until the definitive basic plumage of the mature adult appears, which may take up to eight years in some species (Lovette and Fitzpatrick, 2004). As a new feather forms it pushes out the older feather so that molting and new feather formation are essentially a single process (Lucas and Stettenheim, 1972). Immature (developing) feathers are readily identifiable as they emerge, being encased in a tubular waxy sheath, which is completely removed through preening after the feather cells have died and dried allowing the curled feather vanes to unfurl into a planar structure revealing their pennaceous morphology (Lovette and Fitzpatrick, 2004). The sheath is a keratinized epithelial tube that forms separately from the feather in the outer epidermal collar (Murphy and King, 1986; Lovette and Fitzpatrick, 2004). The presence of the sheath obscures observation of the feather structure within and gives the feather a narrow and solid appearance; the rachis and barbs are only visible where the sheath has been removed (Figs. 1, 2). Molting occurs in living birds for two reasons: during early ontogeny exchanging natal, juvenal, pre-basic, and non-definitive basic plumages; and as adults in the definitive molt cycle associated with an annual renewal of the basic plumage and seasonally associated with an alternate plumage related to breeding and more rarely, a supplemental plumage that provides camouflage (Amadon, 1966; Lucas and Stettenheim, 1972; Lovette and Fitzpatrick, 2004). If a bird goes straight from the juvenal to the definitive basic plumage, the molt strategy is considered simple. If these two plumages are separated by additional molts (first basic, etc.), the molt strategy is termed complex (Lovette and Fitzpatrick, 2004). When a feather is damaged it is not replaced until the next molt. However, immature feathers may occur outside these ontogenetic or annual molt cycles if a feather is lost entirely in which case it is immediately replaced; this feather replacement is not considered a molt (Lovette and Fitzpatrick, 2004).
Feather emergence has not been convincingly documented in any avian specimen from the rich Jehol Biota. However, immature feathers have been proposed to be present in a juvenile specimen of the oviraptorosaur (Maniraptora: Pennaraptora) Similicaudipteryx (Fig. 3) (Prum, 2010), although this identification is not without controversy (Prum, 2010; Xu et al., 2010a, b). The unusual feather traces preserved in Similicaudipteryx STM 4-1 were originally interpreted as representing a distinct feather morphotype, the so-called ‘proximally ribbon-like pennaceous feathers’ (PRPFs) (Xu et al., 2010a, b). Interpreting two-dimensional fossilized traces is notoriously difficult and with only a single juvenile specimen of Similicaudipteryx available, it is difficult to weigh these two competing hypotheses. However, in this case disagreement may be exacerbated by confusing terminologies. Prum (2010) referred to the immature feathers in STM 4-1 as pin-feathers. This hypothesis was rejected by Xu et al. (2010b) based on the large size of the feather structures in question. Although widely used to refer to all immature feathers (Lovette and Fitzpatrick, 2004), the term pin-feather technically refers only to the early stages of feather growth (early immature), when the developing feather is short and entirely encased in its sheath (and thus resembling a pin) (Lucas and Stettenheim, 1972) (Fig. 2A). At this stage the feather would most likely not be visible, blocked from view by other surrounding feathers, unless it belonged to the first incoming natal plumage of an altricial chick (born naked) or a complete molt (all feathers molting at the same time, rare in Neornithes) (Lovette and Fitzpatrick, 2004). As the feather continues to elongate it becomes a blood quill, the mid-immature stage (Figs. 1D-F, 2B). The name derives from the richly vascularized pulp extending up to the pulp cap, where parts of the feather are completing their keratinization (Lucas and Stettenheim, 1972). Late immature refers to the stage in which the distal half of the feather has emerged from the sheath, exposing the pennaceous vanes (Figs. 1A-C, 2C). A feather is considered mature when the pulp has receded into the calamus and the sheath has been completely removed (Lucas and Stettenheim, 1972). Therefore, the argument of Xu et al. (2010b) that the feathers in Similicaudipteryx STM 4-1 are too large to be pin-feathers (early immature) is technically correct. However, these feathers could still represent mid to late stage immature feathers (Figs. 2C, 3).
