 ,?,**,2), 龙勉?,**, 郭兴明
,?,**,2), 龙勉?,**, 郭兴明 ,*,3)
,*,3)BIOMECHANICS AND FUNCTIONAL REGULATIONS OF CD44-LIGAND INTERACTIONS1)
Li Linda*, Ding Qihan?,**, Chen Shenbao?,**, Lü Shouqin ,?,**,2), Long Mian?,**, Guo Xingming
,?,**,2), Long Mian?,**, Guo Xingming ,*,3)
,*,3)通讯作者: 2) 吕守芹, 研究员, 主要研究方向: 生物力学. E-mail:lsq@imech.ac.cn;3) 郭兴明, 教授, 主要研究方向: 生物医学信息检测及处理. E-mail:guoxm@cqu.edu.cn
收稿日期:2020-07-31接受日期:2020-11-13网络出版日期:2021-02-07
| 基金资助: |
Received:2020-07-31Accepted:2020-11-13Online:2021-02-07
作者简介 About authors

摘要
作为一种广谱表达的细胞粘附分子, I型跨膜糖蛋白CD44(cluster of differentiation 44)参与细胞增殖、分化、迁移, 血管生成等生物学过程,对于介导细胞信号转导, 调节组织稳态等功能具有关键作用. 特别地,CD44-选择素、CD44 -透明质酸相互作用介导的细胞粘附动力学在经典炎症反应、肿瘤转移或组织特异的肝脏免疫中具有重要作用.该综述分别从细胞层次粘附动力学、二维与三维条件下的分子层次反应动力学、原子层次微观结构以及胞内信号转导通路等方面综述了CD44 -选择素、CD44 -透明质酸相互作用的研究进展及尚待回答的生物力学问题.力学、物理因素对生命活动的不可或缺性逐渐被研究者们接受,力学医学、力学免疫学、力学组学等新概念相继提出. 生理、病理条件下,CD44 -配体相互作用介导的细胞粘附必将受到血流剪切、基底硬度等力学、物理微环境的调控,但是其调控机制还远不清楚. 基于此,本文就CD44 -配体相互作用相关的未来研究方向做出展望, 主要包括:力学、物理因素如何调控CD44 -配体相互作用介导的细胞粘附动力学及其内在机制;CD44 -配体相互作用反应动力学的力学调控规律及结构基础是什么;以及力学作用下CD44 -配体相互作用原子层次的微观结构如何发生动态演化.本文可为深入理解CD44 -配体相互作用的生物学功能及其结构功能关系提供线索.
关键词:
Abstract
As a widely expressed cellular adhesion molecule, type I transmembrane glycoprotein CD44 is crucial in cell proliferation, differentiation, migration, angiogenesis and other biological processes to induce intracellular signal transduction and regulate tissue homeostasis. Especially, cell adhesion dynamics mediated by CD44-selectin and CD44-hyaluronic acid (HA) interactions play key roles in classic inflammatory cascade, tumor metastasis, or tissue-specific liver immunity. This review discussed the progresses and remaining issues of CD44 selectin and CD44-HA interactions in various aspects of cellular adhesion dynamics, two- and three-dimensional molecular reaction kinetics, atomic microstructural features, and intracellular signal transduction pathways. Nowadays, the importance of mechanical and physical factors to biological activities has been gradually accepted by scientific community. New concepts such as mechanomedicine, mechanoimmunology and mechanomics have been put forward one after another. Under physiological or pathological conditions, cell adhesion mediated by CD44-ligand interactions are regulated by in vivo mechanical and physical cues such as blood shear or tissue stiffness, but their regulatory mechanisms are still unclear. From that on, future perspectives related to CD44-ligand interaction were also proposed in this review as follows: how mechanical and physical factors regulate cellular adhesion dynamics and intrinsic mechanism mediated by CD44-ligand interactions; what the mechanical regulation features of molecular reaction kinetics of CD44-ligand interactions and corresponding structural bases are; and how the atomic-level microstructures of CD44-ligand binding evolve dynamically under mechanical forces. This review provides clues for further understanding the biological functions and structure-function relationship of CD44-ligand interactions.
Keywords:
PDF (3816KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
李林达, 丁奇寒, 陈深宝, 吕守芹, 龙勉, 郭兴明. CD44-配体相互作用的生物力学与功能调控1). 力学学报[J], 2021, 53(2): 539-553 DOI:10.6052/0459-1879-20-313
Li Linda, Ding Qihan, Chen Shenbao, Lü Shouqin, Long Mian, Guo Xingming.
引言
CD44 (cluster of differentiation 44)是一种I型跨膜糖蛋白, 广泛分布在心脏、肝脏、肾脏、脾脏、肺等组织器官中, 表达细胞包括内皮细胞、间充质细胞、造血干细胞, 以及单核、巨噬、嗜中性粒、嗜酸性粒细胞和淋巴细胞等血细胞, 同时还包括中胚层来源的细胞等[1]. CD44在多种生理和病理过程中发挥作用, 如器官形成、造血、淋巴细胞活化、肿瘤转移及免疫反应[2]等, 同时是淋巴癌、前列腺癌、宫颈癌、肿瘤干细胞及早期动脉粥样硬化的标志物[3]. 其中通过CD44-配体相互作用介导的细胞-细胞之间或细胞-基质之间的粘附及胞内信号转导通路是CD44在炎症级联反应、肿瘤转移、淋巴细胞归 巢等生理、病理过程中发挥作用的重要途径. 因此, 考察CD44与不同配体相互作用的反应动力学及其介导的细胞粘附动力学与胞内信号通路特征是阐释其功能的基础. 同时, 炎症级联反应、肿瘤转移或淋巴细胞归巢等过程均发生在血流剪切的力学环境中, 血流对细胞的剪切会转化为CD44-配体相互作用的外力, 从而调控其分子间相互作用. 此外, 免疫细胞的募集、归巢或癌细胞的转移还受到基底硬度等其他力学微环境的调控. 因此, 进一步考察外力作用如何通过调控CD44-配体相互作用反应动力学进而调控细胞粘附动力学从而实现特定生物学功能是理解CD44功 能的另一重要内容. 基于此, 本文将重点介绍CD44-配体相互作用在免疫反应过程中的作用及机制研究进展.1 CD44概述
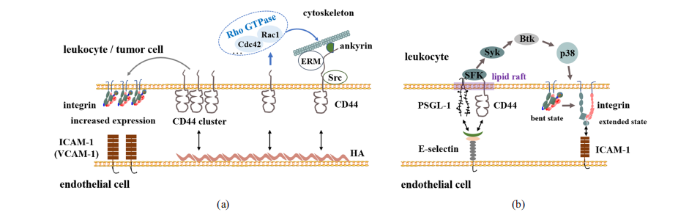
人源CD44是由11号染色体单拷贝编码的跨膜糖蛋白[4], 按照转录方式不同可将CD44前体信使RNA(messenger RNA, mRNA)的20个外显子分为组成型和变异型拼接外显子, 进而分别转录成标准型CD44(CD44s)和变异型CD44(CD44v), 而CD44v又可以通过可变区域的选择性拼接得到不同变异体亚型[4]. 目前发现人类至少有数十种CD44的可变剪切体[1], 其中最常见的亚型包括CD44v4-7, CD44v8-10, CD44v3-10和CD44v1-10[5]. 不同亚型的CD44表达部位不同, CD44s主要分布于间质及造血源性细胞, 而CD44v主要分布于上皮源性细胞和肿瘤细胞. 另外, CD44可进行N糖基化、O糖基化翻译后修饰. 最常见的人源CD44s由 361个氨基酸组成($\sim$ 37.2 kD), 经过糖基化修饰加工[6], 蛋白质分子量可达 80$\sim$100 kD[7]; 而变异型CD44v分子量变化范围约为80$\sim$250 kD. 采用原子力显微镜(atomic force microscopy, AFM)技术扫描CD44蛋白的大小, 结果显示CD44高度为2.3 $\pm$ 1.4 nm, 三维尺寸约为25 nm$\times$30 nm$\times$2.5 nm, 与免疫球蛋白大小相类似[8-9].CD44结构从胞外N末端至胞内C末端包含胞外域、跨膜域和胞内域3部分. 胞外域又可分为氨基末端和近膜域. 氨基末端是含有6个保守半胱氨酸残基的球状结构域, 其中保守半胱氨酸形成的二硫键是N末端球状结构域正确折叠及结构稳定性的基础[10]. 标准型CD44s的氨基末端球状结构域与跨膜段之间是一段由46个氨基酸形成的茎状区[11], 保守性较低, 可被高度糖基化并包含蛋白水解切割位点[12]. 而不同剪接体CD44v的可变区可插入在N末端球状结构域与茎状区之间[13]. CD44跨膜区和胞内区序列高度保守, 其跨膜区结构支持其在细胞膜上的寡聚化(oligomerisation), 并有助于其定位在富含糖脂的膜微区(glycolipid enriched membrane microdomains)[14], 以及其与脂筏的作用[15]. CD44的胞内区含有与细胞骨架锚定蛋白(ankyrin)和ERM (ezrin, radixin, moesin)蛋白 的结合位点. Merlin蛋白(moesin-ezrin-radixin-like protein)也可与CD44胞内端发生相互作用[16]. 对于CD44s, ERM蛋白与细胞骨架锚定蛋白分别与其胞内段的292$\sim$300, 304$\sim$318位氨基酸结合[17]. 由蛋白激酶C(protein kinase C, PKC)触发, CD44胞内尾端丝氨酸(Ser)残基的有序磷酸化和/或去磷酸化, 将增强ERM蛋白与CD44的结合[18]. 激活的ERM蛋白N末端与CD44胞内域结合, 而其C末端与F肌动蛋白结合, 因此, ERM蛋白是将CD44连接至肌动蛋白细胞骨架的桥梁蛋白[19], 可启动CD44下游的胞内信号传导途径, 行使特定的生物学功能. CD44潜在的N糖基化位点(天冬酰胺(Asn)残基)多数位于胞外域的N末端结构域或可变区, 而 O糖基化位点(丝氨酸(Ser)/苏氨酸(Thr)残基)和GAG附着物(GAG attachments) (Ser-Gly多肽)则主要分布在胞外的细胞膜近端区域和可变区[20-21].
2 CD44-配体相互作用在炎症级联反应中的作用
炎症反应(inflammation)是一种由损害或损伤刺激所诱发的适应性反应(adaptive response), 分为急性(acute)和慢性(chronic)炎症反应[22]. 多数急性炎症反应由病毒感染或者机体组织损伤引起, 首先由组织中驻留的巨噬细胞和肥大细胞通过Toll 样受体(toll-like receptors, TLRs)和 NOD 样受体(nucleotide-binding oligomerization-domain protein-like receptors, NLRs)等启动感染识别[23], 随后产生趋化因子、细胞因子、血管活性胺(vasoactive amines)等多种炎性介质, 引发局部炎症, 进而通过炎症级联反应募集血流中的白细胞迁移穿过毛细血管后微静脉(postcapillary venules)到达炎症部位, 吞噬病原体 [24]. 白细胞从血流向炎症部位募集主要包括被血管内皮细胞捕获, 在内皮细胞表面滚动、粘附、爬行, 最后跨内皮迁移等级联过程. 该级联反应由系列受体-配体相互作用介导, 同时受到基质硬度、血流剪切等力学微环境调控. 已有研究表明, 表达在白细胞上的糖蛋白PSGL-1 (P-selectin glycoprotein ligand 1)与表达在内皮细胞上的选择素(selectin)的相互作用主要介导初期的捕获与快速滚动过程, CD44-配体相互作用则主要介导白细胞的慢速滚动, 而整合素-配体相互作用则主要介导后期的稳定粘附、爬行等过程[25]. 下文将重点介绍CD44-配体相互作用在炎症级联反应过程中的作用.2.1 CD44配体—选择素
I型跨膜蛋白选择素是CD44介导细胞-细胞粘附的主要配体之一, 包括P, E与L选择素3个家族成员, 其结构从胞外N末端至胞内C末端依次包括钙型凝集素结构域(calcium-type lectin domain, Lectin)、类上皮生长因子样结构域(epidermal growth factor-like module, EGF), 起粘附补体蛋白作用的多个重复序列(consensus repeats, CR)、跨膜区域(transmembrane, TM)和胞内区(cytoplasmic, Cyto). 三者之间的区别是P, E与L选择素分别含有9, 6, 2个CR结构域, 从而具有不同长度[26-27]. 尽管具有相似结构, 但是3种选择素具有不同生物学功能. L选择素在造血干细胞和成熟白细胞上高表达, 并且L选择素容易水解, 即使在没有化学因子的刺激下, 力学因素如流体剪切即可以使得中性粒细胞(polymorphonuclear neutrophil, PMN)上L选择素发生水解[28]; P选择素主要表达在血小板和内皮细胞, 并分别储存在$\alpha $颗粒和Weibel-Palade小体中, 而促炎刺激可促使P选择素通过细胞内储藏小体与质膜的融合, 从而迅速从$\alpha $颗粒转移至细胞表面; E选择素主要表达在血管内皮细胞上, 其表达水平受到炎症介质的调控. 在人源血管内皮细胞中, 由肿瘤坏死因子$\alpha $ (tumor necrosis factor alpha, TNF-$\alpha )$, 脂多糖(lipopolysaccharide, LPS)和白细胞介素1$\beta $ (interleukin-1$\beta $, IL-1$\beta )$诱导上调表达的主要是E选择素[29].炎症反应中CD44- E选择素相互作用的研究较为充分. 在中性粒细胞参与的炎症反应中, CD44被鉴定为新型E选择素配体, 表明CD44- E选择素相互作用可以介导人源及鼠源中性粒细胞在血管内皮细胞上的粘附[30]. 通过小鼠微血管内活体成像, 发现CD44介导的中性粒细胞的缓慢滚动依赖于E选择素, 而P选择素与PSGL-1相互作用则是初始捕获和快速滚动所必需的[30]. CD44- E选择素相互作用与PSGL-1$-$E选择素相互作用类似, 均可激活中性粒细胞内Src家族激酶(SFK)和下游信号, 进而激活整合素$\alpha _{\rm L}\beta_{2}$变构, 使中性粒细胞发生慢速滚动, 而共同敲除CD44和PSGL-1的小鼠则有效阻止中性粒细胞在炎症组织部位的募集[31]. CD44对于T淋巴细胞在炎症反应中的募集同样具有重要作用. T淋巴细胞上表达的CD44是调控其滚动粘附以及从滚动到稳定粘附的主要配体[32]. 另外, 在人造血干细胞上表达的CD44具有特殊的糖基化, 同时又是E, L选择素的配体, 所以又被称为造血细胞E/L选择素配体HCELL[33]. 通过敲低小鼠CD44基因实验证实, T淋巴细胞上的CD44 / HCELL和PSGL-1是E选择素的主要配体, 而非CD43[34]. 而且, CD44- E选择素相互作用可调控PSGL-1与L选择之间的簇集、进而调控中性粒细胞的慢速滚动过程[35]. 利用超分辨荧光显微镜可进一步考察E选择素结合对CD44分布的调控, 结果表明CD44和胞内肌动蛋白细胞骨架的重组是 E选择素与CD44相互作用以及外部剪切应力共同作用的结果[36]. 相比较而言, P选择素则很少被报道为CD44的配体. 虽然L选择素被认为是CD44的配体, 但作为表达在白细胞同侧的两个分子, CD44与L选择素通过何种相互作用介导炎症反应中白细胞的迁移动力学尚不明确.
简言之, CD44-选择素相互作用, 尤其是E选择素, 是介导炎症反应中免疫细胞募集的重要分子体系. 与 E选择素与E选择素配体-1(ESL-1)、 E选择素与PSGL-1相互作用体系相比, 三者在炎症反应中具有比较明确的功能分工. PSGL-1 主要在初期白细胞捕获过程中起作用; ESL-1 在初期捕获到稳定慢速滚动过程中起作用; 而CD44则主要介导慢速滚动[35].
2.2 CD44配体——透明质酸
透明质酸(hyaluronic acid, HA)是脊椎动物中细胞外基质的重要组成部分. HA是葡萄糖醛酸和N-乙酰氨基葡糖通过交替$\beta $-1,4和$\beta $-1,3糖苷键结合的具有重复二糖单元的线性聚合物, 典型分子量约为1 MDa, 长度可达微米量级, 在多种病理、生理过程(如炎症反应、免疫应答、胚胎发生、 肿瘤发展、 骨关节炎和动脉粥样硬化)中起重要作用[37-39]. 目前观点认为, 低分子量HAHA(LMW-HA)起到促进炎症的作用, 而高分子量HAHA(LMW-HA)具有抑制炎症的作用[40]. 低分子量HA与CD44相互作用可促进细胞释放IL-10和转化生长因子$\beta $1(transforming growth factor-$\beta $1, TGF-$\beta $1), 加剧炎症反应[41]. 低分子量HA还可以上调CD44表达, 同时增加蛋白激酶$\delta $(PKC$\delta )$和蛋白激酶$\varepsilon $(PKC$\varepsilon )$表达, 并对软骨细胞产生损伤, 增强炎症反应. 而中、高分子量HA对软骨细胞无作用, 并且高分子量HA可抑制PKC$\delta $, PKC$\varepsilon$, 核因子$\kappa $B (nuclear factor kappa-B, NF-$\kappa $B)的活化和炎症反应[42]. 另外, HA片段上调CD44和TLR-4表达, 激活NF-$\kappa $B易位并增加有害细胞因子TNF-$\alpha $, IL-6和IL-1$\beta $分泌. 特异性CD44阻断抗体可降低CD44和细胞因子表达水平[43].CD44可通过两种方式与HA相互作用, 一是细胞膜定位的CD44通过特异性相互作用将可溶性HA锚定在细胞膜上; 二是免疫细胞或癌细胞表达的CD44与其他细胞膜表达或锚定的HA相互作用直接介导细胞粘附. 细胞表面高表达的CD44与富含HA的胞外基质相互作用是介导脑癌细胞侵袭的主要分子体系, 并且癌细胞在迁移过程中呈现独特的微触角结构[44]. 与CD44-选择素相互作用类似, CD44-HA相互作用介导的细胞粘附同样在炎症反应中起重要作用, 其中报道最多的是其介导的T淋巴细胞归巢过程. 在化学因子PMA (phorbol 12-myristate 13-acetate)诱导条件下, T淋巴细胞上CD44与内皮细胞上HA相互作用介导其滚动粘附[45]. 而且, T细胞的活化增加其表面CD44与内皮细胞上HA的结合能力. 在体研究表明, 注射超抗原葡萄球菌肠毒素B后, T细胞募集到发炎腹膜部位依赖于CD44和HA之间的相互作用[46]; TNF-$\alpha $诱导的炎症小鼠模型中Th1和Th2细胞在体内的滚动粘附同样依赖于CD44-HA相互作用[47]. 另外, CD44也是整合素VLA-4 (integrin $\alpha_{4}\beta _{1})$-VCAM-1(vascular cellular adhesion molecule 1)相互作用介导T细胞在内皮细胞上稳定粘附的必要条件[48]. 此外, CD44-HA相互作用同样可以介导中性粒细胞向炎症部位的募集[49-50]. 另一方面, CD44-HA相互作用受到多种因素的调控. 细胞表面HA延伸形成的缆状结构可促进巨噬细胞在特定炎症组织的定位[51]. CD44胞外域糖基化程度以及CD44胞质端特定丝氨酸残基磷酸化均会调控其与HA的结合能力[52]. 抗原受体、细胞因子(如IL-2)、肿瘤坏死因子(TNF)以及趋化因子(包括MIP-1$\beta $ (macrophage inflammatory protein 1 $\beta )$, IL-8和RANTES等)均可激活CD44、从而进一步增强其与HA的结合促进T细胞粘附[53]. 而IL-1$\alpha $, IL-1$\beta$, IL-3, 粒细胞巨噬细胞集落刺激因子(GM-CSF)、干扰素$\gamma $ (IFN-$\gamma )$和LPS等均可通过TNF诱导外周血单核细胞上CD44与HA的结合 [54-55]. 综上所述, CD44-HA相互作用是介导生命体免疫反应的重要分子体系.
CD44-HA相互作用在肝脏免疫中的作用更为凸显. HA在肝脏中的表达相对于其他器官更高, 而且主要分布在肝血窦内[56]. 肝血窦作为肝脏内特化的毛细血管网络, 是肝脏免疫发生的主要场所. 与经典炎症级联反应中白细胞的募集动力学不同, 白细胞在肝脏免疫过程中的募集主要发生于肝血窦内, 仅有20%$\sim$30%的白细胞在肝血窦后微静脉粘附. 由于肝血窦内皮细胞上极少量表达选择素, 白细胞在肝血窦内的募集不会发生滚动粘附[57]. 根据诱发因素不同, 肝血窦内的中性粒细胞主要通过两种途径发生募集. 在肝脏无菌炎性损伤中, 凋亡细胞释放的DAMPs(damage associated molecular patterns)直接或间接激活中性粒细胞, 并刺激内皮细胞上调表达ICAM-1(intercellular cell adhesion molecule-1), 进而与中性粒细胞上的粘附分子整合素$\alpha_{\rm M}\beta_{2}$发生相互作用, 介导中性粒细胞粘附, 随后中性粒细胞在肝内驻留的巨噬细胞—枯否氏细胞(kupffer cell)等—分泌的趋化因子梯度引导下进行定向爬行, 到达特定位点进行跨膜迁移进入损伤组织. 而在有菌炎症(如内毒素血症和革兰氏阴性菌败血症等)过程中, 高水平LPS的刺激促炎因子IL-10表达上调、进而抑制$\alpha_{\rm M}\beta _{2}$的表达, 此时CD44取代行使功能, 通过与HA相互作用介导中性粒细胞募集[58]. CD44敲除可有效降低LPS刺激条件下中性粒细胞的粘附[59]. 更进一步, 阻断CD44-HA相互作用有效降低了LPS小鼠肝血窦内中性粒细胞的粘附, 而对其在窦后微小静脉内的滚动粘附过程则没有影响, 而且在此过程中起作用的CD44是中性粒细胞上而不是内皮细胞上表达的CD44[56]. 另外, 一种血清来源的HA相关蛋白SHAP通过与HA共价结合形成HA/SHAP复合物, 可显著增强HA与CD44的相互作用[60].
