 ,1,*, 谢道昕
,1,*, 谢道昕 ,2,*
,2,*New Insight into Strigolactone Signaling
Ruifeng Yao ,1,*, Daoxin Xie
,1,*, Daoxin Xie ,2,*
,2,*通讯作者:
责任编辑: 朱亚娜
收稿日期:2020-05-28接受日期:2020-06-2网络出版日期:2020-07-01
| 基金资助: |
Corresponding authors:
Received:2020-05-28Accepted:2020-06-2Online:2020-07-01

摘要
关键词:
Abstract
Keywords:
PDF (1426KB)摘要页面多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
引用本文
姚瑞枫, 谢道昕. 独脚金内酯信号途径的新发现——抑制子也是转录因子. 植物学报, 2020, 55(4): 397-402 doi:10.11983/CBB20099
Yao Ruifeng, Xie Daoxin.
植物的正常生长发育需要适宜的光照、温度、水分和各种营养元素。在整个生命周期中, 植物还需不断地应对各种环境变化。植物激素是植物自身产生的、微量浓度就能引起植物生理效应的信号分子, 在调节植物生长发育、应对环境变化和防御病虫害等方面具有重要作用, 影响植(作)物的生存、产量和品质。迄今研究得较为深入的植物激素包括生长素、赤霉素、乙烯、细胞分裂素、脱落酸、油菜素内酯、水杨酸、茉莉素和独脚金内酯共9类小分子激素及一些重要的多肽类激素; 同时, 一氧化氮和多胺类生长调节物质也受到高度关注(黎家和李传友, 2019)。
独脚金内酯(strigolactone, SL)是一类新近被鉴定为植物激素的萜类小分子化合物(Gomez-Roldan et al., 2008; Umehara et al., 2008), 其命名基于它最初被发现的功能——促进独脚金属(Striga)寄生杂草的种子萌发, 及其化学结构特征——内酯(lactone)。SL分子早在7.25-12亿年前便已出现, 伴随植物从水生向陆生演化这一重大事件(Waters et al., 2017), 并逐渐演化出一系列重要生物学功能。
作为一种新型植物激素, SL在调控植物生长发育及环境适应性的多个方面具有重要功能, 包括调控植物分枝、茎秆粗细和叶片形态等地上部分的株型, 以及主根长度、侧根和根毛密度等地下部分的株型, 还能介导植物对灰霉菌、干旱及营养匮乏等生物/非生物逆境胁迫的抗性(Wang et al., 2017)。作为根际信号分子, SL从根部分泌到土壤中, 促进丛枝菌根真菌与植物形成菌根共生, 有助于陆生植物吸收营养和水分, 却也可被根寄生杂草利用于促进自身种子萌发, 从而寄生在宿主植物根部。此外, SL还表现出抑制乳腺癌等肿瘤的活性(Zwanenburg and Blanco-Ania, 2018)。
SL如何发挥生物学功能一直是植物领域的重要科学问题。得益于拟南芥(Arabidopsis thaliana)、水稻(Oryza sativa)、豌豆(Pisum sativum)和矮牵牛(Petunia hybrida)等模式植物完备的遗传研究体系, SL的生物合成、转运及信号传导机理研究取得了一系列重要进展(Bürger and Chory, 2020)。SL由全反式- β-胡萝卜素经过一系列酶催化加工而来, 目前已在不同植物中发现了25种以上的天然SL分子。根据其是否具有完整的ABC-ring三环结构, 可将它们分为典型SL和非典型SL两类, 但它们均有1个共同的保守结构——D-ring (图1)。水稻、拟南芥及其它物种(包括寄生植物独脚金(S. hermonthica))中的D14蛋白或其旁系同源蛋白被证明为SL的受体, 发现这些受体蛋白具有双重功能——既作为酶催化SL的水解反应, 又作为受体感知SL信号(Hamiaux et al., 2012; Zhao et al., 2013; de Saint Germain et al., 2016; Yao et al., 2016, 2017, 2018; Uraguchi et al., 2018; Shabek et al., 2018; Seto et al., 2019)。