Fig. 2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 2Illustration of the stages in feather development
A. pin feather, early immature stage; B. blood-quill, mid-immature stage; C. late immature stage; D. mature feather
Fig. 3
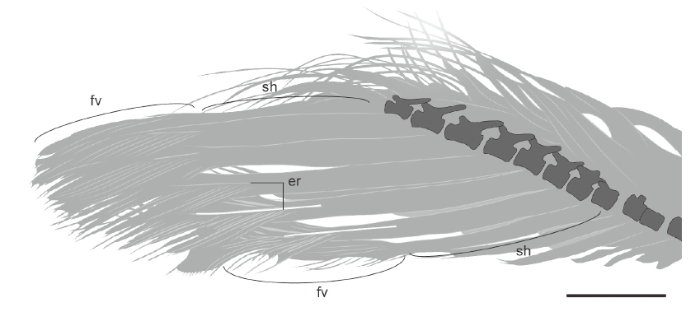 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 3Unusual tail feathers in juvenile Similicaudipteryx STM 4-1, line drawing of rectrices
(modified from S. Abramowicz, O’Connor et al., 2012)
Abbreviations: er. exposed rachis; other abbreviations see
Recently, integumentary data from the Jehol Lagerst?tte is being supplemented by skeletal specimens with associated soft tissue three-dimensionally preserved in Cenomanian (~99 Ma) age Burmese amber (Xing et al., 2016, 2017). One such specimen, HPG-15-1, preserves a cylindrical structure protruding from the caudal region interpreted as an emerging rectrix in the early stages of development (Xing et al., 2017). Identification of this structure as an immature feather is facilitated by the three-dimensional preservation of remains in amber, whereas feather traces in compression fossils are obscured by overlap and their two-dimensional preservation (Xing et al., 2018). Here we describe the preserved integument in four juvenile enantiornithines from the Early Cretaceous Jehol Biota, which we interpret as mid to late immature feathers based on extensive comparison with immature feathers in extant neornithines (Fig. 1). This identification informs on the interpretation of similar integumentary structures in other previously described Jehol specimens. Together with data from previously reported juvenile enantiornithines, we make several inferences regarding the molt pattern in at least some members of this diverse clade.
Fig. 1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 1Immature feathers in juvenile neornithines
A. late immature remiges in Pelecanus occidentalis LACM 86193; B. mid to late immature remiges in Otus asio LACM 100682; C. mid to late immature remiges in Turdus migratorius LACM 100338; D-F. mid immature contour feathers in Tyto alba LACM 100815 (nestling). Note tubular (‘ribbon-like’ in compression fossils) appearance of the proximally sheathed portions of the developing feathers
Abbreviations: fv. feather vane (exposed distal to the proximal developing portion of the feather still encased in the waxy sheath); sh. waxy sheath. Scale bars = 1 cm
Institutional abbreviations GMV, National Geological Museum of China, Beijing, China; GSGM, Gansu Geological Museum, Lanzhou, China; HPG, Hupoge Amber Museum, Tengchong, China; IVPP, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing, China; LACM, Los Angeles County Museum of Natural History, Los Angeles, California, U.S.A.; NIGP, Nanjing Institute of Geology and Paleontology, Nanjing, China; STM, Shandong Tianyu Museum of Nature, Pingyi, Shandong, China; UFRJ-DG, Universidade Federal do Rio de Janeiro, Departmento de Geologia collection, Rio de Janeiro, Brazil.
2 Methods
IVPP V 15564 and V 14980 were studied using a Leica S4E stereo microscope and photographed under normal light using Canon 5D4 digital camera and a Dinolite AM4115ZT. STM 34-1 and STM 34-9 were photographed using a Canon EOS 5D Mark II. Measurements were taken using Fiji (ImageJ) v. 2.0.Laser-stimulated fluorescence (LSF) photography was performed following modified methods after Kaye et al. (2015), using a Nikon D60 with an AF-S Micro NIKKOR 85 mm 1:3.5 G macro lens. The laser used was a 447 nm 400 mW blue Spartan laser pointer (Dragon Lasers) with a Thorlabs EDI-S20-MD mounted engineered diffuser. The diffuser produced a square dot-matrix pattern. During the long-exposure shot required for LSF photography, the combined laser and diffuser were moved back and forth slightly to cover the entire specimen evenly in the light source; otherwise, the photograph showed only tiny dots of light and not a properly fluorescing fossil. To filter out the blue portion of the visible light spectrum, a Midwest Optical LP 470-52 Longpass filter was used.