简言之, CD44-HA相互作用是介导免疫过程中白细胞募集的另一重要分子体系. 但是相对于在经典炎症反应中起重要作用的选择素$-$ PSGL-1、选择素$-$ CD44等分子体系而言, CD44-HA相互作用更具有其器官特异性, 尤其是在肝脏免疫中起重要作用, 但是其分子机制尚不明确, 其主要原因在于HA分子量的多样性及不同组装形式. 比如, 不同分子量的可溶性HA对炎症反应效果不同, 而不同分子量膜定位的HA对介导细胞粘附有什么样的差异?其不同组装形式对细胞粘附的调控?膜定位条件下CD44-HA相互作用反应动力学特性与其他细胞粘附分子配体相互作用反应动力学之间的区别等问题, 目前均不明确.
3 CD44-配体相互作用的反应动力学及力学调控
受体-配体相互作用反应动力学是调控细胞粘附动力学的基础. 分子间相互作用分为三维、二维反应两种形式. 三维反应是指特异性相互作用的分子双方至少有一种在溶液中处于游离态(如血液中血浆蛋白和抗体), 具有空间三维运动自由度, 因而易于与另一种分子发生相互作用; 三维反应表征的是大量分子的统计平均性质, 通常可用确定性化学反应动力学理论来描述, 常用的实验测量方法包括表面等离子共振(surface plasmon resonance, SPR)等. 二维反应是指特异性相互作用的分子双方分别被锚定在两个表面上, 仅具有沿表面面内扩散的二维运动自由度, 但缺乏沿垂直于表面法向运动的自由度(如白细胞或内皮细胞表面的选择素配体相互作用等); 因为接触面受限, 二维反应中参与作用的分子数目少, 表征的是有限数目受体-配体间结合和解离的随机动力学行为; 而且, 二维反应形成的分子复合物为两个相对表面之间提供了直接的物理连接, 因此其反应动力学还受到外力及其他物理因素的调控, 具有力学-化学耦合特征. 常用的实验测量方法包括原子力显微镜、生物膜力探针(biomembrane force probe, BFP)等典型的分子生物力学测量手段[61-62].为进一步理解CD44-选择素、CD44-HA相互作用介导的细胞粘附动力学差异, 以下着重介绍该分子体系的反应动力学特征及其力学调控规律. 目前已报道的分子层次三维反应动力学参数汇总于表1中. 在无外力作用下, E选择素与PSGL-1相互作用的负反应率$k_{\rm off}$与 E选择素与CD44相互作用基本相当, 而正反应率$k_{\rm on}$略低, 进而导致其解离常数$K_{\rm D}(K_{\rm D}=k_{\rm off}$/$k_{\rm on})$略高, 也就是说 E选择素与CD44相互作用结合更快. 虽然不同文献报道的具体数值有差异, 但是二者间的相对趋势保持不变. 如果是 E选择素$-$ PSGL-1, E选择素$-$ CD44两对分子体系与CD44-HA相互作用去比较, 后者的正反应率显著升高, 而负反应率没有太大差异, 最终导致其解离常数$K_{\rm D}$远低于E选择素配体相互作用, 表明CD44-HA相互作用的结合更快、更稳定. 一个例外是当HA分子量为6.4 kD时, 其与CD44相互作用的负反应率$k_{\rm off}$明显高于其他分子量HA, 提示特别低分子量的HA与CD44相互作用较弱. 另外, 从已有数据可以看出, 当HA分子量>100 kD时, 其与CD44相互作用的正反应率从6.4, 31 kD时的$\sim$ 10$^{4}$ M$^{-1}$s$^{-1}$显著升高至10$^{6}$$\sim$10$^{7}$ M$^{-1}$s$^{-1}$, 表明CD44-HA相互作用能力与HA的分子量密切相关. 而只有HA寡糖大于20个残基才能有效与CD44结合[63].
Table 1
表1
表1基于表面等离子共振技术的不同分子体系三维反应动力学参数比较
Table 1
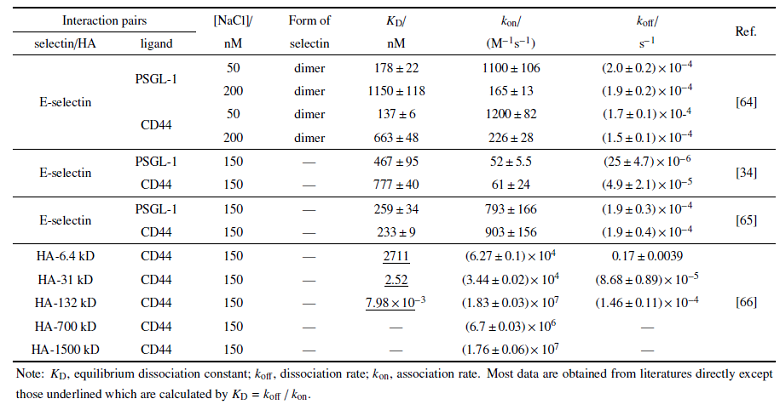 |
新窗口打开|下载CSV
力学调控下不同分子间相互作用反应动力学参数汇总于表2中, 主要是基于AFM技术对不同CD44亚型配体相互作用的测量. 结果显示: 在大致可比的加载率条件下(0.46$\sim$4.58 或0.45$\sim$5.22 nN/s), 不管是单键还是多键相互作用, CD44-HA相互作用断裂力强度约在20$\sim$50 pN, 相应的零力下负反应率分别约为0.3 $\pm$ 0.5, 0.57 $\pm$ 0.11 s$^{-1}$. 不管是变异体CD44v或标准型CD44s, 其与HA相互作用的负反应率显著低于其与P选择素、纤维蛋白或纤维蛋白原的相互作用, 说明CD44-HA相互作用更稳定. 对于同样表达CD44的细胞, AFM探针上直接包被HA与探针上固定包被HA的微珠的测量则表现出明显差异. 前者获得的CD44-HA相互作用的断裂力范围与底板直接包被CD44或含CD44的磷脂双分子层的实验体系基本可比, 约为几十皮牛. 而探针上固定包被有HA的微珠时, 断裂力则增加至nN量级; 这可能与细胞较软, 与微珠接触面大, 从而导致是多键相互作用有关. 另外, 比较不同分子量HA与CD44相互作用发现, 高分子量HA的断裂力更低[67]. 从表2统计也可以看出, 二维条件下获得的CD44-配体相互作用负反应率明显高于三维条件下(表1)的结果, 表明二维约束及外力作用对其反应动力学的调控作用.另外, 针对CD44-选择素、CD44-HA相互作用体系而言, 虽然已有研究报道了二维条件下的相互作用强度及零力下负反应率, 但是负反应率随外力的变化规律则鲜有报道. 需要说明的是, 考虑到不同实验条件、实验手段之间的差异, 导致无法对测量结果进行直接比较, 所以这里没有将选择素与PSGL-1相互作用的结果统计在内.
Table 2
表2
表2基于原子力显微镜技术的不同分子体系的二维反应动力学参数比较
Table 2
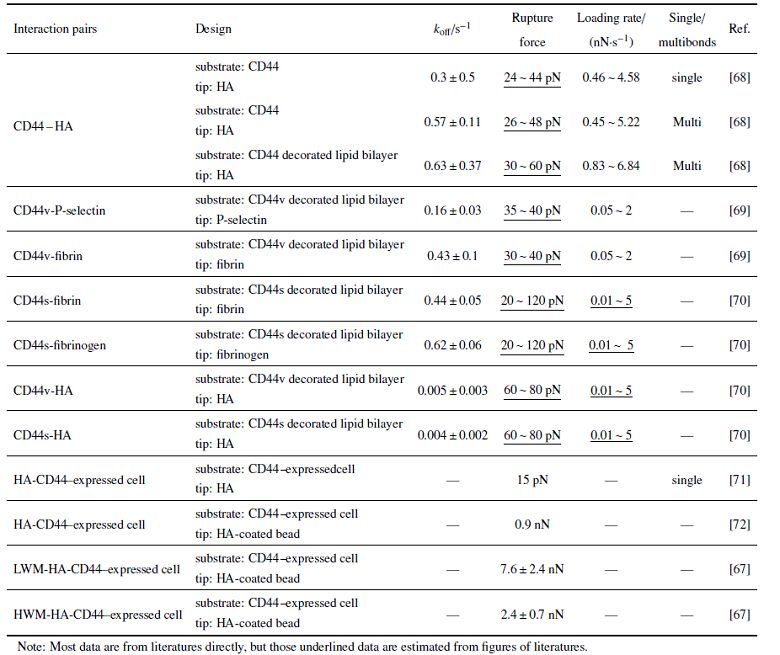 |
新窗口打开|下载CSV
除了上述二维、三维条件下分子层次定量反应动力学测量, 外力作用下CD44-配体相互作用介导的离体细胞层次粘附动力学也有系列报道, 最典型的是模拟血流剪切的平板流动腔技术. 已有研究表明, 虽然生理条件下CD44同为三种选择素的配体[73-74], 但是其结合强度不尽相同. 流体剪切条件下, 结肠癌细胞LS17T, T84等来源的CD44v与选择素相互作用介导的微珠滚动速度表现为E选择素最慢, 次之是P选择素, 而L选择素最快[75-76]. 另一方面, 流体剪切条件下, 表达有CD44的白血病细胞株KG-1a在HA包被的底板上呈现多相粘附特征: 在约0.2 dyn/cm$^{2}$下发生滚动粘附, 而且滚动细胞数目随着流体剪切的增加而增加, 在0.7$\sim$1.0 dyn/cm$^{2}$时滚动细胞数量达到最高, 然后随着流体剪切的进一步增加滚动细胞数量逐渐减少, 抗剪切能力甚至可达100 dyn/cm$^{2}$[77]. 造血祖细胞在包被HA的底板上也存在类似现象, 最优剪切力在1.0 dyn/cm$^{2}$左右[78]. 此外, CD44介导的人源胶质母细胞瘤的粘附和迁移速度取决于HA水凝胶的硬度[79], 表明CD44-HA相互作用除受流体剪切外, 同时受到硬度等力学微环境的调控. 值得注意的是, CD44-HA相互作用介导的细胞粘附动力学随流体剪切呈现的多相特征与选择素$-$ PSGL-1相互作用介导的细胞粘附动力学类似, 而该特征是由外力作用下选择素与PSGL-1相互作用的逆锁键特征决定的[80]. 不同流体剪切条件下白细胞滚动动力学改变的研究结果表明, 外力可以将HA-CD44相互作用从低亲和力状态转变为高亲和力状态[81]. 而CD44-选择素相互作用介导的细胞粘附动力学具有什么样的特征?外力如何调控等问题, 则鲜有报道.
综上所述, CD44-选择素/HA相互作用不论是在分子层次的反应动力学还是在细胞层次的粘附动力学, 以及力学调控规律的研究还不完善. 而CD44不同剪接体或糖基化修饰等的多样性、HA不同分子量大小或组装方式, 以及不同实验条件、手段的差异等, 导致现有数据之间难以进行直接比较, 从而无法更好地阐释其生物学功能.
4 CD44-配体相互作用的微观结构基础
结构决定功能, 分子微观结构特征决定了分子间相互作用反应动力学, 进而决定细胞层次粘附动力学及其生物学功能. 因此, 明确CD44-配体相互作用的微观结构特征有利于更好阐释CD44-配体相互作用的差异以及在炎症级联反应过程中的作用. 已有研究通过系列氨基酸位点突变实验表明CD44胞外氨基末端的球状结构域(32$\sim$132位氨基酸)是CD44的配体—胶原蛋白, 层粘连蛋白, 纤连蛋白以及细胞表面受体(如E/L选择素)—的主要结合位点[82-83], 且该结构域内的二硫键对于CD44与HA的结合也至关重要[84]. 此外, CD44的胞外区有两段高度保守的BX7B多肽片段, 其中一段为38$\sim$46位氨基酸片段, 参与其与HA的结合(其中B代表精氨酸Arg或赖氨酸Lys, X7代表任何7个非酸性氨基酸). 另一个BX7B片段位于第一个片段"下游"约100个氨基酸的位置, 同样可以与HA作用[85]. 但是其微观结构特征尚不清楚.目前报道的原子层次精细结构主要包括CD44 N-末端的HABD(HA binding domain)结构域[86], 以及HABD-HA相互作用的复合物结构[87-88]. 通过比较HA结合前后的构象变化, 提出了CD44-HABD结构域存在两种不同程度的变构效应: 一是HA结合导致HABD结构域的Link domain C-末端扩展区的有序$\beta $9-sheet变成高度无序的loop区, 并从Link domain脱离, 而且HABD的C-末端片段在HA结合状态下柔性增加. 根据该构象差异, 将未结合状态和HA结合状态下的HABD构象分别称为"有序(O)"和"部分无序(PD)"构象. 超过90%的HABD被认为在HA结合状态下采用PD状态, 也就是说PD构象具有更高的亲和性[89]. 二是HA的结合导致HABD上R45位(人源蛋白对应于R41位)精氨酸位点附近的loop区发生取向变化, 进而导致其与HA结合能力的调整[88] (图1(a)和图1(b)). CD44-HA相互作用主要是静电与范德华相互作用, 虽然通过结构生物学手段获得的复合物结构显示HA与HABD的结合仅存在一种结合模式, 而后续的分子模拟则预测HA与HABD的结合可以存在3种不同取向, 分别是晶体结合模式, 平行结合模式和直立结合模式, 其中晶体结合模式结合能力最强, 后两个是亚稳状态[90], 体现出CD44-HA相互作用的复杂性.
图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1CD44-配体相互作用的微观结构特征: (a)细胞粘附分子PSGL-1、CD44、E选择素与HA的分布及相互作用网络; (b) PSGL-1的N末端糖基sLe$^{X}$作用在E选择素Lectin结构域的钙离子附近; (c) HA作用导致CD44 HABD结构域发生构象改变
Fig.1Microstructural features of CD44-ligands interactions: (a) Schematic interaction network among PSGL-1, CD44, E-selectin and HA; (b) conformational allostery of CD44 HABD domain induced by HA binding; (c) crystalized E-selectin-sLe$^{X}$ interaction complex
HA的结合可诱导CD44构象改变、进而调控其结合能力的观点得到一系列研究的支持, 并验证了外力调控其相互作用的重要性. 在此基础上考察外力在CD44介导的细胞(微珠)滚动中的作用, 发现当HABD通过C末端标签连接到微珠时, 滚动行为仅在高剪切应力时才稳定发生, 表明高剪切应力下HABD从O构象转变到PD构象, 增强了其与HA的相互作用, 从而更好地抵抗流体剪切的作用. 而采用O构象或PD构象的HABD突变体包被的微珠则没有上述随流体剪切增高而粘附增强的现象. 分子动力学模拟表明, 作用于C末端的外力可破坏C末端区域和该结构域主体结构之间的相互作用, 从而实现了从HABD结构域从O构象态到PD构象态的变构; 同时在C末端施加的外力可更快地诱导高亲和力PD的构象、增强HABD-HA相互作用, 从而更有效介导白细胞的炎症反应和造血祖细胞归巢[81]. 此外, CD44-HA相互作用介导的细胞粘附和迁移速度取决于HA水凝胶的硬度, 提示基于CD44的信号传导具有机械敏感性[79], 进一步支持了力学因素调控CD44不同亲和态构象进而调控其与HA的结合能力的观点.
虽然关于CD44 HABD结构域及HABD-HA相互作用的微观结构特征有系列报道, 但是CD44其他结构域或不同糖基化的微观结构特征及其对HA结合的贡献还不清楚. 另外, 尽管选择素的微观结构研究相对完善, 包括构象动力学及其与配体PSGL-1相互作用的特征[91-94] (图1(a)和图1(b)), 但是CD44-选择素相互作用的微观结构特征还尚无报道. 因此, 进一步从微观结构层次考察CD44-选择素相互作用特征及其力学调控规律, 是深入理解其结构功能-关系的基础.
5 CD44-配体相互作用介导的胞内信号通路
HA是与CD44结合进而通过胞内信号通路调控细胞迁移、生长与增殖等功能的主要配体之一. 在调控细胞迁移方面: CD44通过其胞内结构域与细胞骨架相关蛋白相互作用进而调控细胞迁移[95-96]. ERM是桥接CD44与胞质肌动蛋白的主要蛋白之一[5], 其结合位点位于CD44胞质段的碱性氨基酸序列[97]. HA结合CD44导致蛋白激酶C(PKC)激活, 使得CD44胞内端磷酸化, 增强其与ERM蛋白的结合, 进而加强CD44与细胞骨架的相互作用, 促进细胞迁移[18,98-99]. 另外, HA与CD44的结合可以促进c-Src激酶募集至CD44部位并激活c-Src, 继而增加细胞骨架蛋白cortactin的酪氨酸磷酸化. Cortactin的酪氨酸磷酸化减弱了其交联丝状肌动蛋白的能力, 从而调节细胞的迁移能力, 促进细胞的募集[100]. HA与CD44相互作用还可以激活RhOGTPase(如Cdc42, Rac1等)信号, 该信号通过不同的效应分子来调控细胞骨架的重组, 进而调控细胞迁移. HA-CD44相互作用可以通过Cdc42调节F肌动蛋白进而调节细胞骨架, 促进细胞的募集[101]; HA与某些表达CD44的细胞结合也可激活Rac1信号传导, 进而调控细胞膜皱褶结构或细胞运动. CD44v3与 Tiam1之间的相互作用促进了Rac1信号转导和细胞骨架介导的乳腺肿瘤迁移[102]. HA的结合促进CD44与癌蛋白Vav家族成员Vav2蛋白的相互作用, 维持Rac1和Ras活化, 促进卵巢肿瘤细胞生长和迁移增加[103] (图2(a)). 另外, HA与CD44相互作用还可通过不同信号通路调控细胞的生长、增殖、存活等. CD44-HA相互作用可通过Rho激活诱导PI3K, 再由PI3K激活丝氨酸/苏氨酸激酶(Akt), 进而促进细胞增殖和存活[104]. 同时, CD44还通过HA合成酶HAS2, HA及Akt信号通路之间的正反馈回路诱导乳腺癌细胞中Akt信号的持续激活, 最终克服凋亡并维持细胞存活[105]. 外源性HA通过骨髓巨噬细胞样细胞上两种不同的HA受体增强造血功能. 其中一种是HA$-$CD44与激活p38丝裂原活化蛋白(MAP)激酶的途径相关, CD44-HA增强了细胞的增殖[106]. HA$-$CD44相互作用还可通过细胞外信号相关激酶1和2(ERK1/2)介导内皮细胞的活化和增殖[107]; 或通过激活ERK2, 进一步磷酸化Elk-1, 促进细胞迁移以及增殖[101]. CD44也可以作为共同受体而行使生物学功能, 通过与肝细胞生长因子HGF结合, CD44v6与Met以及HGF形成三体复合物并促进Met激活, 去除CD44胞质尾部依旧可以激活Met, 但需有CD44胞质尾部与ERM蛋白相互作用、才能激活Ras-MAPK途径[108]. CD44v6-ECM结合还促进PI3K / Akt途径激活和Met转录[109-110].图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2介导细胞粘附与迁移的CD44胞内信号通路: (a) HA与CD44相互作用启动的CD44胞内信号通路; (b) E选择素与CD44相互作用启动的CD44胞内信号通路
Fig.2CD44-mediated intracellular signaling pathways for cell adhesion and migration: (a) CD44-HA interaction mediated intracellular signaling pathways; (b) CD44- E-selectin interaction mediated intracellular signaling pathways
另一蛋白Merlin在CD44胞内端的结合位点与ERM存在竞争性作用, Merlin的激活发生在ERM蛋白失活之后. Merlin的激活引起皮质肌动蛋白细胞骨架的重组, 同时阻止Ras激活以及Ras依赖性信号转导, 并抑制了多种受体酪氨酸激酶的信号传递[5]. 丝氨酸/苏氨酸蛋白激酶PAK2可使Merlin磷酸化[111], 但同时导致Merlin失活并抑制其与CD44的结合[99]. 高细胞密度或高分子量HA的加入会触发Merlin的去磷酸化, 从而导致生长抑制复合物的形成, 使得细胞增殖受限[5]. 因此, ERM蛋白和Merlin蛋白具有控制细胞增殖的"开关"作用.
除上述HA结合启动CD44胞内信号通路在细胞生长、增殖、细胞骨架重组等过程中的作用外, CD44作为细胞粘附分子则呈现出不同的信号通路. 从现象上来讲, CD44-HA相互作用介导的T细胞外渗同时依赖于整合素VLA-4与配体VACM-1相互作用[112], 而CD44胞内端缺失则破坏了VLA-4整合素介导的T细胞稳定粘附和外渗[113], 提示CD44可能通过胞内某种信号通路与VLA-4整合素协同作用. CD44-HA相互作用也可增加整合素信号传导, 从而导致细胞铺展[114]. 高表达CD44的结肠癌细胞通过CD44交联或低分子量HA刺激可诱导$\beta _{2}$整合素$\alpha_{\rm L}\beta_{2}$的表达, 并进一步通过$\alpha _{\rm L}\beta _{2}$-ICAM-1相互作用促进癌细胞在内皮细胞上的粘附和跨内皮迁移, 通过加入PKC酶抑制剂, 可阻断该过程的发生[115]. 类似地, CD44交联导致MDA-MB-435S或Hs578T乳腺癌细胞系上整合素$\alpha_{\rm L}\beta _{2}$与VLA-4表达上调, 并进一步通过整合素-配体相互作用促进乳腺癌细胞跨内皮迁移[116] (图2(a)). E选择素结合也可触发CD44胞内信号、从而激活整合素. E选择素与CD44相互作用可以介导中性粒细胞的慢速滚动过程, 其原因在于E选择素的结合可通过脂筏上CD44胞内端SFK $\to$ Syk $\to$ Btk $\to$ p38的信号通路激活整合素$\alpha_{\rm L}\beta _{2}$, 进而通过$\alpha_{\rm L}\beta _{2}$-ICAM-1相互作用促进细胞的慢速滚动[31] (图2(b)).
正常生理条件下CD44成簇定位于细胞膜脂筏上, 因此脂筏的破坏与否对其与配体相互作用的能力、胞内信号的传递及相应生物学功能有明显调控作用. 通过甲基-$\beta$-环糊精(M$\beta$CD)消耗膜胆固醇、降低CD44在脂筏中的聚集, 可增强T细胞上CD44与HA的结合, 进而增加了生理流动条件下T细胞发生滚动粘附的细胞数量[117]. 而高水平的胆固醇促进CD44进入脂筏, CD44-Ezrin结合力减弱, 抑制肿瘤细胞的迁移和侵袭[118]. 另外, 多数Src家族激酶可被特定的脂质修饰, 而这些脂质可以将Src激酶引导至具有高胆固醇和糖磷脂含量的脂筏区域. 因此, CD44与脂筏中的c-Src激酶直接结合可促进HA介导的c-Src激酶活性并介导细胞骨架调节的细胞迁移[119]. 高分子量HA可以加强CD44成簇, 而HA片段似乎没有作用[120-121]. 有趣的是, 寡糖HA孵育细胞后, 能够减少先前由高分子量HA诱导形成的CD44簇[122].
简言之, CD44通过复杂的胞内信号网络介导细胞的生长、增殖、迁移等多种生物学过程, 而且受到力学、CD44糖修饰、CD44簇集及不同配体作用等多种因素调控. 作为细胞粘附分子, HA或E选择素的结合均可通过CD44胞内信号通路激活整合素, 进而通过整合素-配体相互作用实现免疫细胞募集、癌细胞迁移等过程的级联反应. 需要说明的是, 就目前的报道可以发现, HA与CD44相互作用主要是激活T淋巴细胞或癌细胞上整合素的表达上调, 进而通过增强整合素-配体相互作用的亲和力(avidity), 促进细胞间粘附或跨膜迁移; 而 E选择素与CD44相互作用则主要是激活中性粒细胞上整合素构象的改变, 进而通过增强整合素配体相互作用的亲和性(affinity), 促进细胞间的粘附或跨膜迁移, 从而表明不同配体与CD44相互作用介导不同的生物学功能. 作为在肝脏免疫中起重要作用的分子体系, HA与CD44相互作用如何调控中性粒细胞在肝血窦内的募集, 其与整合素之间存在怎样的协同作用等问题尚不清楚.
6 结论与展望
作为干细胞鉴定或疾病检测、病程发生发展的标志物, 胞内多种复杂信号网络的触发分子, 以及介导细胞粘附的重要受体, CD44分子的生物学重要性毋庸置疑, 其相应内在机制的研究也日趋深入. 随着人们对生命活动规律及内在机制认知的加深, 研究者们逐渐认识到力学、物理因素对生命活动的不可或缺性, 重要研究进展包括力学因素对发育[123-127]、遗传[128]、免疫[129]等重要生命活动的调控, 并催生了力学医学、力学免疫学、力学组学新概念, 发展了生物力学与力学生物学等交叉研究领域, 但是其内在机制还远不清楚. 如上所述, CD44作为细胞膜表达分子, 其与配体HA或选择素相互作用介导的细胞粘附受到生理力学、物理微环境的调控, 包括流体剪切、基质硬度等.虽然目前关于CD44与配体相互作用微观结构特征、分子反应动力学、细胞粘附动力学及胞内信号通路均有报道, 但是力学因素调控CD44与配体相互作用的规律及内在机制还远不清楚, 均需深入研究. 在不同力学因素对细胞粘附动力学的调控规律及内在机制方面: 主要包括外力作用下CD44与选择素相互作用介导的细胞粘附动力学具有什么样的特征; 外力作用下不同分子量膜定位的HA与CD44相互作用介导的细胞粘附动力学差异; 其不同组装形式对细胞粘附的调控等. 在分子相互作用反应动力学的调控规律及结构基础方面: 主要包括分子层次CD44与选择素/HA相互作用的力学调控规律, 及相互间的差异; 不同分子量HA-CD44相互作用的力学调控规律, 及其组装方式的影响; 原子层次CD44与选择素相互作用特征及其力学调控特征等. 特别地, 作为肝脏免疫过程中起重要作用的分子体系, 尚待考察的问题包括其独特力学、物理微环境如何调控CD44与HA相互作用介导的细胞粘附动力学?其生物学功能如何体现其组织特异性等.
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 4]
[本文引用: 1]
[本文引用: 1]
URLPMID [本文引用: 1]
URLPMID [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURLPMID [本文引用: 1]