图1
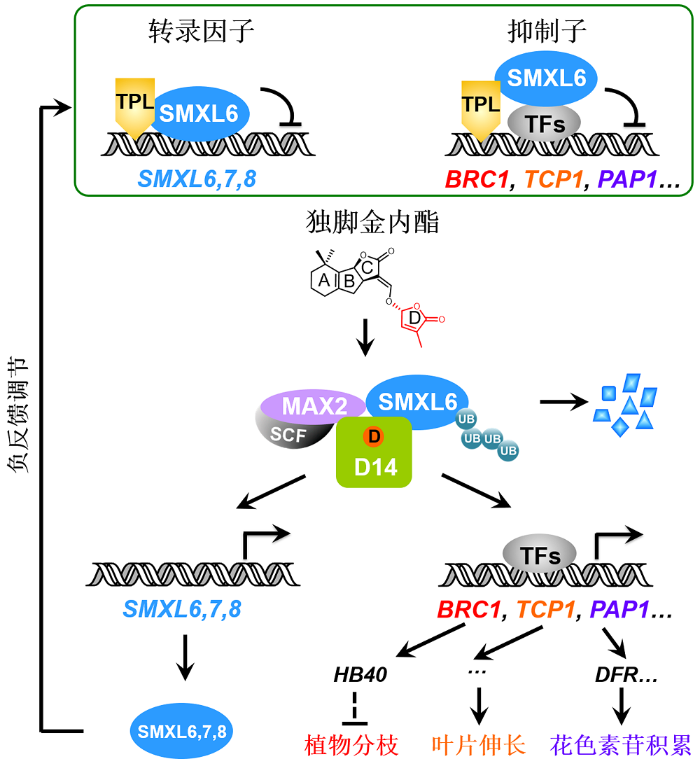 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1独脚金内酯信号通路中抑制子SMXL6,7,8的双重功能工作模型
独脚金内酯信号通路中的SMXL6,7,8是具有双重功能的新型抑制子: SMXL6,7,8作为抑制子招募TPL共抑制子并直接结合下游转录因子抑制其转录活性, 从而阻遏独脚金内酯(SL)响应基因的表达; 同时SMXL6,7,8又作为转录因子直接结合并抑制SMXL6,7,8基因的启动子。SL被D14感知, 诱导SMXL6,7, 8-D14-MAX2复合体形成, 导致SMXL6,7,8通过泛素化-蛋白酶体途径降解, 从而解除SMXL6,7,8对下游转录因子以及自身基因启动子的抑制, 一方面激活BRC1、TCP1和PAP1等响应基因的转录, 最终调控植物分枝、叶片伸长和花色素苷积累等生物学过程; 另一方面解除对SMXL6,7,8启动子的抑制, 激活SMXL6,7,8自身基因的表达, 形成维持SL通路稳态的负反馈调控体系。SCF: Skp1-Cullin-F-box; UB: 泛素
Figure 1Working model for the dual-function repressors SMXL6,7,8 in strigolactone signaling
SMXL6,7,8 in the strigolactone signaling pathway act as novel repressors with dual functions: SMXL6,7,8 act as repressors that recruit TPL co-repressor proteins and bind transcription factors to inhibit their transcriptional activity, thereby suppressing expression of strigolactone (SL)-responsive genes; meanwhile, SMXL6,7,8 also serve as transcription factors that directly bind and inhibit the promoters of SMXL6,7,8 genes. SL is perceived by D14 to trigger formation of SMXL6,7,8-D14-MAX2 complex and further induce SMXL6,7,8 degradation via the ubiquitination-proteasome pathway. The SL-induced SMXL6,7,8 degradation releases transcription factors to activate expression of the SL-responsive genes such as BRC1, TCP1 and PAP1 essential for plant branching, leaf elongation, and anthocyanin biosynthesis, respectively. Such SMXL6,7,8 degradation also de- represses the SMXL6,7,8 suppression on the SMXL6,7,8 promoters to activate the expression of SMXL6,7,8 genes, which forms a negative feedback regulation loop that maintains the homeostasis of SL pathway. SCF: Skp1-Cullin-F- box; UB: Ubiquitin