3 Description
3.1 IVPP V 14980
IVPP V 14980 consists of a fully articulated partial skeleton of a young juvenile, laterally preserved in a slab and counterslab (Fig. 4). It can be assigned to the Enantiornithes based on the presence of a Y-shaped furcula, minor metacarpal that projects farther distally than the major metacarpal, and metatarsal IV that is more slender than metatarsals II and IV with the trochlea reduced to a single condyle (Chiappe and Walker, 2002). The specimen is considered a juvenile based on its proportionately large cranium with proportionately large orbit, unossified sternum, and the absence of fusion between the distal carpals and metacarpals, proximal tarsals and the tibia, and the distal tarsals and the tarsometatarsus (Hu and O’Connor, 2017). The remains of seven unusual feathers are visible in both slabs projecting from the caudal margin of the proximal carpometacarpus and the ulna (Fig. 4). As preserved, the feathers are 2.7-5.1 mm long. Some of these remains are clearly missing their proximal ends and potentially the distal ends may also be incomplete. Barbs protrude from the distal 13%-53% of the feathers (Fig. 4C). Proximal to the visible barbs the feathers are solidly colored, featureless (lacks indication of barbs or rachis), and narrow with parallel margins, overall having a strap-like or ‘ribbon-like’ appearance (‘ribbon-like’ is used here to describe the morphology of proximal portions of feathers that appear solidly colored and strap-like, meaning the width is constant, without evidence of structural elements such as barbs or a rachis; it does not refer to a specific extinct feather morphotype). These unusual feather traces are here interpreted as probable immature feathers.Fig. 4
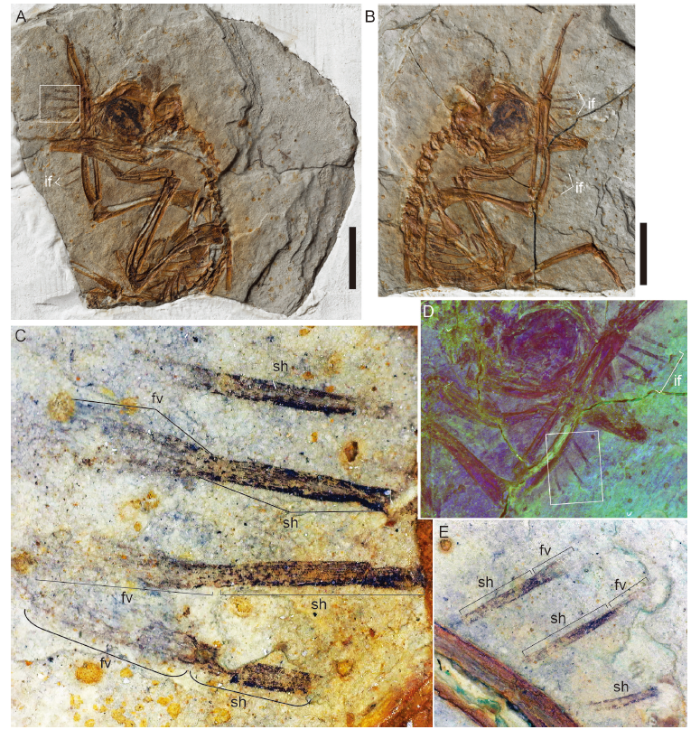 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 4Juvenile enantiornithine IVPP V 14980 preserving probable immature feathers
A. slab A; B. slab B; C. close up of boxed area marked in A showing details of the immature feathers along the distal ulna and proximal carpometacarpus; D. slab B under laser-stimulated fluorescence; E. boxed region in D enlarged to show details of the immature feathers under normal light
Abbreviations: if. immature feathers; other abbreviations see
3.2 IVPP V 15564
Previously described with regards to preserved sternal ossifications (Zheng et al., 2012), IVPP V 15564 consists of a nearly complete and articulated juvenile enantiornithine ventrally exposed preserved in a slab (Fig. 5A) and counter-slab. The remains of three proximally narrow feathers with distal barbs are visible on the dorsal margin of the left humerus with traces of another three feathers projecting from the caudal margin of the distal left ulna and wrist (Fig. 5A-C). These are interpreted as probable immature feathers. Two incomplete remiges are preserved cranially projecting from the manus; one preserves only the calamus region and the other preserves approximately the proximal 33%-50% of the feather (Fig. 5A, B). Their proximal ends are featureless but barbs can be faintly observed for most of the preserved length of the more complete remix. The probable immature feathers on the humerus extend from the distal end of the deltopectoral crest to just before the mid-point of this element, measuring 6.3-8.5 mm in length. Barbs visibly protrude in approximately the distal half (41%-58%) of the feather (Fig. 5C). The feathers preserved near the wrist are shorter (2.8-4.3 mm). Barbs are only visible protruding in the distal most portion (14%-18%) of the feathers. V 15564 additionally preserves a pair of elongate ‘rachis-dominated’ tail feathers (RDFs) (Wang et al., 2014). The feather remains are only faintly preserved along the proximal three-quarters and darkly preserved distally (Fig. 5D). The feather remains are roughly equal in width for their entire length, being featureless throughout, and flexed so that they are ventrally concave. This unusual preservation may suggest that these tail feathers are also immature.Fig. 5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 5Juvenile enantiornithine IVPP V 15564 preserving probable immature feathers
A. slab A; B. left forelimb (enlarged from boxed region indicated in A);C. boxed region in B enlarged to show detail of the immature feathers on the proximal humerus;D. possible late stage developing tail feathers (boxed region indicated in A) under laser-stimulated fluorescence
Abbreviations: rm. remige; other abbreviations see Figs. 1, 4. Scale bar = 1 cm
3.3 STM 34-1