Recent genomewide analyses of alternative splicing (AS) indicate that up to 70% of human genes may have alternative splice forms, suggesting that AS together with various posttranslational modifications plays a major role in the production of proteome complexity. Splice-site selection under normal physiological conditions is regulated in the developmental stage in a tissue type-specific manner by changing the concentrations and the activity of splicing regulatory proteins. Whereas spliceosomal errors resulting in the production of aberrant transcripts rarely occur in normal cells, they seem to be an intrinsic property of cancer cells. Changes in splice-site selection have been observed in various types of cancer and may affect genes implicated in tumor progression (for example, CD44, MDM2, and FHIT) and in susceptibility to cancer (for example, BRCA1 and APC). Splicing defects can arise from inherited or somatic mutations in cis-acting regulatory elements (splice donor, acceptor and branch sites, and exonic and intronic splicing enhancers and silencers) or variations in the composition, concentration, localization, and activity of regulatory proteins. This may lead to altered efficiency of splice-site recognition, resulting in overexpression or down-regulation of certain splice variants, a switch in splice-site usage, or failure to recognize splice sites correctly, resulting in cancer-specific splice forms. At least in some cases, changes in splicing have been shown to play a functionally significant role in tumorigenesis, either by inactivating tumor suppressors or by gain of function of proteins promoting tumor development. Moreover, cancer-specific splicing events may generate novel epitopes that can be recognized by the host's immune system as cancer specific and may serve as targets for immunotherapy. Thus, the identification of cancer-specific splice forms provides a novel source for the discovery of diagnostic or prognostic biomarkers and tumor antigens suitable as targets for therapeutic intervention.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURLPMID [本文引用: 1]