李家洋团队和中国农业科学院作物科学研究所万建民团队在2013年分别发现了水稻SL信号通路的抑制子D53 (Jiang et al., 2013; Zhou et al., 2013)。D53基因发生显性突变产生的功能获得性突变体d53对SL不敏感, 呈现矮化多分蘖表型。SL可促进D14与D53及F-box蛋白D3互作, 诱导D53泛素化修饰和降解, 从而抑制植物分枝。D53中含有EAR基序, 李家洋团队发现D53能够招募TPL共抑制子(co-repressor), 进而结合转录因子IPA1蛋白并抑制其转录激活功能(Jiang et al., 2013; Song et al., 2017; Ma et al., 2017)。此外, 他们还发现D53通过拮抗SL诱导的细胞分裂素降解酶基因OsCKX9转录, 促进细胞分裂素的积累(Duan et al., 2019)。李家洋团队进一步鉴定了水稻D53在拟南芥中的同源蛋白, 发现拟南芥SMXL2、SMXL6、SMXL7和SMXL8是SL信号通路的抑制子, 其中SMXL2通过SL和KAR (karrikin)两条信号通路调控下胚轴伸长(Wang et al., 2020b), 而SMXL6、SMXL7和SMXL8 (SMXL6,7,8)调控植物分枝和叶片形态(Wang et al., 2015)。
李家洋团队近期的研究发现SMXL6,7,8是具有抑制子和转录因子双重功能的新型抑制子, 初步阐明了SL调控植物分枝、叶片伸长和花色素苷积累的转录调控机理(Wang et al., 2020a) (图1)。他们还通过对SL类似物rac-GR24手性异构体GR244DO处理的拟南芥进行转录组分析, 成功鉴定了401个响应基因(约90%为新发现的SL响应基因), 揭示SL可能通过调控细胞骨架发挥生理功能, 并发现SL通过诱导抗旱关键基因AFL1的表达调控植物抗旱性(Wang et al., 2020a)。该研究建立了SMXL6,7,8依赖EAR基序抑制BRC1表达进而降低脱落酸(ABA)含量、促进植物分枝的转录调控途径(图1)。他们发现抑制腋芽伸长的关键转录因子BRC1可激活其靶基因HB40的表达, 促进ABA的合成, 抑制腋芽伸长从而减少植物分枝; 而SMXL6则依赖其EAR基序抑制BRC1基因的表达, 抑制ABA合成, 促进植物分枝。GR244DO诱导抑制子SMXL6的降解, 释放转录因子BRC1, 激活HB40的表达, 提高ABA含量, 从而抑制植物分枝(Wang et al., 2020a) (图1)。
此外, 该研究发现调节叶片发育的TCP1基因以依赖D14的方式受到GR244DO的诱导, 进而通过一系列遗传表型和基因表达分析证明SMXL6,7,8通过抑制TCP1表达而抑制叶片伸长, 且该抑制作用依赖其EAR基序, 从而建立了SL控制叶片发育的转录调控途径(图1)。研究还发现SL通过解除SMXL6,7,8对转录因子PAP1、PAP2、MYB113和MYB114的抑制作用, 激活花色素苷合成基因DFR、ANS和TT7的表达, 从而促进花色素苷的积累(Wang et al., 2020a) (图1)。