Previously described with regards to ossification patterns in enantiornithines, STM 34-1 represents a nearly complete and articulated juvenile laterally exposed preserved in a slab and counterslab (Zheng et al., 2012). Mature primaries are preserved on the right wing; mature secondaries can be observed on the left wing (Fig. 6A). Body feathers are preserved along the dorsal margin of the body from the braincase to the free caudal vertebrae, ventral to the pygostyle, and on the tibiotarsus. Dense feathering is preserved associated with both humeri and the cranial margin of the wing. The body feathers appear to be immature although interpretations are obscured in most areas by the density of the preserved feathers (whereas identification is much clearer in IVPP V 15564 and V 14980 because the preserved feathers are very sparse with no overlap). The proximal portions of many of the body feathers are dark, featureless, narrow, and strap-like (parallel margins) whereas the distal most portions are lighter in color and barbs are visible. This morphology is clearest in regions where the feather preservation is sparser, such as along the tibiotarsus (Fig. 6A). The overall morphology of the body feathers strongly resembles the immature feathers in some juvenile neornithines in which a majority of the feather remains sheathed and the feathers have a curved appearance and are oriented perpendicular to the body (Fig. 1D-F). The feathers in STM 34-1 are also reminiscent of the unusual feathers preserved in Cruralispennia V 21711 (Fig. 7).Fig. 6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 6Juvenile enantiornithines preserving possible immature feathers
A. STM 34-1 with mature remiges and densely preserved body feathers that appear to be immature; B. STM 34-9 with sparsely preserved probable immature body feathers and a pair of rachis-dominated feathers; C. close up of the area marked in B showing early and mid-immature stage feathers on the forelimb. Scale bars = 1 cm
Fig. 7
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPTFig. 7Unusual feathers in Cruralispennia IVPP V 21711
A. photograph of the full slab; B. close up of the unusual feathers on the tibiotarsus
Scale bar in A equals 1 cm; scale bar in B equals 5 mm. Abbreviations see
3.4 STM 34-9
Described by Zheng et al. (2012) with regards to the morphology of the sternum, STM 34-9 is a nearly complete and articulated juvenile enantiornithine dorsally exposed preserved in a slab and counterslab. It is the only juvenile described here that is not from the Jiufotang Formation but from the older Yixian Formation (Zheng et al., 2012). STM 34-9 has sparsely preserved body feathers located on the neck, wings and abdomen (Fig. 6B, C). These feathers have a solid appearance for most of their length with barbs visible distally in some of the better preserved feathers, suggesting the body feathers preserved in this specimen may be immature. A pair of RDFs is also preserved. Similar to the body feathers these rectrices have a dark, solid appearance along the proximal two-thirds of their length. These tail feathers are poorly preserved but barbs appear to be visible along the distal third. The unusual preservation and curved appearance of these RDFs may suggest they are immature.4 Discussion
Based on gross anatomical observations and comparison with living birds, we suggest that the unusual integumentary structures in IVPP V 15564 and V 14980 that are proximally narrow with distally located barbs - appearing ‘ribbon-like’ - are best interpreted as immature feathers partially encased in a waxy keratinous sheath. Similarly, we infer that the unusual morphology of the feathers in STM 34-1 and STM 34-9 may also be due to immaturity although conclusions are more equivocal due to the large degree of overlap in STM 34-1 and relatively poorer preservation in STM 34-9. In immature feathers the vanes are folded within the sheath, giving the feather a temporarily narrow, tubular appearance, in which rachis and barbs cannot be distinguished (Figs. 1, 2). Reduced into two-dimensional traces, this might appear ‘proximally ribbon-like’ and/or to superficially resemble a hypertrophied rachis. The rachis and barbs only become visible distally in mid and late stage immature feathers (Figs. 1A-C, 2B, C) as the distal portions of the sheath dry out and begin to fall away or are removed by preening exposing the barbs and allowing the vanes to unfurl. The identification of immature feathers in juvenile enantiornithines is unsurprising. Living birds go through a number of molts and plumages early in their ontogeny before reaching the definitive basic plumage characteristic of the mature adult (Lucas and Stettenheim, 1972; Gill, 2007).4.1 Identification of probable immature feathers