The stem cell niche provides a regulatory microenvironment for cells as diverse as totipotent embryonic stem cells to cancer stem cells (CSCs) which exhibit stem cell-like characteristics and have the capability of regenerating the bulk of tumor cells while maintaining self-renewal potential. The transmembrane glycoprotein CD44 is a common component of the stem cell niche and exists as a standard isoform (CD44s) and a range of variant isoforms (CD44v) generated though alternative splicing. CD44 modulates signal transduction through post-translational modifications as well as interactions with hyaluronan, extracellular matrix molecules and growth factors and their cognate receptor tyrosine kinases. While the function of CD44 in hematopoietic stem cells has been studied in considerable detail, our knowledge of CD44 function in tissue-derived stem cell niches remains limited. Here we review CD44s and CD44v in both hematopoietic and tissue-derived stem cell niches, focusing on their roles in regulating stem cell behavior including self-renewal and differentiation in addition to cell-matrix interactions and signal transduction during cell migration and tumor progression. Determining the role of CD44 and CD44v in normal stem cell, CSC and (pre)metastatic niches and elucidating their unique functions could provide tools and therapeutic strategies for treating diseases as diverse as fibrosis during injury repair to cancer progression.
DOIURLPMID [本文引用: 2]

The dynamic assembly and disassembly of membrane cytoskeleton junctional complexes is critical in cell migration. Here we describe a novel phosphorylation mechanism that regulates the hyaluronan receptor CD44. In resting cells, CD44 is constitutively phosphorylated at a single serine residue, Ser325. After protein kinase C is activated, a switch in phosphorylation results in CD44 being phosphorylated solely at an alternative residue, Ser291. Using fluorescence resonance energy transfer (FRET) monitored by fluorescence lifetime imaging microscopy (FLIM) and chemotaxis assays we show that phosphorylation of Ser291 modulates the interaction between CD44 and the cytoskeletal linker protein ezrin in vivo, and that this phosphorylation is critical for CD44-dependent directional cell motility.
[本文引用: 1]
DOIURLPMID [本文引用: 1]

It is now generally accepted that CD44 is a cell adhesion receptor and that hyaluronan is one of its ligands. Like many cell adhesion receptors, CD44 is broadly distributed, and its ligand, hyaluronan, is a common component of extracellular matrices and extracellular fluids. Yet a great variety of responses has been reported to result from CD44 ligation. These include cell adhesion, cell migration, induction (or at least support) of hematopoietic differentiation, effects on other cell adhesion mechanisms, and interaction with cell activation signals. This diversity of responses indicates that downstream events following ligand binding by CD44 may vary depending on the cell type expressing CD44 and on the environment of that cell. CD44 is expressed on cells in the early stages of hematopoiesis and has been shown to participate in at least some aspects of the hematopoietic process. In mature lymphocytes, CD44 is upregulated in response to antigenic stimuli and may participate in the effector stage of immunological responses. Along with other adhesion receptors that show alterations in expression after activation, CD44 probably contributes to differences in the recirculation patterns of different lymphocyte subpopulations. CD44 ligand-binding function on lymphocytes is strictly regulated, such that most CD44-expressing cells do not constitutively bind ligand. Ligand-binding function may be activated as a result of differentiation, inside-out signaling, and/or extracellular stimuli. This regulation, which in some situations can be rapid and transient, potentially provides exquisite specificity to what would otherwise be a common interaction. CD44 is not a single molecule, but a diverse family of molecules generated by alternate splicing of multiple exons of a single gene and by different posttranslational modifications in different cell types. It is not yet clear how these modifications influence ligand-binding function. The significance of the multiple isoforms of CD44 is not understood, but association of some isoforms with malignancies has been observed. And in at least some experimental systems, a contribution of CD44 isoforms to metastatic behavior has been demonstrated.
DOIURLPMID [本文引用: 1]

CD44 is a ubiquitous multistructural and multifunctional cells surface adhesion molecule involved in cell-cell and cell-matrix interactions. Twenty exons are involved in the genomic organization of this molecule. The first five and the last 5 exons are constant, whereas the 10 exons located between these regions are subjected to alternative splicing, resulting in the generation of a variable region. Differential utilization of the 10 variable region exons, as well as variations in N-glycosylation, O-glycosylation, and glycosaminoglycanation (by heparan sulfate or chondroitin sulfate), generate multiple isoforms (at least 20 are known) of different molecular sizes (85-230 kDa). The smallest CD44 molecule (85-95 kDa), which lacks the entire variable region, is standard CD44 (CD44s). As it is expressed mainly on cells of lymphohematopoietic origin, CD44s is also known as hematopoietic CD44 (CD44H). CD44s is a single-chain molecule composed of a distal extracellular domain (containing, the ligand-binding sites), a membrane-proximal region, a transmembrane-spanning domain, and a cytoplasmic tail. The molecular sequence (with the exception of the membrane-proximal region) displays high interspecies homology. After immunological activation, T lymphocytes and other leukocytes transiently upregulate CD44 isoforms expressing variant exons (designated CD44v). A CD44 isform containing the last 3 exon products of the variable region (CD44V8-10, also known as epithelial CD44 or CD44E), is preferentially expressed on epithelial cells. The longest CD44 isoform expressing in tandem eight exons of the variable region (CD44V3-10) was detected in keratinocytes. Hyaluronic acid (HA), an important component of the extracellular matrix (ECM), is the principal, but by no means the only, ligand of CD44. Other CD44 ligands include the ECM components collagen, fibronectin, laminin, and chondroitin sulfate. Mucosal addressin, serglycin, osteopontin, and the class II invariant chain (Ii) are additional, ECM-unrelated, ligands of the molecule. In many, but not in all cases, CD44 does not bind HA unless it is stimulated by phorbol esters, activated by agonistic anti-CD44 antibody, or deglycosylated (e.g., by tunicamycin). CD44 is a multifunctional receptor involved in cell-cell and cell-ECM interactions, cell traffic, lymph node homing, presentation of chemokines and growth factors to traveling cells, and transmission of growth signals. CD44 also participates in the uptake and intracellular degradation of HA, as well as in transmission of signals mediating hematopoiesis and apoptosis. Many cancer cell types as well as their metastases express high levels of CD44. Whereas some tumors, such as gliomas, exclusively express standard CD44, other neoplasms, including gastrointestinal cancer, bladder cancer, uterine cervical cancer, breast cancer and non-Hodgkin's lymphomas, also express CD44 variants. Hence CD44, particularly its variants, may be used as diagnostic or prognostic markers of at least some human malignant diseases. Furthermore, it has been shown in animal models that injection of reagents interfering with CD44-ligand interaction (e.g., CD44s- or CD44v-specific antibodies) inhibit local tumor growth and metastatic spread. These findings suggest that CD44 may confer a growth advantage on some neoplastic cells and, therefore, could be used as a target for cancer therapy. It is hoped that identification of CD44 variants expressed on cancer but not on normal cells will lead to the development of anti-CD44 reagents restricted to the neoplastic growth.
DOIURLPMID [本文引用: 1]

Inflammation underlies a wide variety of physiological and pathological processes. Although the pathological aspects of many types of inflammation are well appreciated, their physiological functions are mostly unknown. The classic instigators of inflammation - infection and tissue injury - are at one end of a large range of adverse conditions that induce inflammation, and they trigger the recruitment of leukocytes and plasma proteins to the affected tissue site. Tissue stress or malfunction similarly induces an adaptive response, which is referred to here as para-inflammation. This response relies mainly on tissue-resident macrophages and is intermediate between the basal homeostatic state and a classic inflammatory response. Para-inflammation is probably responsible for the chronic inflammatory conditions that are associated with modern human diseases.
[本文引用: 1]
DOIURLPMID [本文引用: 1]

Scientists who study neutrophils often have backgrounds in cell biology, biochemistry, haematology, rheumatology or infectious disease. Paradoxically, immunologists seem to have a harder time incorporating these host-defence cells into the framework of their discipline. The recent literature discussed here indicates that it is appropriate for immunologists to take as much interest in neutrophils as in their lymphohaematopoietic cousins with smooth nuclei. Neutrophils inform and shape immune responses, contribute to the repair of tissue as well as its breakdown, use killing mechanisms that enrich our concepts of specificity, and offer exciting opportunities for the treatment of neoplastic, autoinflammatory and autoimmune disorders.
DOIURLPMID [本文引用: 1]

Neutrophils have traditionally been thought of as simple foot soldiers of the innate immune system with a restricted set of pro-inflammatory functions. More recently, it has become apparent that neutrophils are, in fact, complex cells capable of a vast array of specialized functions. Although neutrophils are undoubtedly major effectors of acute inflammation, several lines of evidence indicate that they also contribute to chronic inflammatory conditions and adaptive immune responses. Here, we discuss the key features of the life of a neutrophil, from its release from bone marrow to its death. We discuss the possible existence of different neutrophil subsets and their putative anti-inflammatory roles. We focus on how neutrophils are recruited to infected or injured tissues and describe differences in neutrophil recruitment between different tissues. Finally, we explain the mechanisms that are used by neutrophils to promote protective or pathological immune responses at different sites.
URLPMID [本文引用: 1]
DOIURLPMID [本文引用: 1]

Selectins are a family of three cell adhesion molecules (L-, E-, and P-selectin) specialized in capturing leukocytes from the bloodstream to the blood vessel wall. This initial cell contact is followed by the selectin-mediated rolling of leukocytes on the endothelial cell surface. This represents the first step in a cascade of molecular interactions that lead to leukocyte extravasation, enabling the processes of lymphocyte recirculation and leukocyte migration into inflamed tissue. The central importance of the selectins in these processes has been well documented in vivo by the use of adhesion-blocking antibodies as well as by studies on selectin gene-deficient mice. This review focuses on the molecular mechanisms that regulate expression and function(s) of the selectins and their ligands. Cell-surface expression of the selectins is regulated by a variety of different mechanisms. The selectins bind to carbohydrate structures on glycoproteins, glycolipids, and proteoglycans. Glycoproteins are the most likely candidates for physiologically relevant ligands. Only a few glycoproteins are appropriately glycosylated to allow strong binding to the selectins. Recently, more knowledge about the structure and the regulated expression of some of the carbohydrates on these ligands necessary for selectin binding has been accumulated. For at least one of these ligands, the physiological function is now well established. A novel and exciting aspect is the signaling function of the selectins and their ligands. Especially in the last two years, convincing data have been published supporting the idea that selectins and glycoprotein ligands of the selectins participate in the activation of leukocyte integrins.
[本文引用: 1]
URLPMID [本文引用: 1]

We used in vitro and in vivo approaches to examine whether tumor necrosis factor-alpha (TNF-alpha) and oncostatin M (OSM), cytokines that bind to distinct classes of receptors, differentially regulate expression of P- and E-selectin in murine and primate endothelial cells. In human umbilical vein endothelial cells, TNF-alpha rapidly increased mRNA for E-selectin but not P-selectin. OSM elicited little or no change in mRNA for E-selectin, but induced a delayed and prolonged increase in P-selectin mRNA. TNF-alpha and OSM did not cooperate to further enhance P- or E-selectin mRNA. Intravenous infusion of Escherichia coli, which markedly elevates plasma lipopolysaccharide and TNF-alpha, increased mRNA for E-selectin but not P-selectin in baboons. In murine bEnd.3 endothelioma cells, TNF-alpha and OSM individually and cooperatively increased mRNA and protein for both P- and E-selectin. Intravenous injection of these cytokines also individually and cooperatively increased mRNA for P- and E-selectin in mice. We conclude that the murine P- and E-selectin genes respond to both TNF-alpha and OSM, whereas the primate P- and E-selectin genes have much more specialized responses. Such differences should be considered when extrapolating the functions of P- and E-selectin in murine models of inflammation to humans.
[本文引用: 2]
DOIURLPMID [本文引用: 2]

In inflamed venules, neutrophils rolling on E-selectin induce integrin alpha(L)beta(2)-dependent slow rolling on intercellular adhesion molecule-1 by activating Src family kinases (SFKs), DAP12 and Fc receptor-gamma (FcRgamma), spleen tyrosine kinase (Syk), and p38. E-selectin signaling cooperates with chemokine signaling to recruit neutrophils into tissues. Previous studies identified P-selectin glycoprotein ligand-1 (PSGL-1) as the essential E-selectin ligand and Fgr as the only SFK that initiate signaling to slow rolling. In contrast, we found that E-selectin engagement of PSGL-1 or CD44 triggered slow rolling through a common, lipid raft-dependent pathway that used the SFKs Hck and Lyn as well as Fgr. We identified the Tec kinase Bruton tyrosine kinase as a key signaling intermediate between Syk and p38. E-selectin engagement of PSGL-1 was dependent on its cytoplasmic domain to activate SFKs and slow rolling. Although recruiting phosphoinositide-3-kinase to the PSGL-1 cytoplasmic domain was reported to activate integrins, E-selectin-mediated slow rolling did not require phosphoinositide-3-kinase. Studies in mice confirmed the physiologic significance of these events for neutrophil slow rolling and recruitment during inflammation. Thus, E-selectin triggers common signals through distinct neutrophil glycoproteins to induce alpha(L)beta(2)-dependent slow rolling.
[本文引用: 1]
DOIURLPMID [本文引用: 1]