抑制子SMXL6,7,8也是转录因子, 可通过直接结合DNA调控基因的表达(Wang et al., 2020a)。SMXL6蛋白直接结合SMXL6,7,8基因的启动子并抑制其转录, SMXL7也可结合SMXL6,7,8基因的启动子, SMXL8则可结合SMXL7基因的启动子, 而SMXL7基因启动子的ATAACAA基序是SMXL6蛋白结合并抑制SMXL7基因的启动子所必需的。这些结果表明, SMXL6,7,8可作为转录因子直接结合DNA并负调控SMXL6,7,8自身基因的转录, 从而维持自身的稳态和适度的SL信号响应(图1, 图2A)。
图2
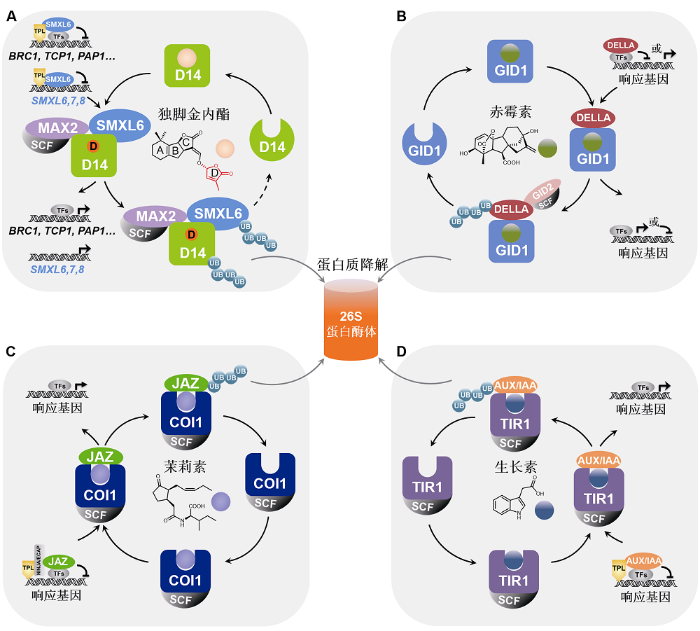 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2独脚金内酯、赤霉素、茉莉素及生长素信号途径中抑制子的功能比较
植物激素独脚金内酯(A)、赤霉素(B)、茉莉素(C)及生长素(D)信号传导途径中的抑制子DELLA、AUX/IAA、JAZ和D53/SMXL均通过结合下游信号蛋白(转录因子)调控其转录活性, 从而阻遏激素响应基因的表达。激素分子被相应的受体识别后激活其信号传导链,诱导抑制子通过泛素化-蛋白酶体途径降解, 促进响应基因表达并介导相应的生物学功能。独脚金内酯(SL)信号传导途径中的抑制子SMXL6,7,8同时还作为转录因子直接结合并抑制SMXL6,7,8基因的启动子; SL诱导SMXL6,7,8降解, 从而解除SMXL6,7,8对自身基因启动子的抑制, 激活SMXL6,7,8自身基因的表达, 形成维持SL通路稳态的负反馈调控体系(A)。
Figure 2Comparison of the repressor proteins in strigolactone, gibberellin, jasmonate and auxin signaling pathways
The repressor proteins D53/SMXL, DELLA, JAZ, and AUX/IAA in the signaling pathways of strigolactone (A), gibberellin (B), jasmonate (C) and auxin (D) bind and inhibit downstream transcription factors, thereby suppressing the expression of hormone-responsive genes. Hormone molecule is recognized by corresponding receptor protein and activates the signal transduction chain to induce the degradation of the repressor protein via ubiquitination-proteasome pathway, then triggering response gene expression and related biological processes. Moreover, the repressor proteins SMXL6,7,8 in strigolactone (SL) signaling pathway can also directly bind and inhibit the promoter of SMXL6,7,8 gene as transcription factors. SL induces the degradation of SMXL6,7,8 to release its repression on the SMXL6,7,8 promoters to activate the expression of SMXL6,7,8 genes, forming a negative feedback regulation loop (A) essential for the homeostasis of SL pathway.