Two-dimensionally preserved immature feathers appear superficially ‘ribbon-like’ for a significant portion of their proximal length. Similar ‘proximally ribbon-like’ (or ‘wire-like’) feather structures have been previously described in two specimens from the Jehol Biota - IVPP V 21711 the holotype of enantiornithine Cruralispennia multidonta (inferred to be a subadult; Fig. 7) and a juvenile specimen of the basal oviraptorosaur Similicaudipteryx STM 4-1 (Fig. 3). Each taxon is currently inferred, at least by some, to possess a unique feather morphotype that is now extinct (Xu et al., 2010a; Wang et al., 2017). Juvenile Similicaudipteryx STM 4-1 preserves rectrices that are described as ‘ribbon-like’ along their proximal two-thirds and normal pennaceous in appearance in the distal third, interpreted both as an unusual feather morphotype (the so-called proximally ribbon-like pennaceous feathers or PRPFs) (Xu et al., 2010a, b) and alternatively as pin-feathers (Prum, 2010). Cruralispennia V 21711 preserves feathers described as “narrow and wire-like almost the entire length, only distally fraying into individual hair-like barbs that account for <10% the length of the feather,” which were considered a distinct (and previously undescribed) feather morphotype in the original description (Wang et al., 2017). The feathers in Cruralispennia were originally described as present on the wrist and tibiotarsus (Wang et al., 2017). The feathers in V 21711 are densely preserved surrounding the skeleton (Fig. 7). Although details of the plumage are heavily obscured by a high degree of overlap, re-examination suggests similar feather structures may additionally be present on other regions of the body (e.g., lateral body feathers). The descriptions of the feathers in both Similicaudipteryx STM 4-1 and Cruralispennia V 21711 is consistent with the unusual feathers preserved in the juvenile enantiornithines described here, as well as that of mid-immature feathers in extant birds (Fig. 1D-F). Although two-dimensional preservation makes it nearly impossible to interpret feathers in compression fossils unequivocally, we feel the unusual feather structures preserved in enantiornithines V 14980, V 15564, STM 34-1, STM 34-9, and V 21711 can best be interpreted as immature feathers based on the following lines of evidence:First, these unusual feather structures co-occur with normal feathers throughout different tracts of the body in STM 34-1 and Cruralispennia V 21711. In extant birds, feathers that are ornamental in structure and not just color typically occur in discrete regions, whereas the feathers here in question have a patchy distribution throughout many regions of the body consistent with a molt in which the entire plumage is in the process of being replaced so that immature feathers appear in every tract on the body. Filoplumes, specialized sensory feathers, occur throughout the plumage and have barbs only distal on the rachis, thus superficially resembling the purported immature feathers described here, although filoplumes differ in that they are very small and have a narrow, delicate rachis compared to normal feathers (Lucas and Stettenheim, 1972; Lovette and Fitzpatrick, 2004). The width of the proximally strap-like portion of the feathers in question is greater than the rachis of normal body feathers in other Jehol birds (Wang et al., 2018) making it unlikely that these are filoplumes. Furthermore, such a robust rachis would impede on the sensory function of the filoplume, which must be delicate in order to sense aerodynamic disturbances (Lucas and Stettenheim, 1972). It is unknown when such sensory feathers evolved in Aves (or a more inclusive clade of pennaraptorans), although it is unlikely (although not impossible) given their diminutive size in extant birds, that these would be the only feather type to preserve in specimens such as V 14980 (Fig. 4).
Second, the anatomical location of these feathers in Cruralispennia V 21711 (as well as V 14980, V 15564, STM 34-1, and STM 34-9) is inconsistent with the previous interpretation of these feathers in V 21711 as a unique morphotype of ornamental feather (Wang et al., 2017) - wiry ornamental feathers projecting craniolaterally from the wrist would presumably impede flight. However, immature feathers in neornithines often protrude in unusual directions while incased in sheath (Fig. 1C), as also observed in STM 34-1 (Fig. 6A).
Third, the large number of loose feathers associated with V 21711 supports interpretations this bird was molting at the time of death and immature feathers would not be unexpected in juveniles such as V 14980, V 15564, STM 34-1, and STM 34-9 (Zheng et al., 2012), presumably exchanging their juvenal plumage for a more mature basic or pre-basic plumage. Immature feathers in the presumably subadult holotype of C. multidonta, may be related to reproductive activity (ushering in an alternate plumage) or seasonal changes in plumage (supplemental plumage). However, the most likely interpretation is that they are part of an annual molt as alternate and supplemental plumages are comparatively less common within Neornithes (Lovette and Fitzpatrick, 2004).
An alternative interpretation is that these feathers might represent unusual taphonomic artifacts resultant from the lacustrine depositional environment since preservation in water can sometimes deform feathers (Foth, 2012). However, this interpretation is not supported given the selectiveness of the purported distortion throughout the plumage of STM 34-1 and V 21711. This also does not explain the frequency of such distortion in juvenile specimens.