Despite great strides in our knowledge of the genetic and epigenetic changes underlying malignancy, we have limited information on the molecular basis of metastasis. Over 90% of cancer deaths are caused by spread of tumor cells from a primary site to distant organs and tissues, highlighting the pressing need to define the molecular effectors of cancer metastasis. Mounting evidence suggests that circulating tumor cells (CTCs) home to specific tissues by hijacking the normal leukocyte trafficking mechanisms. Cancer cells characteristically express CD44, and there is increasing evidence that hematopoietic cell E-/L-selectin ligand (HCELL), a sialofucosylated glycoform of CD44, serves as the major selectin ligand on cancer cells, allowing interaction of tumor cells with endothelium, leukocytes, and platelets. Here, we review the structural biology of CD44 and of HCELL, and present current data on the function of these molecules in mediating organ-specific homing/metastasis of CTCs.
DOIURLPMID [本文引用: 1]

Selectins guide the traffic of activated T-cells through the blood stream by mediating their tethering and rolling onto inflamed endothelium, in this way acting as beacons to help navigate them to sites of inflammation. Here, we present a comprehensive analysis of E-selectin ligands expressed on activated human T-cells. We identified several novel glycoproteins that function as E-selectin ligands. Specifically, we compared the role of P-selectin glycoprotein ligand-1 (PSGL-1) and CD43, known E-selectin ligands, to CD44, a ligand that has not previously been characterized as an E-selectin ligand on activated human T-cells. We showed that CD44 acts as a functional E-selectin ligand when expressed on both CD4(+) and CD8(+) T-cells. Moreover, the CD44 protein carries a binding epitope identifying it as hematopoietic cell E- and/or L-selectin ligand (HCELL). Furthermore, by knocking down these ligands individually or together in primary activated human T-cells, we demonstrated that CD44/HCELL, and not CD43, cooperates with PSGL-1 as a major E-selectin ligand. Additionally, we demonstrated the relevance of our findings to chronic autoimmune disease, by showing that CD44/HCELL and PSGL-1, but not CD43, from T-cells isolated from psoriasis patients, bind E-selectin.
DOIURLPMID [本文引用: 2]

The selectins and their ligands are required for leukocyte extravasation during inflammation. Several glycoproteins have been suggested to bind to E-selectin in vitro, but the complete identification of its physiological ligands has remained elusive. Here, we showed that E-selectin ligand-1 (ESL-1), P-selectin glycoprotein ligand-1 (PSGL-1), and CD44 encompassed all endothelial-selectin ligand activity on neutrophils by using gene- and RNA-targeted loss of function. PSGL-1 played a major role in the initial leukocyte capture, whereas ESL-1 was critical for converting initial tethers into steady slow rolling. CD44 controlled rolling velocity and mediated E-selectin-dependent redistribution of PSGL-1 and L-selectin to a major pole on slowly rolling leukocytes through p38 signaling. These results suggest distinct and dynamic contributions of these three glycoproteins in selectin-mediated neutrophil adhesion and signaling.
DOIURLPMID [本文引用: 1]

Hematopoietic stem/progenitor cell (HSPC) homing occurs via cell adhesion mediated by spatiotemporally organized ligand-receptor interactions. Although molecules and biological processes involved in this multistep cellular interaction with endothelium have been studied extensively, molecular mechanisms of this process, in particular the nanoscale spatiotemporal behavior of ligand-receptor interactions and their role in the cellular interaction, remain elusive. We introduce a microfluidics-based super-resolution fluorescence imaging platform and apply the method to investigate the initial essential step in the homing, tethering, and rolling of HSPCs under external shear stress that is mediated by selectins, expressed on endothelium, with selectin ligands (that is, CD44) expressed on HSPCs. Our new method reveals transient nanoscale reorganization of CD44 clusters during cell rolling on E-selectin. We demonstrate that this mechanical force-induced reorganization is accompanied by a large structural reorganization of actin cytoskeleton. The CD44 clusters were partly disrupted by disrupting lipid rafts. The spatial reorganization of CD44 and actin cytoskeleton was not observed for the lipid raft-disrupted cells, demonstrating the essential role of the spatial clustering of CD44 on its reorganization during cell rolling. The lipid raft disruption causes faster and unstable cell rolling on E-selectin compared with the intact cells. Together, our results demonstrate that the spatial reorganization of CD44 and actin cytoskeleton is the result of concerted effect of E-selectin-ligand interactions, external shear stress, and spatial clustering of the selectin ligands, and has significant effect on the tethering/rolling step in HSPC homing. Our new experimental platform provides a foundation for characterizing complicated HSPC homing.
DOIURLPMID [本文引用: 1]

Production of the glycosaminoglycan hyaluronan is increased at sites of inflammation, often correlating with the accumulation of leukocytes. Mounting evidence suggests that this polysaccharide can be organized into a wide variety of molecular architectures by its association with specific binding proteins, leading to the formation of fibrils and cable-like structures involving a large number of hyaluronan chains. We propose that hyaluronan cross-linking is part of a protective mechanism, promoting adhesion of leukocytes to the hyaluronan complexes rather than enabling contact with inflammation-promoting receptors on the underlying tissues. Leukocytes are thus maintained in a non-activated state by appropriate receptor clustering or receptor co-engagement. Additionally, hyaluronan networks might serve as scaffolds to prevent the loss of extracellular matrix components during inflammation and to sequester proinflammatory mediators.
DOIURLPMID

Accumulation and turnover of extracellular matrix components are the hallmarks of tissue injury. Fragmented hyaluronan stimulates the expression of inflammatory genes by a variety of immune cells at the injury site. Hyaluronan binds to a number of cell surface proteins on various cell types. Hyaluronan fragments signal through both Toll-like receptor (TLR) 4 and TLR2 as well as CD44 to stimulate inflammatory genes in inflammatory cells. Hyaluronan is also present on the cell surface of epithelial cells and provides protection against tissue damage from the environment by interacting with TLR2 and TLR4. Hyaluronan and hyaluronan-binding proteins regulate inflammation, tissue injury, and repair through regulating inflammatory cell recruitment, release of inflammatory cytokines, and cell migration. This review focuses on the role of hyaluronan as an immune regulator in human diseases.
DOIURL [本文引用: 1]
[本文引用: 1]
DOIURLPMID [本文引用: 1]

Allergic asthma is a chronic inflammatory disease of the airways characterized by excessive eosinophilic and lymphocytic inflammation with associated changes in the extracellular matrix (ECM) resulting in airway wall remodeling. Hyaluronan (HA) is a nonsulfated glycosaminoglycan ECM component that functions as a structural cushion in its high molecular mass (HMM) but has been implicated in metastasis and other disease processes when it is degraded to smaller fragments. However, relatively little is known about the role HA in mediating inflammatory responses in allergy and asthma. In the present study, we used a murine Aspergillus fumigatus inhalational model to mimic human disease. After observing in vivo that a robust B cell recruitment followed a massive eosinophilic egress to the lumen of the allergic lung and corresponded with the detection of low molecular mass HA (LMM HA), we examined the effect of HA on B cell chemotaxis and cytokine production in the ex vivo studies. We found that LMM HA functioned through a CD44-mediated mechanism to elicit chemotaxis of B lymphocytes, while high molecular mass HA (HMM HA) had little effect. LMM HA, but not HMM HA, also elicited the production of IL-10 and TGF-beta1 in these cells. Taken together, these findings demonstrate a critical role for ECM components in mediating leukocyte migration and function which are critical to the maintenance of allergic inflammatory responses.
DOIURLPMID [本文引用: 1]

BACKGROUND: Hyaluronan (HA) fragments elicit the expression of inflammatory mediators through a mechanism involving the CD44 receptor. This study investigated the effects of HA at different molecular weights on PMA-induced inflammation in mouse chondrocytes. METHODS: mRNA and related protein levels were measured for CD44, PKCdelta, PKCepsilon, TNF-alpha, IL-1beta, MMP-13, and iNOS in chondrocytes, untreated or PMA treated, with and without the addition of HA. The level of NF-kB activation was also assayed. RESULTS: CD44, PKCdelta, and PKCepsilon mRNA expression resulted higher than controls in chondrocytes treated with PMA. PMA also induced NF-kB up-regulation and increased TNF-alpha, IL-1beta, MMP-13, and iNOS expression. HA treatment produced different effects: low MW HA up-regulated CD44 expression, increased PKCdelta and PKCepsilon levels, and enhanced inflammation in untreated chondrocytes; while in PMA-treated cells it increased CD44, PKCdelta, PKCepsilon, NF-kB, TNF-alpha, IL-1beta, MMP-13, and iNOS expression and enhanced the effects of PMA; medium MW HA did not exert action; high MW HA had no effect on untreated chondrocytes; however, it reduced PKCdelta, PKCepsilon, NF-kB activation and inflammation in PMA-stimulated cells. Specific CD44 blocking antibody was utilised to confirm CD44 as the target of HA modulation. GENERAL SIGNIFICANCE: These data suggest that HA via CD44 may modulate inflammation via its different molecular mass.
DOIURLPMID [本文引用: 1]

Small degradation fragments of hyaluronan (HA) may stimulate an inflammatory response in a variety of tissues at the injury site. HA oligosaccharides are endogenous ligands for the cluster determinant 44 (CD44) receptor as well as for toll-like receptor 4 (TLR-4). Previous data have shown that HA fragments may induce pro-inflammatory cytokine expression by interacting with both the CD44 receptor and TLR-4. CD44 and TLR-4 stimulation activates different inflammatory pathways that culminate with the activation of the transcriptional nuclear factor kappaB (NF-kappaB) which is responsible for the expression of inflammation mediators such as tumor necrosis factor alpha (TNF-alpha), interleukin-6 (IL-6) and interleukin-1 beta (IL-1beta). The aim of this study was to investigate the inflammatory effects of very small HA oligosaccharides on both TLR-4 and CD44 involvement in normal human articular chondrocytes. Adding HA fragments to chondrocyte cultures up-regulated CD44 and TLR-4 expression, activated NF-kappaB translocation and increased the pro-inflammatory cytokines TNF-alpha, IL-6 and IL-1beta. The addition of a specific CD44 blocking antibody reduced CD44 and all inflammatory cytokine expression as well as protein production. However, cytokine expression remained significantly higher than in untreated chondrocytes. TLR-4 expression was not affected. The treatment with TLR-4 blocking antibody decreased TLR-4 and inflammatory cytokine expression, although cytokine expression was significantly higher than in control cells. CD44 expression was unaffected. The addition of both CD44 and TLR-4 blocking antibodies significantly reduced CD44, TLR-4 and inflammatory cytokine expression.
DOIURLPMID [本文引用: 1]

The structure and mechanics of many connective tissues are dictated by a collagen-rich extracellular matrix (ECM), where collagen fibers provide topological cues that direct cell migration. However, comparatively little is known about how cells navigate the hyaluronic acid (HA)-rich, nanoporous ECM of the brain, a problem with fundamental implications for development, inflammation, and tumor invasion. Here, we demonstrate that glioblastoma cells adhere to and invade HA-rich matrix using microtentacles (McTNs), which extend tens of micrometers from the cell body and are distinct from filopodia. We observe these structures in continuous culture models and primary patient-derived tumor cells, as well as in synthetic HA matrix and organotypic brain slices. High-magnification and superresolution imaging reveals McTNs are dynamic, CD44-coated tubular protrusions containing microtubules and actin filaments, which respectively drive McTN extension and retraction. Molecular mechanistic studies reveal that McTNs are stabilized by an interplay between microtubule-driven protrusion, actomyosin-driven retraction, and CD44-mediated adhesion, where adhesive and cytoskeletal components are mechanistically coupled by an IQGAP1-CLIP170 complex. McTNs represent a previously unappreciated mechanism through which cells engage nanoporous HA matrix and may represent an important molecular target in physiology and disease.
[本文引用: 1]
DOIURLPMID [本文引用: 1]

Leukocytes extravasate from the blood into inflammatory sites through complementary ligand interactions between leukocytes and endothelial cells. Activation of T cells increases their binding to hyaluronate (HA) and enables CD44-mediated primary adhesion (rolling). This rolling could be induced in vivo in murine Vbeta8(+) T cells in response to specific superantigen stimulation; it was initially found in lymph nodes, then in peripheral blood, and finally within the peritoneum, the original inflamed site. The migration of Vbeta8(+) cells into the peritoneal cavity was dependent on CD44 and HA, as shown by inhibition studies. Thus, CD44-HA interactions can target lymphocytes to specific extralymphoid effector sites.
DOIURLPMID [本文引用: 1]

Localization of circulating lymphocytes to a site of inflammation is paramount for the development and maintenance of an immune response. In vitro studies using cell lines have previously demonstrated that rolling and adhesion of lymphocytes on endothelium requires CD44 interactions with hyaluronan (HA). To date, whether CD44 has a role in mediating CD4(+)-polarized T-helper 1 (Th1) and Th2 lymphocyte interactions with the endothelium in vivo is yet to be determined. In this study we used intravital microscopy to demonstrate that both Th1 and Th2 lymphocytes use CD44 to roll and adhere to tumor necrosis factor-alpha (TNFalpha)-activated microvasculature. Furthermore, chimeric studies imply that CD44 expression by both the endothelium and lymphocytes is essential for these interactions to occur. HA was also necessary for T cell-endothelial cell interactions in vivo and Th1 and Th2 cells rolled on immobilized HA in vitro via CD44. In vitro, both Th1 and Th2 lymphocytes have increased expression of CD44 and greater binding of fluorescent HA than naive cells. The interactions of Th1 and Th2 cells were entirely dependent upon both P-selectin and CD44 in vivo, but did not appear to be counter ligands in vitro. Taken together, these results suggest that CD44 and HA are key to both Th1 and Th2 lymphocyte interactions with the TNFalpha-activated endothelium and raises the possibility of cooperativity between the P-selectin/PSGL-1 and HA/CD44 pathways for Th1 and Th2 rolling in vivo.
DOIURLPMID [本文引用: 1]

The cell adhesion molecule CD44 is expressed on the majority of immune cells and is responsible for mediating adhesion to the extracellular matrix glycosaminoglycan, hyaluronan. The binding of CD44 to hyaluronan is induced on T lymphocytes after activation by antigen and on monocytes after stimulation by inflammatory agents. Under inflammatory conditions, CD44 on endothelial cells presents hyaluronan to CD44 on activated T lymphocytes and mediates a rolling interaction under flow conditions. This rolling interaction together with chemokine signaling upregulates integrin-mediated adhesion, which induces cell arrest and leads to subsequent migration to the inflammatory site. Studies with monoclonal antibodies against CD44 in mouse models of chronic inflammatory disease showed reduced disease severity attributed to reduced leukocyte recruitment. More recent investigations, taking advantage of the availability of CD44 null mice, further established a role for CD44 in leukocyte recruitment to inflammatory sites. These studies also revealed a role for CD44 in limiting the inflammatory response and resolving inflammation in models of lung injury and hepatitis. Here we describe the contributions of CD44 and hyaluronan to an inflammatory response and discuss the role of CD44 in both promoting and resolving inflammation in various mouse models of inflammatory disease.
DOIURLPMID [本文引用: 1]