此前的研究表明, SMXL6,7,8招募TPL/TPR共抑制子(Wang et al., 2015), 直接结合下游转录因子(如BES1、水稻IPA1在拟南芥中的同源蛋白SPL9及SPL15)并抑制这些转录因子对BRC1基因的转录, 进而调控植物分枝(Fang et al., 2020; Hu et al., 2020; Xie et al., 2020)。D53/SMXL6,7,8类似于赤霉素信号途径的DELLA、生长素信号途径的AUX/IAA及茉莉素信号途径的JAZ等抑制子, 均可通过直接结合下游信号蛋白(转录因子)抑制其转录活性, 从而阻遏激素响应基因的表达。当体内激素含量上升时, 激素分子通过激活信号传导链降解抑制子、释放下游转录因子,从而诱导激素响应基因的表达, 以调控相应的生物学功能(图1, 图2)。与赤霉素、生长素及茉莉素类似, SL在诱导其抑制子D53/SMXL6,7,8降解的同时却促进D53/SMXL6,7,8基因的表达(Jiang et al., 2013; Stanga et al., 2013; Wang et al., 2015), 但是其具体调控机制并不清楚。
李家洋团队最新的研究发现抑制子SMXL6,7,8也是转录因子, 揭示了SL诱导SMXL6,7,8自身基因表达的调控机制, 并初步阐明了SL调控植物分枝、叶片伸长和花色素苷积累的转录调控机理(Wang et al., 2020a), 为探索植物激素作用机理提供了新思路, 具有重要科学意义和应用前景。SMXL6,7,8作为新型抑制子, 既招募TPL/TPR共抑制子并结合下游转录因子(如BES1和SPL9/15蛋白)抑制其转录活性, 从而阻遏SL响应基因的表达, 同时, 又作为转录因子直接结合SMXL6,7,8自身基因的启动子并抑制其表达。当植物体内SL含量上升时, SL被受体D14感知从而诱导SMXL6,7,8-D14-MAX2复合体形成, 导致SMXL6,7, 8通过泛素化-蛋白酶体途径降解, 解除了SMXL6,7,8对下游转录因子的抑制, 进而激活SL响应基因(如BRC1、TCP1和PAP1)的转录, 最终调控植物分枝、叶片伸长和花色素苷积累等生物学过程; 同时, SL诱导SMXL6,7,8的降解也解除了SMXL6,7,8对其自身的转录抑制, 从而激活SMXL6,7,8基因的表达。SL既诱导SMXL6,7,8蛋白的降解又激活SMXL6,7,8基因的表达, 形成了一个精细调控SMXL6,7,8丰度所必需的反馈调控回路(图1, 图2A)。
赤霉素、茉莉素和生长素信号途径中的抑制子(DELLA、JAZ和AUX/IAA)可招募共抑制子并结合下游转录因子, 但这些抑制子是否具有转录因子的功能尚不清楚(图2B-D), 且这些抑制子是否类似于SMXL6,7,8可以作为转录因子直接结合自身基因的启动子进而调控自身基因的表达, 还有待进一步验证。拟南芥SMXL家族的其它成员以及SMXL6,7,8在水稻中的直系同源蛋白D53和D53-Like是否也具有转录因子的功能? SMXL6,7,8作为转录因子是否直接结合并调控其它基因的启动子? SMXL6,7,8除了通过抑制ABA的生物合成调控植物分枝之外(Wang et al., 2020a), 是否也通过调控生长素和细胞分裂素的合成控制植物分枝? 对这些问题的回答将有助于进一步解析SL信号传导途径, 以及深入理解SL调控植物生长发育的分子机制。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
DOI:10.1016/j.tplants.2019.12.009URLPMID:31948791 [本文引用: 1]

Strigolactones (SLs) are a class of plant hormones involved in several biological processes that are of great agricultural concern. While initiating plant-fungal symbiosis, SLs also trigger germination of parasitic plants that pose a major threat to farming. In vascular plants, SLs control shoot branching, which is linked to crop yield. SL research has been a fascinating field that has produced a variety of different signaling models, reflecting a complex picture of hormone perception. Here, we review recent developments in the SL field and the crystal structures that gave rise to various models of receptor activation. We also highlight the increasing number of discovered SL molecules, reflecting the existence of cross-kingdom SL communication.