Although without further material interpretations are tenuous, we consider that the tail feathers in Similicaudipteryx STM 4-1 are also best interpreted as immature. As immature pennaceous feathers unfurl from their sheaths the proximal most portion of the exposed vane (at the distal-most portion of the sheath) forms a distinct V-shaped morphology (Fig. 1A) that can also be clearly observed in STM 4-1 (Fig. 3). This feature is unfortunately not visible in the immature body feathers in juvenile enantiornithines, probably due to their small size and poor preservation. The presence of immature feathers in the juvenile Similicaudipteryx STM 4-1 is almost certainly related to ontogeny and the appearance of the juvenal plumage. This is supported by the fact that all the immature tail feathers appear to be in the same stage of development, whereas in post-juvenal molts tail feathers are renewed in sequence beginning with the medial pair (Lucas and Stettenheim, 1972; Gill, 2007).
4.2 Rachis-dominated feathers: immature or poorly preserved
Juvenile enantiornithines V 15564 and STM 34-9 both preserve a pair of elongate rectrices that appear unusual when compared to RDFs preserved in subadult-adult specimens (Figs. 6, 7). In both specimens the tail traces are preserved in lateral view and the feathers are slightly curved. The feather remains are darkly colored throughout their preserved length and largely featureless, whereas in the “proximally ribbon-like” portion of RDFs (which consists of rachis) preserved in subadult or adult specimens of Confuciusornis and enantiornithines the proportionately wide rachis is typically observed as an empty space demarcated laterally by faint dark margins that are distally continuous with the pennaceous vane, and marked by a medial stripe (e.g., Confuciusornis V 13156, Eopengornis STM 24-1, enantiornithine indet. GSGM-07-CM-001) (O’Connor et al., 2012; Wang et al., 2014). In the entire preserved portion of the RDFs in V 15564 and STM 34-9 these features are not visible. Instead the entire feather is preserved dark and “ribbon-like” although the proximal two thirds is considerably lighter. Furthermore, the RDFs preserved in all previously described specimens including other juveniles (e.g., UFRJ-DG 031 Av and STM 34-7; Table 1) are perfectly straight (Zheng et al., 2012; de Souza Carvalho et al., 2015), whereas the feathers in V 15564 and STM 34-9 are distinctly flexed. This featureless morphology and curvature may suggest the RDFs in V 15564 and STM 34-9 are still encased in the keratinous feather sheath (mid to late immature feathers). In this interpretation, the darker distal portion is presumably the vaned, melanosome bearing portion of the RDF. The ornamental tail feathers in the pin-tailed ornithuromorph Archaeorhynchus STM 7-11 appear similarly solid and featureless and may also be immature feathers (Wang et al., 2018).Table 1
Table 1List of neonate and juvenile enantiornithines preserving plumage
| Specimen No. | Feathers | Remiges | RDFs | body coverts | mature feathers | immature feathers | No. Sternal ossifications |
|---|---|---|---|---|---|---|---|
| IVPP V 14980 | × | body feathers (wing) | 4? | ||||
| IVPP V 155641) | × | remix, body feathers (wing), RDFs? | 3 | ||||
| IVPP V 142872) | × | remiges, body feathers | 0 | ||||
| STM 34-11) | × | × | × | remiges | body feathers (neck, wing, abdomen, legs) | 4 | |
| STM 34-91) | × | × | × | wing/neck/abdomen body feathers, RDFs? | 4 | ||
| STM 34-21) | × | × | × | remiges, body feathers | 3 | ||
| STM 34-71) | × | × | × | × | remiges, body feathers, RDFs (pair) | 4 | |
| GMV-21583) | × | ×? | - | ×? | possible remiges | possible body feathers | 3 |
| GMV-21593) | × | × | ×? | ? | remiges, possible tail and body feathers | none (visibly preserved) | 4 |
| GMV-2156/NIGP-1307234) | × | × | ? | remiges, possible body feathers | none (visibly preserved) | 3 | |
| HPG-15-15) | remiges, body feathers (primitive) | rectrices (RDFs) | ? | ||||
| UFRJ-DG 031 Av6) | × | × | × | rectrices, body feathers | ? | ||
| MPCM-LH-261897) | × | × | × | remiges, body feathers | 3 |
新窗口打开|下载CSV
The only previous report of immature avian feathers in the Cretaceous fossil record is a developing rectrix preserved protruding from the tail region in an enantiornithine neonate preserved trapped in amber (HPG-15-1) (Xing et al., 2017). This is considered one of the paired RDFs commonly found in enantiornithines, with the second feather in the pair poorly preserved, bent back against the body (Xing et al., 2017). The developing rectrix is preserved in a cylindrical sheath with very short barbs just visible beginning to protrude from the distal tip (see Xing et al., 2017:fig. 7). Parts of the sheath appear to have been taphonomically lost but because of its small size and inclusion in amber, which is cloudy in some parts, details of the developing feather inside are not visible. Despite these limitations, three-dimensional preservation makes it much easier to interpret the fossilized integumentary structures and the observed morphology is fully consistent with early-immature stage developing feathers in extant birds. Loss of the sheath and exposure of the distally projecting barbs while the feather is still so immature is probably abnormal and a result of entrapment in amber and subsequent taphonomic processes. The fact the developing tail feathers are early immature suggests that HPG-15-1 represents an earlier ontogenetic stage than V 15564 and STM 34-9, in which the immature RDFs are proportionately much longer (i.e., more mature). This also suggests that juvenile enantiornithine STM 34-7 (Zheng et al., 2012) is more mature than HPG-15-1, V 15564, and STM 34-9 with regards to plumage, given that the preserved RDFs are mature (fully developed). However, it is possible that these feathers appeared at different times in different enantiornithine lineages and therefore any inference regarding ontogenetic maturity based on plumage is at this time tentative at best.