CD44, the leukocyte adhesion receptor for hyaluronan, has been considered a therapeutic target on the basis of the robust anti-inflammatory effect of CD44-specific antibodies in animal models of immune-mediated diseases. However, CD44 deficiency does not provide substantial protection against inflammation. Using intravital video microscopy in a murine model of rheumatoid arthritis, we show that CD44 deficiency and anti-CD44 antibody treatment exert disparate effects on leukocyte recruitment in inflamed joints. Leukocyte rolling, which is increased in CD44-deficient mice, is promptly abrogated in anti-CD44-treated wild-type mice. CD44-specific antibodies also trigger platelet deposition on granulocytes and subsequent depletion of this leukocyte subset in the circulation. These in vivo effects require CD44 cross-linking and are reproducible with an antibody against Gr-1, a molecule that, like CD44, is highly expressed on granulocytes. Anticoagulant pretreatment, which prevents platelet deposition, mitigates both granulocyte depletion and the suppressive effect of CD44-specific antibody on joint swelling. Our observations suggest that cross-linking of prominent cell surface molecules, such as CD44 or Gr-1, can initiate a rapid self-elimination program in granulocytes through engagement of the coagulation system. We conclude that the robust anti-inflammatory effect of CD44-specific antibodies in arthritis is primarily the result of their ability to trigger granulocyte depletion.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID [本文引用: 1]
DOIURLPMID [本文引用: 1]

In the present study, we examined the expression and distribution of both hyaluronan and its cell-surface receptor (CD44) during lung development in the mouse. Hyaluronan was detected by a specific binding probe, termed b-PG, which is a biotinylated mixture of proteoglycan and link protein from cartilage. Using this probe in an enzyme-linked assay, the amount of hyaluronan in relation to protein content was found to decrease as lung development progressed. In addition, histochemical staining of the embryonic lungs revealed that during early stages, relatively large amounts of hyaluronan were present in the interstitium. However, as development progressed, much of this was lost, and in the adult, hyaluronan was restricted to the regions surrounding the major blood vessels, bronchi, and bronchioles. In contrast to hyaluronan, the amount of CD44 increased as a function of development, as determined by the rat monoclonal antibody, KM-201. Histochemical staining with this antibody showed that the receptor was primarily associated with macrophages and to a lesser extent with adult bronchial and bronchiolar epithelium, vascular smooth muscle, and endothelial cells. As development progressed, the macrophages expressing CD44 increased in number, and this increase was temporarily correlated with the decrease in hyaluronan content. In addition, histochemical staining revealed that some of these macrophages contained hyaluronan in their cytoplasm, suggesting that macrophages had internalized hyaluronan from the extracellular matrix. This possibility was further supported by the fact that when newborn mice were injected with the KM-201 monoclonal antibody, which blocks the interaction between hyaluronan and the receptor, the number of hyaluronan-containing macrophages in the lungs decreased while the concentration of hyaluronan increased. Taken together, these results suggest that macrophages can internalize hyaluronan during lung development and could possibly play a significant role in its removal.
[本文引用: 2]
DOIURLPMID [本文引用: 1]

Neutrophils have traditionally been thought of as simple foot soldiers of the innate immune system with a restricted set of pro-inflammatory functions. More recently, it has become apparent that neutrophils are, in fact, complex cells capable of a vast array of specialized functions. Although neutrophils are undoubtedly major effectors of acute inflammation, several lines of evidence indicate that they also contribute to chronic inflammatory conditions and adaptive immune responses. Here, we discuss the key features of the life of a neutrophil, from its release from bone marrow to its death. We discuss the possible existence of different neutrophil subsets and their putative anti-inflammatory roles. We focus on how neutrophils are recruited to infected or injured tissues and describe differences in neutrophil recruitment between different tissues. Finally, we explain the mechanisms that are used by neutrophils to promote protective or pathological immune responses at different sites.
DOIURL [本文引用: 1]
DOIURLPMID [本文引用: 1]

A hallmark feature of inflammation is the orchestrated recruitment of neutrophils from the bloodstream into inflamed tissue. Although selectins and integrins mediate recruitment in many tissues, they have a minimal role in the lungs and liver. Exploiting an unbiased in vivo functional screen, we identified a lung and liver homing peptide that functionally abrogates neutrophil recruitment to these organs. Using biochemical, genetic, and confocal intravital imaging approaches, we identified dipeptidase-1 (DPEP1) as the target and established its role as a physical adhesion receptor for neutrophil sequestration independent of its enzymatic activity. Importantly, genetic ablation or functional peptide blocking of DPEP1 significantly reduced neutrophil recruitment to the lungs and liver and provided improved survival in models of endotoxemia. Our data establish DPEP1 as a major adhesion receptor on the lung and liver endothelium and identify a therapeutic target for neutrophil-driven inflammatory diseases of the lungs.
DOIURL [本文引用: 1]
URLPMID [本文引用: 1]

Receptor-ligand interactions in blood flow are crucial to initiate the biological processes as inflammatory cascade, platelet thrombosis, as well as tumor metastasis. To mediate cell adhesions, the interacting receptors and ligands must be anchored onto two apposing surfaces of two cells or a cell and a substratum, i.e., the two-dimensional (2D) binding, which is different from the binding of a soluble ligand in fluid phase to a receptor, i.e., three-dimensional (3D) binding. While numerous works have been focused on 3D kinetics of receptor-ligand interactions in immune systems, 2D kinetics and its regulations have less been understood, since no theoretical framework and experimental assays have been established until 1993. Not only does the molecular structure dominate 2D binding kinetics, but the shear force in blood flow also regulates cell adhesions mediated by interacting receptors and ligands. Here we provided the overview of current progresses in 2D bindings and regulations. Relevant issues of theoretical frameworks, experimental measurements, kinetic rates and binding affinities, and force regulations, were discussed.
DOIURL [本文引用: 1]
DOIURLPMID [本文引用: 1]

The role of the CD44 cytoplasmic domain in cells displaying constitutive or inducible hyalu ronan (HA) binding mediated by wild-type CD44 was investigated using mutant CD44 constructs with the cytoplasmic domain truncated or replaced by foreign sequences. In cell lines in which wild-type CD44 bound HA constitutively, chimeric constructs consisting of the CD44 external domain and the transmembrane plus cytoplasmic domains of beta5 integrin bound as well as wild-type CD44, arguing that the specific sequence of the cytoplasmic and transmembrane domains was not critical for HA binding by the external domain. The cytoplasmic domain sequence did not contribute to the 'inducible' phenotype in cell lines which did not bind HA constitutively, but which could be induced to bind by CD44-specific mAb. Tailless CD44 was inducible in these cells, as was chimeric CD44 with the integrin beta5 cytoplasmic domain. Dimer- or oligomerization of CD44 by addition of AP1510 to cells containing CD44 / FKBP chimeric constructs caused a modest enhancement of HA binding in cells that bound constitutively, but did not alter the inducible phenotype. This result suggests that clustering of CD44 from inside the cell is not a sufficient 'inside-out' signal to activate HA binding in inducible cells.
DOIURL
DOIURLPMID

CD34 is routinely used to identify and isolate human hematopoietic stem/progenitor cells (HSPCs) for use clinically in bone marrow transplantation, but its function on these cells remains elusive. Glycoprotein ligands on HSPCs help guide their migration to specialized microvascular beds in the bone marrow that express vascular selectins (E- and P-selectin). Here, we show that HSPC-enriched fractions from human hematopoietic tissue expressing CD34 (CD34(pos)) bound selectins, whereas those lacking CD34 (CD34(neg)) did not. An unbiased proteomics screen identified potential glycoprotein ligands on CD34(pos) cells revealing CD34 itself as a major vascular selectin ligand. Biochemical and CD34 knockdown analyses highlight a key role for CD34 in the first prerequisite step of cell migration, suggesting that it is not just a marker on these cells. Our results also entice future potential strategies to investigate the glycoforms of CD34 that discriminate normal HSPCs from leukemic cells and to manipulate CD34(neg) HSPC-enriched bone marrow or cord blood populations as a source of stem cells for clinical use.
DOIURLPMID

Hyaluronan (HA), a naturally occurring glycosaminoglycan, exerts different biological functions depending on its molecular weight ranging from 4000-10M Da. While high Mw HA (HMw-HA) is considered as anti-inflammatory, low Mw HA (LMw-HA) has been reported to activate an innate immune response. In addition, opposing effects on cell proliferation mediated by the HA receptor CD44, have also been reported for high and low Mw HA. We have previously demonstrated that HMw-HA can be covalently attached to the surface of lipid nanoparticles (NPs), endowing the carriers with long circulation and active targeting towards HA-receptors (CD44 and CD168) highly expressed on tumors. Here we present a small library of HA-coated NPs distinguished only by the Mw of their surface anchored HA ranging from 6.4 kDa to 1500 kDa. All types of NPs exerted no effect on macrophages, T cells and ovarian cancer cells proliferation. In addition, no induction of cytokines or complement activation was observed. The affinity towards the CD44 receptor was found to be solely controlled by the Mw of the NPs surface-bound HA, from extremely low binding for LMw-HA to binding with high affinity for HMw-HA. These findings have major implications for the use of HA in nanomedicine as LMw-HA surface modified-NPs could be a viable option for the replacement of polyethylene glycol (PEG) when passive delivery is required, lacking adverse effects such as complement activation and cytokine induction, while HMw-HA-coated NPs could be used for active targeting to CD44 overexpressing tumors and aberrantly activated leukocytes in inflammation.
DOIURLPMID [本文引用: 1]

Hyaluronan (HA) and its principal receptor CD44 are known to be involved in regulating tumor cell dissemination and metastasis. The direct correlation of CD44-HA interaction on proliferation and invasion of tumor cells in dependence on the molecular weight and the presentation form of HA is not fully understood because of lack of appropriate matrix models. To address this issue, we reconstituted 3D collagen (Coll I) matrices and functionalized them with HA of molecular weight of 30-50kDa (low molecular weight; LMW-HA) and 500-750kDa (high molecular weight; HMW-HA). A post-modification strategy was applied to covalently immobilize HA to reconstituted fibrillar Coll I matrices, resulting in a non-altered Coll I network microstructure and stable immobilization over days. Functionalized Coll I matrices were characterized regarding topological and mechanical characteristics as well as HA amount using confocal laser scanning microscopy, colloidal probe force spectroscopy and quantitative Alcian blue assay, respectively. To elucidate HA dependent tumor cell behavior, BRO melanoma cell lines with and without CD44 receptor expression were used for in vitro cell experiments. We demonstrated that only soluble LMW-HA promoted cell proliferation in a CD44 dependent manner, while HMW-HA and immobilized LMW-HA did not. Furthermore, an enhanced cell invasion was found only for immobilized LMW-HA. Both findings correlated with a very strong and specific adhesive interaction of LMW-HA and CD44+ cells quantified in single cell adhesion measurements using soft colloidal force spectroscopy. Overall, our results introduce an in vitro biomaterials model allowing to test presentation mode and molecular weight specificity of HA in a 3D fibrillar matrix thus mimicking important in vivo features of tumor microenvironments. STATEMENT OF SIGNIFICANCE: Molecular weight and presentation form (bound vs. soluble) of hyaluronan (HA) are intensively discussed as key regulators in tumor progression and inflammation. We introduce 3D fibrillar collagen matrices with defined microstructure and stiffness allowing the presentation of specific molecular weight forms of HA in soluble and bound manner. Mimicking in that way important in vivo features of tumor microenvironments, we found that only low molecular weight HA (LMW-HA) in soluble form promoted proliferation of a melanoma cell line (BRO), while it enhanced cell invasion in bound form. The molecular weight specificity of LMW-HA was verified to be CD44 receptor dependent and was correlated to adhesive ligand-receptor interactions in quantitative colloidal force spectroscopy at single cell level.
DOIURLPMID

Glycosaminoglycans (GAGs), a category of linear, anionic polysaccharides, are ubiquitous in the extracellular space, and important extrinsic regulators of cell function. Despite the recognized significance of mechanical stimuli in cellular communication, however, only few single molecule methods are currently available to study how monovalent and multivalent GAG.protein bonds respond to directed mechanical forces. Here, we have devised such a method, by combining purpose-designed surfaces that afford immobilization of GAGs and receptors at controlled nanoscale organizations with single molecule force spectroscopy (SMFS). We apply the method to study the interaction of the GAG polymer hyaluronan (HA) with CD44, its receptor in vascular endothelium. Individual bonds between HA and CD44 are remarkably resistant to rupture under force in comparison to their low binding affinity. Multiple bonds along a single HA chain rupture sequentially and independently under load. We also demonstrate how strong non-covalent bonds, which are versatile for controlled protein and GAG immobilization, can be effectively used as molecular anchors in SMFS. We thus establish a versatile method for analyzing the nanomechanics of GAG.protein interactions at the level of single GAG chains, which provides new molecular-level insight into the role of mechanical forces in the assembly and function of GAG-rich extracellular matrices.
DOIURLPMID

CD44 is a multifunctional glycoprotein that binds to hyaluronan and fibrin(ogen). Alternative splicing is responsible for the generation of numerous different isoforms, the smallest of which is CD44s. Insertion of variant exons into the extracellular membrane proximal region generates the variant isoforms (CD44v). Here, we used force spectroscopy to delineate the biophysical and molecular requirements of CD44-HA and CD44-fibrin(ogen) interactions at the single-molecule level. CD44v-HA and CD44s-HA single bonds exhibit similar kinetic and micromechanical properties because the HA-binding motif on CD44 is common to all of the isoforms. Although this is the primary binding site, O- and N-linked glycans and sulfation also contribute to the tensile strength of the CD44-HA bond. The CD44s-fibrin pair has a lower unstressed dissociation rate and a higher tensile strength than CD44s-fibrinogen but is weaker than the CD44-HA bond. In contrast to CD44-HA binding, the molecular interaction between CD44 and fibrin(ogen) is predominantly mediated by the chondroitin sulfate and dermatan sulfate on CD44. Blocking sulfation on CD44s modestly decreases the tensile strength of CD44s-fibrin(ogen) binding, which is in stark contrast to CD44v-fibrin interaction. Collectively, the results obtained by force spectroscopy in conjunction with biochemical interventions enable us to delineate the biophysical parameters and molecular constituents of CD44 binding to hyaluronan and fibrin(ogen).
DOIURLPMID

Interaction of cells with hyaluronan (HA) rich extracellular matrix involves the membrane receptor CD44. HA-CD44 interactions are particularly important in the development of glioma pathogenesis for its implication in tumor cells spreading. Highly motile states rely on the spaciotemporal regulation of HA-CD44 interactions occurring in specific cytoskeletal-supported membrane organization such as microvilli or the leading edge observed in migrating cell. We used AFM-based force measurement to probe the HA-CD44 interaction at localized regions at the surface of living glioma cells expressing high level of the CD44 standard isoform. We show that unstimulated cells interact with HA over their entire surfaces and are highly deformable when force is exerted on individual HA molecules bound to membrane CD44 receptors. Conversely, in PKC-activated cells the probed interactions are concentrated at the leading edge of the cells with reduced membrane deformability. Taken together, our results show that PKC-enhanced motility in glioma cells is associated with a redistribution of CD44 receptors at the leading edges concomitant with a stiffer anchoring of CD44 to the cell surface involving the actin cytoskeleton.
DOIURL
[本文引用: 1]
DOIURLPMID [本文引用: 1]

We previously have obtained operational evidence of a hematopoietic cell L-selectin ligand expressed on normal human hematopoietic cells and on leukemic blasts. Using a technique developed in our laboratory for analyzing and identifying adhesion molecules, we show here that hematopoietic cell L-selectin ligand is a specialized glycoform of CD44. This L-selectin ligand activity of CD44 requires sialofucosylated N-linked glycans and is sulfation-independent. These data provide important insights on the structural biology of CD44 and reveal a role for this protein as an L-selectin ligand on human hematopoietic cells.
DOIURLPMID [本文引用: 1]