DOI:10.1038/nchembio.2147URLPMID:27479744 [本文引用: 1]

Strigolactone plant hormones control plant architecture and are key players in both symbiotic and parasitic interactions. They contain an ABC tricyclic lactone connected to a butenolide group, the D ring. The DWARF14 (D14) strigolactone receptor belongs to the superfamily of alpha/beta-hydrolases, and is known to hydrolyze the bond between the ABC lactone and the D ring. Here we characterized the binding and catalytic functions of RAMOSUS3 (RMS3), the pea (Pisum sativum) ortholog of rice (Oryza sativa) D14 strigolactone receptor. Using new profluorescent probes with strigolactone-like bioactivity, we found that RMS3 acts as a single-turnover enzyme that explains its apparent low enzymatic rate. We demonstrated the formation of a covalent RMS3-D-ring complex, essential for bioactivity, in which the D ring was attached to histidine 247 of the catalytic triad. These results reveal an undescribed mechanism of plant hormone reception in which the receptor performs an irreversible enzymatic reaction to generate its own ligand.
DOI:10.1073/pnas.1810980116URLPMID:31235564 [本文引用: 1]

Strigolactones (SLs), a group of terpenoid lactones derived from carotenoids, are plant hormones that control numerous aspects of plant development. Although the framework of SL signaling that the repressor DWARF 53 (D53) could be SL-dependently degraded via the SL receptor D14 and F-box protein D3 has been established, the downstream response genes to SLs remain to be elucidated. Here we show that the cytokinin (CK) content is dramatically increased in shoot bases of the rice SL signaling mutant d53 By examining transcript levels of all the CK metabolism-related genes after treatment with SL analog GR24, we identified CYTOKININ OXIDASE/DEHYDROGENASE 9 (OsCKX9) as a primary response gene significantly up-regulated within 1 h of treatment in the wild type but not in d53 We also found that OsCKX9 functions as a cytosolic and nuclear dual-localized CK catabolic enzyme, and that the overexpression of OsCKX9 suppresses the browning of d53 calli. Both the CRISPR/Cas9-generated OsCKX9 mutants and OsCKX9-overexpressing transgenic plants showed significant increases in tiller number and decreases in plant height and panicle size, suggesting that the homeostasis of OsCKX9 plays a critical role in regulating rice shoot architecture. Moreover, we identified the CK-inducible rice type-A response regulator OsRR5 as the secondary SL-responsive gene, whose expression is significantly repressed after 4 h of GR24 treatment in the wild type but not in osckx9 These findings reveal a comprehensive plant hormone cross-talk in which SL can induce the expression of OsCKX9 to down-regulate CK content, which in turn triggers the response of downstream genes.
URLPMID:31837469 [本文引用: 1]
DOI:10.1038/nature07271URLPMID:18690209 [本文引用: 1]

A carotenoid-derived hormonal signal that inhibits shoot branching in plants has long escaped identification. Strigolactones are compounds thought to be derived from carotenoids and are known to trigger the germination of parasitic plant seeds and stimulate symbiotic fungi. Here we present evidence that carotenoid cleavage dioxygenase 8 shoot branching mutants of pea are strigolactone deficient and that strigolactone application restores the wild-type branching phenotype to ccd8 mutants. Moreover, we show that other branching mutants previously characterized as lacking a response to the branching inhibition signal also lack strigolactone response, and are not deficient in strigolactones. These responses are conserved in Arabidopsis. In agreement with the expected properties of the hormonal signal, exogenous strigolactone can be transported in shoots and act at low concentrations. We suggest that endogenous strigolactones or related compounds inhibit shoot branching in plants. Furthermore, ccd8 mutants demonstrate the diverse effects of strigolactones in shoot branching, mycorrhizal symbiosis and parasitic weed interaction.