4.3 Enantiornithine molt patterns
A late-stage enantiornithine embryo from the Jehol Lagerst?tte (IVPP V 14238) preserves traces of developing remiges (flight feathers of the wing) described as “feather sheets” (Zhou and Zhang, 2004); given that the enantiornithine is unhatched, these feathers are very likely mid to late stage immature feathers. Their large size precludes them from being early immature feathers. These feather traces and the plumage in HPG-15-1 strongly suggest that members of the Enantiornithes were born fully fledged and capable of flight soon after hatching, somewhat resembling the super-precocial megapodes, the only group of neornithines in which neonates are similarly born fledged and capable of flight (Zhou and Zhang, 2004; Jones and G?th, 2008; Xing et al., 2017). Megapodes do not fly immediately, requiring nearly two days to dig themselves out of their mounds during which they preen off their feather sheaths and let their feathers dry (Jones and G?th, 2008). Similarly, hatchling enantiornithines would have had to wait until their feather sheaths were removed and their feathers dry before attempting flight. Although ecological and behavioural differences clearly exist between enantiornithines and megapodes (e.g., enantiornithines were arboreal and not mound-nesters), megapodes represent the precocial extreme in extant neornithines and thus the closest analogue for enantiornithine development, for which all evidence indicates a form of extreme precociality (Elzanowski, 1981; Zhou and Zhang, 2004; Xing et al., 2017).We do not consider the sparse plumage preserved in specimens such as V 15564, V 14980, and STM 34-9 to reflect the in vivo condition and thus to represent evidence of sparse altricial-like plumage in some juvenile enantiornithines. Rather, we consider the sparse plumage to be a preservational artifact. This inference is supported by the fact the skeleton in these and all known juvenile enantiornithine specimens are similarly well ossified, which is strongly suggestive of precocial development (Elzanowski, 1981; Starck, 1993). Although we cannot begin to explain the selectivity of the feather preservation in these specimens, we tentatively suggest that the presence of a feather sheath may in some circumstances have aided in the preservation of some of these feathers. Taphonomy is an incredibly complex subject with every possible subdivision of an organism (from organs to cells, and from the plumage to individual feathers and feather parts) representing a unique chemical microenvironment subject to different forms of preservation, producing specimens with vastly different degrees and forms of preservation. However, attempting to account for these preservational differences is clearly beyond the scope of this paper.
The juvenal plumage is marked by the first appearance of pennaceous feathers (Lovette and Fitzpatrick, 2004). The presence of pennaceous feathers upon hatching indicates the absence of a downy natal plumage, which was also suggested for Similicaudipteryx and may represent the primitive pennaraptoran condition (Xu et al., 2010a). Most living birds have one, in some cases more, natal plumage (Lucas and Stettenheim, 1972). All evidence for the Enantiornithes currently indicates a form of super-precociality (hatching fledged with a high degree of skeletal ossification, fairly slow post-natal growth) (Elzanowski, 1981; Zhou and Zhang, 2004; Zheng et al., 2012; Xing et al., 2017), which excludes the presence of a natal plumage based on the presence of pennaceous remiges in hatchlings. A similar pattern is observed in megapodes, which hatch with fully pennaceous plumage and achieve their adult plumage within several weeks in some species, before they reach adult size (Jones and G?th, 2008). The juvenile enantiornithine trapped in amber, HPG-15-1, indicates that although the wings consisted of fully developed remiges, the juvenal plumage in at least some lineages consisted of a sparse coat of primitive feather morphotypes covering other parts of the body (Xing et al., 2017), and was thus very different from the juvenal plumage of super precocial neornithines (i.e., that of megapode neonates) (Jones and G?th, 2008), and unlike that of any extant bird.