The initial selectin-dependent events that mediate tumor cell tethering to platelets, leukocytes, and vascular endothelium can regulate the extravasation and colonization of metastatic cells into distant tissues. Little is known, however, about the identity of selectin counter-receptors on tumor cells, which facilitate the metastatic process. To address this issue, we performed SDS-PAGE analysis of membrane proteins, metabolic inhibition studies, blot rolling assays, and cell-free flow-based adhesion experiments using microbeads coated with CD44 immunoprecipitated from carcinomas and purified selectins as substrate. Here, we demonstrate that variant isoforms of CD44 (CD44v) on LS174T colon carcinoma cells possess P-/L-/E-selectin binding activity, in contrast to the standard isoform of CD44 (CD44s) on hematopoietic-progenitor cells (HPCs), which is primarily an L-/E-selectin ligand. Moreover, the selectin-binding determinants on CD44v from LS174T cells are sialofucosylated structures displayed on O-linked glycans, akin to those on P-selectin glycoprotein ligand-1, but distinct from the HECA-452-reactive N-glycans on CD44s expressed on HPCs. Using flow-based adhesion assays, we systematically characterize shear-dependent LS174T CD44 vs. HL60 CD44s adhesion to E-/P-/L-selectin. The novel finding that CD44v are selectin ligands offers a unifying perspective on the apparent enhanced metastatic potential associated with tumor cell CD44v overexpression and the critical role of selectins in metastasis.
[本文引用: 1]
DOIURLPMID [本文引用: 1]

Leukemic cells and human hematopoietic progenitor cells expressing CD44 receptors have the ability to attach and roll on hyaluronan. We investigated quantitatively the adhesion behavior of leukemic cell lines and hematopoietic progenitor cells on thin films of the polysaccharides hyaluronan and alginate in a microfluidic system. An applied flow enhances the interaction between CD44-positive cells and hyaluronan if a threshold shear stress of 0.2 dyn/cm(2) is exceeded. At shear stress approximately 1 dyn/cm(2), the cell rolling speed reaches a maximum of 15 mum/s. Leukemic Jurkat and Kasumi-1 cells lacking CD44-expression showed no adhesion or rolling on the polysaccharides whereas the CD44-expressing leukemic cells KG-1a, HL-60, K-562, and hematopoietic progenitor cells attached and rolled on hyaluronan. Interestingly, the observations of flow-induced cell rolling are related to those found in the recruitment of leukocytes to inflammatory sites and the mechanisms of stem-cell homing into the bone marrow.
DOIURLPMID [本文引用: 1]

We previously demonstrated that leukemia cell lines expressing CD44 and hematopoietic progenitor cells (HPC) from umbilical cord blood (CB) showed rolling on hyaluronic acid (HA)-coated surfaces under physiological shear stress. In the present study, we quantitatively assessed the interaction of HPC derived from CB, mobilized peripheral blood (mPB) and bone marrow (BM) from healthy donors, as well as primary leukemia blasts from PB and BM of patients with acute myeloid leukemia (AML) with HA. We have demonstrated that HPC derived from healthy donors showed relative homogeneous rolling and adhesion to HA. In contrast, highly diverse behavioral patterns were found for leukemia blasts under identical conditions. The monoclonal CD44 antibody (clone BU52) abrogated the shear stress-induced rolling of HPC and leukemia blasts, confirming the significance of CD44 in this context. On the other hand, the immobile adhesion of leukemia blasts to the HA-coated surface was, in some cases, not or incompletely inhibited by BU52. The latter property was associated with non-responsiveness to induction chemotherapy and subsequently poor clinical outcome.
DOIURLPMID [本文引用: 2]

UNLABELLED: The high-molecular-weight glycosaminoglycan, hyaluronic acid (HA), makes up a significant portion of the brain extracellular matrix. Glioblastoma multiforme (GBM), a highly invasive brain tumor, is associated with aberrant HA secretion, tissue stiffening, and overexpression of the HA receptor CD44. Here, transcriptomic analysis, engineered materials, and measurements of adhesion, migration, and invasion were used to investigate how HA/CD44 ligation contributes to the mechanosensing and invasive motility of GBM tumor cells, both intrinsically and in the context of Arg-Gly-Asp (RGD) peptide/integrin adhesion. Analysis of transcriptomic data from The Cancer Genome Atlas reveals upregulation of transcripts associated with HA/CD44 adhesion. CD44 suppression in culture reduces cell adhesion to HA on short time scales (0.5-hour postincubation) even if RGD is present, whereas maximal adhesion on longer time scales (3 hours) requires both CD44 and integrins. Moreover, time-lapse imaging demonstrates that cell adhesive structures formed during migration on bare HA matrices are more short lived than cellular protrusions formed on surfaces containing RGD. Interestingly, adhesion and migration speed were dependent on HA hydrogel stiffness, implying that CD44-based signaling is intrinsically mechanosensitive. Finally, CD44 expression paired with an HA-rich microenvironment maximized three-dimensional invasion, whereas CD44 suppression or abundant integrin-based adhesion limited it. These findings demonstrate that CD44 transduces HA-based stiffness cues, temporally precedes integrin-based adhesion maturation, and facilitates invasion. IMPLICATIONS: This study reveals that the CD44 receptor, which is commonly overexpressed in GBM tumors, is critical for cell adhesion, invasion, and mechanosensing of an HA-based matrix.
DOIURLPMID [本文引用: 1]

Bonds between adhesion molecules are often mechanically stressed. A striking example is the tensile force applied to selectin-ligand bonds, which mediate the tethering and rolling of flowing leukocytes on vascular surfaces. It has been suggested that force could either shorten bond lifetimes, because work done by the force could lower the energy barrier between the bound and free states ('slip'), or prolong bond lifetimes by deforming the molecules such that they lock more tightly ('catch'). Whereas slip bonds have been widely observed, catch bonds have not been demonstrated experimentally. Here, using atomic force microscopy and flow-chamber experiments, we show that increasing force first prolonged and then shortened the lifetimes of P-selectin complexes with P-selectin glycoprotein ligand-1, revealing both catch and slip bond behaviour. Transitions between catch and slip bonds might explain why leukocyte rolling on selectins first increases and then decreases as wall shear stress increases. This dual response to force provides a mechanism for regulating cell adhesion under conditions of variable mechanical stress.
DOIURLPMID [本文引用: 2]

CD44 is the receptor for hyaluronan (HA) and mediates cell rolling under fluid shear stress. The HA-binding domain (HABD) of CD44 interconverts between a low-affinity, ordered (O) state and a high-affinity, partially disordered (PD) state, by the conformational change of the C-terminal region, which is connected to the plasma membrane. To examine the role of tensile force on CD44-mediated rolling, we used a cell-free rolling system, in which recombinant HABDs were attached to beads through a C-terminal or N-terminal tag. We found that the rolling behavior was stabilized only at high shear stress, when the HABD was attached through the C-terminal tag. In contrast, no difference was observed for the beads coated with HABD mutants that constitutively adopt either the O state or the PD state. Steered molecular dynamics simulations suggested that the force from the C terminus disrupts the interaction between the C-terminal region and the core of the domain, thus providing structural insights into how the mechanical force triggers the allosteric O-to-PD transition. Based on these results, we propose that the force applied from the C terminus enhances the HABD-HA interactions by inducing the conformational change to the high-affinity PD transition more rapidly, thereby enabling CD44 to mediate lymphocyte trafficking and hematopoietic progenitor cell homing under high-shear conditions.
DOIURLPMID [本文引用: 1]

CD44 is a cellular adhesion molecule expressed in many different types of cells that may be a receptor for hyaluronic acid, laminin, collagen or fibronectin. In this study, we determined whether CD44 participated in the adhesion of three human colorectal carcinoma cell lines (KM-12c, CCL 188 and MIP-101) to laminin, collagen and hyaluronic acid. All lines were positive for the epithelial form of CD44 (CD44E) with a molecular weight of approximately 160 kD. All of them bound significantly to laminin and type IV collagen but not to hyaluronic acid in a solid phase adhesion assay. Three monoclonal antibodies to CD44 (Hermes 1, Hermes 3 and J173) significantly blocked the binding of colorectal carcinoma lines to laminin and collagen whereas another antibody to CD44 (50B4) bound to cells but did not inhibit adhesion. Only Hermes 1 completely abolished the binding to hyaluronic acid by a human B lymphoblastoid cell line, JY, that expressed the 90 kD haematopoietic form of CD44. Soluble hyaluronate inhibited the adhesion of JY cells to solid phase hyaluronate but did not inhibit adhesion to laminin and collagen by the colorectal carcinoma lines. Thus, (a) CD44E participates in the adhesion of colorectal carcinoma cells to laminin and type IV collagen and (b) the binding site for laminin and collagen on CD44E is different from the site for hyaluronic acid.
[本文引用: 1]
DOIURLPMID [本文引用: 1]

The glycosaminoglycan hyaluronan (HA), a major component of extracellular matrices, and cell surface receptors of HA have been proposed to have pivotal roles in cell proliferation, migration, and invasion, which are necessary for inflammation and cancer progression. CD44 and receptor for HA-mediated motility (RHAMM) are the two main HA-receptors whose biological functions in human and murine inflammations and tumor cells have been investigated comprehensively. HA was initially considered to be only an inert component of connective tissues, but is now known as a
DOIURLPMID [本文引用: 1]

CD44 is a widely expressed cell adhesion molecule that has been implicated in a variety of biological processes including lymphopoiesis, angiogenesis, wound healing, leukocyte extravasation at inflammatory sites, and tumor metastasis. The adhesive function of CD44, like other molecules involved in inducible adhesion, is tightly regulated. Post-translational modifications, isoform expression, aggregation state, and protein associations all can affect the ligand binding properties of CD44, and these can vary depending on the cell type and the activation state of the cell. The most extensively characterized ligand for CD44 is hyaluronan, a component of the extracellular matrix. Interactions between CD44 and hyaluronan can mediate both cell-cell and cell-extracellular matrix adhesion. In the immune system, both the selectin molecules and CD44 have been implicated in the initial binding of leukocytes to endothelial cells at an inflammatory site. Sulfation is required for selectin-mediated leukocyte-endothelial cell interactions, and, recently, inducible sulfation also was shown to regulate CD44-mediated leukocyte adhesion to endothelial cells. Sulfation, therefore, may be important in the regulation of cell adhesion at inflammatory sites. In this commentary we have reviewed the molecular aspects of CD44 and the mechanisms that regulate its binding to hyaluronan. In addition, we have summarized the role of CD44 and hyaluronan in mediating leukocyte-endothelial cell interactions and have discussed how this interaction may be regulated. Finally, we examined the potential role of sulfation as an inducible means to regulate CD44-mediated leukocyte adhesion and as a more general mechanism to regulate leukocyte-endothelial cell interactions.
DOIURLPMID [本文引用: 1]

Adhesive interactions involving CD44, the cell surface receptor for hyaluronan, underlie fundamental processes such as inflammatory leukocyte homing and tumor metastasis. Regulation of such events is critical and appears to be effected by changes in CD44 N-glycosylation that switch the receptor
[本文引用: 1]
DOIURLPMID [本文引用: 2]

Regulation of transient interactions between cells and the ubiquitous matrix glycosaminoglycan hyaluronan is crucial to such fundamental processes as embryonic development and leukocyte homing. Cd44, the primary cell surface receptor for hyaluronan, binds ligand via a lectin-like fold termed the Link module, but only after appropriate functional activation. The molecular details of the Cd44-hyaluronan interaction and hence the structural basis for this activation are unknown. Here we present the first crystal structure of Cd44 complexed with hyaluronan. This reveals that the interaction with hyaluronan is dominated by shape and hydrogen-bonding complementarity and identifies two conformational forms of the receptor that differ in orientation of a crucial hyaluronan-binding residue (Arg45, equivalent to Arg41 in human CD44). Measurements by NMR indicate that the conformational transition can be induced by hyaluronan binding, providing further insight into possible mechanisms for regulation of Cd44.
DOIURLPMID [本文引用: 1]

The hyaluronan (HA) receptor CD44 mediates cell adhesion in leukocyte trafficking and tumor metastasis. Our previous nuclear magnetic resonance (NMR) studies revealed that the CD44 hyaluronan-binding domain (HABD) alters its conformation upon HA binding, from the ordered (O) to the partially disordered (PD) conformation. Here, we demonstrate that the HABD undergoes an equilibrium between the O and PD conformations, in either the presence or absence of HA, which explains the seemingly contradictory X-ray and NMR structures of the HA-bound HABD. An HABD mutant that exclusively adopts the PD conformation displayed a higher HA affinity than the wild-type. Rolling of the cells expressing the mutant CD44 was less efficient than those expressing the wild-type, due to the decreased tether frequency and the slow cellular off rate. Considering that the mutant CD44, devoid of the low-affinity state, exhibited impaired rolling, we conclude that the coexistence of the high- and low-affinity states of the HABD is essential for the CD44-mediated rolling.
DOIURLPMID [本文引用: 1]

Hyaluronan is a polyanionic, megadalton-scale polysaccharide, which initiates cell signaling by interacting with several receptor proteins including CD44 involved in cell-cell interactions and cell adhesion. Previous studies of the CD44 hyaluronan binding domain have identified multiple widespread residues to be responsible for its recognition capacity. In contrast, the X-ray structural characterization of CD44 has revealed a single binding mode associated with interactions that involve just a fraction of these residues. In this study, we show through atomistic molecular dynamics simulations that hyaluronan can bind CD44 with three topographically different binding modes that in unison define an interaction fingerprint, thus providing a plausible explanation for the disagreement between the earlier studies. Our results confirm that the known crystallographic mode is the strongest of the three binding modes. The other two modes represent metastable configurations that are readily available in the initial stages of the binding, and they are also the most frequently observed modes in our unbiased simulations. We further discuss how CD44, fostered by the weaker binding modes, diffuses along HA when attached. This 1D diffusion combined with the constrained relative orientation of the diffusing proteins is likely to influence the aggregation kinetics of CD44. Importantly, CD44 aggregation has been suggested to be a possible mechanism in CD44-mediated signaling.
DOIURLPMID [本文引用: 1]

P-, E- and L-selectin constitute a family of cell adhesion receptors that mediate the initial tethering and rolling of leukocytes on inflamed endothelium as a prelude to their firm attachment and extravasation into tissues. The selectins bind weakly to sialyl Lewisx (SLe(X))-like glycans, but with high-affinity to specific glycoprotein counterreceptors, including PSGL-1. Here, we report crystal structures of human P- and E-selectin constructs containing the lectin and EGF (LE) domains co-complexed with SLe(X). We also present the crystal structure of P-selectin LE co-complexed with the N-terminal domain of human PSGL-1 modified by both tyrosine sulfation and SLe(X). These structures reveal differences in how E- and P-selectin bind SLe(X) and the molecular basis of the high-affinity interaction between P-selectin and PSGL-1.
DOIURLPMID

Allostery of P-selectin lectin (Lec) domain followed by an epithelial growth factor (EGF)-like domain is essential for its biological functionality, but the underlying pathways have not been well understood. Here the molecular dynamics simulations were performed on the crystallized structures to visualize the dynamic conformational change for state 1 (S1) or state 2 (S2) Lec domain with respective bent (B) or extended (E) EGF orientation. Simulations illustrated that both S1 and S2 conformations were unable to switch from one to another directly. Instead, a novel S1' conformation was observed from S1 when crystallized B-S1 or reconstructed
DOIURLPMID

The allostery of P-selectin has been studied extensively with a focus on the Lec and EGF domains, whereas the contribution of the CR domain remains unclear. Here, molecular dynamics simulations (MDS) combined with homology modeling were preformed to investigate the impact of the CR domain on P-selectin allostery. The results indicated that the CR domain plays a role in the allosteric dynamics of P-selectin in two ways. First, the CR1 domain tends to stabilize the low affinity of P-selectin during the equilibration processes with the transition inhibition from the S1 to S1' state by restraining the extension of the bent EGF orientation, or with the relaxation acceleration of the S2 state by promoting the bending of the extended EGF orientation. Second, the existence of CR domain increases intramolecular extension prior to complex separation, increasing the time available for the allosteric shift during forced dissociation with a prolonged bond duration. These findings further our understanding of the structure-function relationship of P-selectin with the enriched micro-structural bases of the CR domain.
DOIURLPMID [本文引用: 1]