IVPP V 15564, V 14980, STM 34-1, and STM 34-9 probably capture one of the first post-hatching molts. V 15564, STM 34-9, and HPG-15-1 preserve what appear to be developing RDFs. The presence of this feature in several juvenile specimens, including fully formed feathers in STM34-7 and UFRJ-DG 031 Av, clearly indicates these ornamental tail feathers appear at a very early ontogenetic stage (Zheng et al., 2012). Evidence from HPG-15-1 suggests that RDFs may appear in the first post-hatching molt in at least one enantiornithine lineage (Xing et al., 2017). If sexually-dimorphic tail ornaments appear in the first molt, it suggests that enantiornithines had only two plumages and went immediately from the juvenal plumage into the adult basic plumage in their first molt. Similarly, megapodes hatch without their tail feathers, which appear after two weeks in the Brush-turkey (Alectura lathami), achieving basic plumage within four weeks of hatching (Jones and G?th, 2008). In contrast, most extant birds require several annual molts before they achieve the definitive basic plumage (Lovette and Fitzpatrick, 2004; Gill, 2007). This suggests that enantiornithine molting patterns were much simpler than that of most neornithines, suggesting the complexity observed in the crown clade is limited to a subset of avians crown-ward of the Enantiornithes and may have co-evolved with rapid growth strategies in the Ornithuromorpha, in which reproductive maturity follows skeletal maturity (enantiornithines show the opposite condition) (O’Connor et al., 2014). However, given the paucity of relevant data in the fossil record it is unlikely we will ever fully understand molting strategies in stem birds and their early evolution in crown Aves with any great certainty.
In other juvenile enantiornithines STM 34-2 and STM 34-7 (Zheng et al., 2012) the plumage is well preserved but immature feathers are not observed (Table 1). The identification of immature feathers in some juvenile specimens and their clear absence in others (e.g., STM 34-2, 34-7) has the potential to inform on the relative age of a particular specimen. However, when comparing degree of sternal ossification between specimens with the presence or absence of immature feathers, no pattern is apparent (Table 1). Previous attempts to correlate degree of sternal ossification with other signs of maturity (e.g., size) have also failed to identify any useful patterns (Zheng et al., 2012; Knoll et al., 2018). This is unsurprising given the apparent diversity in growth strategies gleaned from the results of sporadic histological studies of enantiornithines (Wang et al., 2017; O’Connor et al., 2018) as well as the variation in molt patterns observed in living birds that most likely would have also been present to some degree in enantiornithines (Gill, 2007; Jones and G?th, 2008). The utility of immature feathers to assess maturity is likely further exasperated by the fact that immature feathers are ephemeral features (Swank, 1955) and thus it may be that they are rarely captured by the fossil record. In taxa in which this is a slow, drawn out process lasting months (Lucas and Stettenheim, 1972), evidence of molting is less obvious and may not be detectable in the halo of overlapping feathers that most often surrounds the skeleton in compression fossils in which feathers are preserved. However, the greatest factor preventing the use of feathers to assess maturity is the differential preservation of feathers between specimens, which at this time cannot be accounted for.
5 Conclusions
The preserved integument of four juvenile enantiornithines is described. Unusual traces are morphologically consistent with their interpretation as immature feathers. Detailed examination of gross morphology and comparison with extant birds suggests that some reported proximally ribbon-like (or wire-like) feather morphotypes may in fact represent immature feathers partially encased in sheaths. However, at this time, all interpretations of delicate integumentary structures strictly drawn from observations from compression fossils should be regarded as equivocal. In the future it may be possible to lend further support to this hypothesis through histochemistry or advanced viewing techniques (e.g., SEM). The sum of the currently available evidence suggests that enantiornithines had simple molt patterns compared to living birds, potentially only possessing the juvenal plumage they hatched with and the basic plumage of the adult, which appears far prior to the advent of both reproductive and skeletal maturity.参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 3]
[本文引用: 1]
[本文引用: 4]
[本文引用: 1]
[本文引用: 6]
DOIURL [本文引用: 1]
[本文引用: 2]
[本文引用: 1]
[本文引用: 12]
[本文引用: 11]
[本文引用: 1]
[本文引用: 1]
[本文引用: 3]
[本文引用: 1]
[本文引用: 1]
URL [本文引用: 4]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 5]
[本文引用: 3]
[本文引用: 2]
[本文引用: 1]
[本文引用: 11]
[本文引用: 1]
[本文引用: 5]
[本文引用: 5]
[本文引用: 2]
[本文引用: 1]
[本文引用: 12]
[本文引用: 1]
[本文引用: 1]
[本文引用: 5]