Catch bonds, whose lifetimes are prolonged by force, have been observed in selectin-ligand interactions and other systems. Several biophysical models have been proposed to explain this counterintuitive phenomenon, but none was based on the structure of the interacting molecules and the noncovalent interactions at the binding interface. Here we used molecular dynamics simulations to study changes in structure and atomic-level interactions during forced unbinding of P-selectin from P-selectin glycoprotein ligand-1. A mechanistic model for catch bonds was developed based on these observations. In the model,
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURLPMID [本文引用: 2]

The neurofibromatosis-2 (NF2) gene encodes merlin, an ezrin-radixin-moesin-(ERM)-related protein that functions as a tumor suppressor. We found that merlin mediates contact inhibition of growth through signals from the extracellular matrix. At high cell density, merlin becomes hypo-phosphorylated and inhibits cell growth in response to hyaluronate (HA), a mucopolysaccharide that surrounds cells. Merlin's growth-inhibitory activity depends on specific interaction with the cytoplasmic tail of CD44, a transmembrane HA receptor. At low cell density, merlin is phosphorylated, growth permissive, and exists in a complex with ezrin, moesin, and CD44. These data indicate that merlin and CD44 form a molecular switch that specifies cell growth arrest or proliferation.
[本文引用: 1]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
URLPMID [本文引用: 1]
DOIURLPMID [本文引用: 1]

Tumor cells nearly invariably evolve sustained PI3K/Akt signaling as an effective means to circumvent apoptosis and maintain survival. However, for those tumor cells that do not acquire PI3K/Akt mutations to achieve this end, the underlying mechanisms have remained obscure. Here, we describe the discovery of a splice isoform-dependent positive feedback loop that is essential to sustain PI3K/Akt signaling in breast cancer. Splice isoform CD44s promoted expression of the hyaluronan synthase HAS2 by activating the Akt signaling cascade. The HAS2 product hyaluronan further stimulated CD44s-mediated Akt signaling, creating a feed-forward signaling circuit that promoted tumor cell survival. Mechanistically, we identified FOXO1 as a bona fide transcriptional repressor of HAS2. Akt-mediated phosphorylation of FOXO1 relieved its suppression of HAS2 transcription, with FOXO1 phosphorylation status maintained by operation of the positive feedback loop. In clinical specimens of breast cancer, we established that the expression of CD44s and HAS2 was positively correlated. Our results establish a positive feedback mechanism that sustains PI3K/Akt signaling in tumor cells, further illuminating the nearly universal role of this pathway in cancer cell survival. Cancer Res; 77(14); 3791-801. (c)2017 AACR.
URLPMID [本文引用: 1]

The glycosaminoglycan hyaluronate (HA) is part of the extracellular environment in bone marrow. We show here that HA activates signal transduction cascades important for hemopoiesis. In myeloid and lymphoid long-term bone marrow cultures (LTBMC), treatment with hyaluronidase (HA'ase) results in reduced production of both progenitor and mature cells. Exogeneous HA added to LTBMC had the opposite effect: it enhanced hematopoiesis. The effect of HA is mediated through two different HA receptors on bone marrow macrophage-like cells, one of which is CD44 while the other is unknown. HA induces bone marrow macrophages to secrete IL-1beta (CD44-dependent) and IL-6 (CD44-independent). The two receptors address different signal transduction pathways: CD44 links to a pathway activating p38 protein kinase while the other yet unknown receptor induces Erk activity. There was no difference of the effect of HA and HA'ase on hematopoiesis in LTBMC and on cytokine production by macrophages in CD44-deficient mice compared with wild-type mice, indicating that the CD44 hyaluronate receptor and its signal transduction can be compensated for. Our data suggest a regulatory role for the extracellular matrix component HA in hematopoiesis and show the induction of signal transduction by HA receptors.
URLPMID [本文引用: 1]

DOIURLPMID [本文引用: 1]

Receptors tyrosine kinases (RTKs) and cell adhesion molecules (CAMs) present on the cell surface sense the surrounding environment and influence the fate of cells. For a long time, it was believed that these molecules were working independently and that the sole binding of a ligand was enough to activate the RTK. It is now apparent that there is, in fact, a very tight connection between RTKs and CAMs and that they work in concert. The CAMs influence the activation, the signaling, or the internalization of the RTKs. Some CAMs have similar functions and are therefore interchangeable. CD44 isoforms exemplify the flexibility of these interactions as they can collaborate with several RTKs and can also be substituted by other CAMs with similar functions. In several instances, CAMs not only control the activation of the receptor by presenting the ligand but also regulate the downstream signaling by organizing a signalosome complex. Furthermore, the functions of the CAMs can be controlled by the cellular environment and the binding to their ligands.
[本文引用: 1]
DOIURLPMID [本文引用: 1]

A major topic covered at the First International Symposium on Cancer Metastasis and the Lymphovascular System was the molecular mechanisms of metastasis. This has become of major interest in recent years as we have discovered new metastasis-related genes and gained understanding of the molecular events of lymphatic metastasis. The symposium covered new aspects and important questions related to the events of metastasis in both humans and animals. The basic and clinical related research covered in this topic represented many disciplines. The presentations showed novel findings and at the same time, raised many new unanswered questions, indicating the limited knowledge we still have regarding the molecular events of metastasis. The hope is that further unraveling of the direct and indirect molecular events of lymphatic metastasis will lead to new approaches in developing effective therapeutics.
[本文引用: 1]
[本文引用: 1]
DOIURLPMID [本文引用: 1]

CD44 on activated T cells can initiate contact and mediate rolling on hyaluronan on endothelial cells. We have shown that the integrin VLA-4 is used preferentially over LFA-1 in conjunction with this rolling interaction for firm adhesion. Here, we show by coimmunoprecipitation and transfection studies that CD44 associates with VLA-4 but not LFA-1 on the plasma membrane of immune cells. Absence of the cytoplasmic portion of CD44 abrogates this coassociation and attendant firm adhesion. Moreover, in an in vivo model of lymphocyte homing, cells expressing only the truncated form of CD44 together with VLA-4 fail to traffic to an inflamed site, thereby defining a discrete biological role for the cytoplasmic domain. These studies demonstrate a molecular mechanism whereby coanchoring within a single bimolecular complex between a primary and secondary adhesion molecule regulates a cell's ability to firmly adhere, providing a fundamental alteration to the paradigm of leukocyte extravasation.
DOIURLPMID [本文引用: 1]

Changes in tissue and organ stiffness occur during development and are frequently symptoms of disease. Many cell types respond to the stiffness of substrates and neighboring cells in vitro and most cell types increase adherent area on stiffer substrates that are coated with ligands for integrins or cadherins. In vivo cells engage their extracellular matrix (ECM) by multiple mechanosensitive adhesion complexes and other surface receptors that potentially modify the mechanical signals transduced at the cell/ECM interface. Here we show that hyaluronic acid (also called hyaluronan or HA), a soft polymeric glycosaminoglycan matrix component prominent in embryonic tissue and upregulated during multiple pathologic states, augments or overrides mechanical signaling by some classes of integrins to produce a cellular phenotype otherwise observed only on very rigid substrates. The spread morphology of cells on soft HA-fibronectin coated substrates, characterized by formation of large actin bundles resembling stress fibers and large focal adhesions resembles that of cells on rigid substrates, but is activated by different signals and does not require or cause activation of the transcriptional regulator YAP. The fact that HA production is tightly regulated during development and injury and frequently upregulated in cancers characterized by uncontrolled growth and cell movement suggests that the interaction of signaling between HA receptors and specific integrins might be an important element in mechanical control of development and homeostasis.
URLPMID [本文引用: 1]

For cancer metastasis, tumor cells present in the circulation must first adhere to the endothelium. Integrins lymphocyte function-associated antigen (LFA) 1 and very late antigen 4 play a central role in leukocyte adhesion to the endothelium and subsequent migration into tissues. The majority of tumor cells derived from solid cancers including colorectal cancer do not express suitable adhesion receptors, LFA-1 and very late antigen 4. We investigated the mechanisms of adhesion and transendothelial migration of cancer cells using colorectal carcinoma cell lines. Our results showed the following novel features of CD44 on the cells: (a) colon cancer cells express high levels of CD44; (b) stimulation of cancer cells by CD44 cross-linking or fragmented hyaluronan markedly induces the expression of LFA-1s, some of which reveal an activation epitope on the cells; (c) CD44 cross-linking induces F-actin polymerization in the cell cortex; (d) fragmented hyaluronan induces up-regulation of the activation epitope of LFA-1, which is mediated through protein kinase C; (e) stimulation of CD44 augments the LFA-1-mediated adhesion of cancer cells to endothelial cells and intercellular adhesion molecule 1-transfected cells and facilitates transendothelial migration; (f) stimulation of CD44 also induces expression of the hepatocyte growth factor (HGF) receptor c-Met on cancer cells; and (g) HGF further amplifies the LFA-1-mediated adhesion of cells prestimulated by CD44-derived signaling. Our results indicated that stimulation by CD44 induces
DOIURLPMID [本文引用: 1]

CD44, a widely expressed cell surface glycoprotein, plays a major role in cell-cell adhesion, cell-substrate interaction, lymphocyte homing, and tumor metastasis. For tumor metastasis to occur through the blood vessel and lymphatic vessel pathway, the tumor cells must first adhere to endothelial cells. Recent studies have shown that high expression of CD44 in certain types of tumors is associated with the hematogenic spread of cancer cells. However, the functional relevance of CD44 to tumor cell metastasis remains unknown. In this study, we investigated the mechanisms of CD44 cross-linking-induced adhesion and transendothelial migration of tumor cells using MDA-MB-435S breast cancer cell line. Breast cancer cells were found to express high levels of CD44. Using flow cytometric analysis and immunofluorescence staining, we demonstrated that cross-linking of CD44 resulted in a marked induction of the expression of lymphocyte function-associated antigen-1 (LFA-1) and very late antigen-4 (VLA-4) by exocytosis. These results were also observed with the Hs578T breast cancer cell line. Furthermore, LFA-1- and VLA-4-mediated adhesion and transendothelial cancer cell migration were also studied. Anti-LFA-1 mAb or anti-VLA-4 mAb alone had no effect on adhesion or transendothelial cancer cell migration, but were able to inhibit both of these functions when added together. This shows that CD44 cross-linking induces LFA-1 and VLA-4 expression in MDA-MB-435S cells and increases integrin-mediated adhesion to endothelial cells, resulting in the transendothelial migration of breast cancer cells. These observations provide direct evidence of a new function for CD44 that is involved in the induction of LFA-1 and VLA-4 expression by exocytosis in MDA-MB-435S cells. Because these induced integrins promote tumor cell migration into the target tissue, it may be possible to suppress this by pharmacological means, and thus potentially cause a reduction in invasive capability and metastasis.
[本文引用: 1]
DOIURLPMID [本文引用: 1]

Cholesterol plays a vital role in modulating the action of membrane proteins critical to cellular function. The effect of serum cholesterol on the prognosis of hepatocellular carcinoma (HCC) patients remains uncertain. Here, we report that high levels of cholesterol predict good survival and low disease recurrence after surgery. Cholesterol could significantly suppress migration and invasion of HCC cells and restrain metastasis of HCC in mice. High levels of cholesterol promoted CD44 translocation into lipid rafts and attenuated CD44-Ezrin binding, which are crucial for cell migration and cancer metastasis. The suppressive effect of cholesterol on HCC metastasis was abolished by the downregulation of CD44 or its palmitoylation inhibitor, which blocked CD44 localization in lipid rafts. Furthermore, pharmacologically promoting CD44 retention inside lipid rafts obviously attenuated HCC migration and invasion, providing a potential therapeutic strategy to prolong the survival of HCC patients.
DOIURLPMID [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOIURLPMID [本文引用: 1]

Renewal of integumentary organs occurs cyclically throughout an organism's lifetime, but the mechanism that initiates each cycle remains largely unknown. In a miniature pig model of tooth development that resembles tooth development in humans, the permanent tooth did not begin transitioning from the resting to the initiation stage until the deciduous tooth began to erupt. This eruption released the accumulated mechanical stress inside the mandible. Mechanical stress prevented permanent tooth development by regulating expression and activity of the integrin beta1-ERK1-RUNX2 axis in the surrounding mesenchyme. We observed similar molecular expression patterns in human tooth germs. Importantly, the release of biomechanical stress induced downregulation of RUNX2-wingless/integrated (Wnt) signaling in the mesenchyme between the deciduous and permanent tooth and upregulation of Wnt signaling in the epithelium of the permanent tooth, triggering initiation of its development. Consequently, our findings identified biomechanical stress-associated Wnt modulation as a critical initiator of organ renewal, possibly shedding light on the mechanisms of integumentary organ regeneration.
DOIURLPMID

Angiocrine signals derived from endothelial cells are an important component of intercellular communication and have a key role in organ growth, regeneration and disease(1-4). These signals have been identified and studied in multiple organs, including the liver, pancreas, lung, heart, bone, bone marrow, central nervous system, retina and some cancers(1-4). Here we use the developing liver as a model organ to study angiocrine signals(5,6), and show that the growth rate of the liver correlates both spatially and temporally with blood perfusion to this organ. By manipulating blood flow through the liver vasculature, we demonstrate that vessel perfusion activates beta1 integrin and vascular endothelial growth factor receptor 3 (VEGFR3). Notably, both beta1 integrin and VEGFR3 are strictly required for normal production of hepatocyte growth factor, survival of hepatocytes and liver growth. Ex vivo perfusion of adult mouse liver and in vitro mechanical stretching of human hepatic endothelial cells illustrate that mechanotransduction alone is sufficient to turn on angiocrine signals. When the endothelial cells are mechanically stretched, angiocrine signals trigger in vitro proliferation and survival of primary human hepatocytes. Our findings uncover a signalling pathway in vascular endothelial cells that translates blood perfusion and mechanotransduction into organ growth and maintenance.
[本文引用: 1]
DOIURLPMID [本文引用: 1]

Mechanical forces play critical roles in the function of living cells. However, the underlying mechanisms of how forces influence nuclear events remain elusive. Here, we show that chromatin deformation as well as force-induced transcription of a green fluorescent protein (GFP)-tagged bacterial-chromosome dihydrofolate reductase (DHFR) transgene can be visualized in a living cell by using three-dimensional magnetic twisting cytometry to apply local stresses on the cell surface via an Arg-Gly-Asp-coated magnetic bead. Chromatin stretching depended on loading direction. DHFR transcription upregulation was sensitive to load direction and proportional to the magnitude of chromatin stretching. Disrupting filamentous actin or inhibiting actomyosin contraction abrogated or attenuated force-induced DHFR transcription, whereas activating endogenous contraction upregulated force-induced DHFR transcription. Our findings suggest that local stresses applied to integrins propagate from the tensed actin cytoskeleton to the LINC complex and then through lamina-chromatin interactions to directly stretch chromatin and upregulate transcription.
DOIURLPMID [本文引用: 1]

The immune response is orchestrated by a variety of immune cells. The function of each cell is determined by the collective signals from various immunoreceptors, whose expression and activity depend on the developmental stages of the cell and its environmental context. Recent studies have highlighted the presence of mechanical force on several immunoreceptor-ligand pairs and the important role of force in regulating their interaction and function. In this Perspective, we use the T cell antigen receptor as an example with which to review the current understanding of the mechanosensing properties of immunoreceptors. We discuss the types of forces that immunoreceptors may encounter and the effects of force on ligand bonding, conformational change and the triggering of immunoreceptors, as well as the effects of force on the downstream signal transduction, cell-fate decisions and effector function of immune cells.