DOI:10.1016/j.cub.2012.08.007URLPMID:22959345 [本文引用: 1]

Strigolactones are a recently discovered class of plant hormone involved in branching, leaf senescence, root development, and plant-microbe interactions. They are carotenoid-derived lactones, synthesized in the roots and transported acropetally to modulate axillary bud outgrowth (i.e., branching). However, a receptor for strigolactones has not been identified. We have identified the DAD2 gene from petunia, an ortholog of the rice and Arabidopsis D14 genes, and present evidence for its roles in strigolactone perception and signaling. DAD2 acts in the strigolactone pathway, and the dad2 mutant is insensitive to the strigolactone analog GR24. The crystal structure of DAD2 reveals an alpha/beta hydrolase fold containing a canonical catalytic triad with a large internal cavity capable of accommodating strigolactones. In the presence of GR24 DAD2 interacts with PhMAX2A, a central component of strigolactone signaling, in a GR24 concentration-dependent manner. DAD2 can hydrolyze GR24, with mutants of the catalytic triad abolishing both this activity and the ability of DAD2 to interact with PhMAX2A. The hydrolysis products can neither stimulate the protein-protein interaction nor modulate branching. These observations suggest that DAD2 acts to bind the mobile strigolactone signal and then interacts with PhMAX2A during catalysis to initiate an SCF-mediated signal transduction pathway.
DOI:10.1016/j.xplc.2019.100014URL [本文引用: 1]
DOI:10.1038/nature12870URL [本文引用: 3]

Strigolactones (SLs) are a group of newly identified plant hormones that control plant shoot branching. SL signalling requires the hormone-dependent interaction of DWARF14 (D14), a probable candidate SL receptor, with DWARF3 (D3), an F-box component of the Skp-Cullin-F-box (SCF) E3 ubiquitin ligase complex. Here we report the characterization of a dominant SL-insensitive rice (Oryza sativa) mutant dwarf 53 (d53) and the cloning of D53, which encodes a substrate of the SCFD3 ubiquitination complex and functions as a repressor of SL signalling. Treatments with GR24, a synthetic SL analogue, cause D53 degradation via the proteasome in a manner that requires D14 and the SCFD3 ubiquitin ligase, whereas the dominant form of D53 is resistant to SL-mediated degradation. Moreover, D53 can interact with transcriptional co-repressors known as TOPLESS-RELATED PROTEINS. Our results suggest a model of SL signalling that involves SL-dependent degradation of the D53 repressor mediated by the D14-D3 complex.
DOI:10.1126/sciadv.1601217URLPMID:28630893 [本文引用: 1]

TOPLESS are tetrameric plant corepressors of the conserved Tup1/Groucho/TLE (transducin-like enhancer of split) family. We show that they interact through their TOPLESS domains (TPDs) with two functionally important ethylene response factor-associated amphiphilic repression (EAR) motifs of the rice strigolactone signaling repressor D53: the universally conserved EAR-3 and the monocot-specific EAR-2. We present the crystal structure of the monocot-specific EAR-2 peptide in complex with the TOPLESS-related protein 2 (TPR2) TPD, in which the EAR-2 motif binds the same TPD groove as jasmonate and auxin signaling repressors but makes additional contacts with a second TPD site to mediate TPD tetramer-tetramer interaction. We validated the functional relevance of the two TPD binding sites in reporter gene assays and in transgenic rice and demonstrate that EAR-2 binding induces TPD oligomerization. Moreover, we demonstrate that the TPD directly binds nucleosomes and the tails of histones H3 and H4. Higher-order assembly of TPD complexes induced by EAR-2 binding markedly stabilizes the nucleosome-TPD interaction. These results establish a new TPD-repressor binding mode that promotes TPD oligomerization and TPD-nucleosome interaction, thus illustrating the initial assembly of a repressor-corepressor-nucleosome complex.
DOI:10.1038/s41467-018-08124-7URLPMID:30643123 [本文引用: 1]

The perception mechanism for the strigolactone (SL) class of plant hormones has been a subject of debate because their receptor, DWARF14 (D14), is an alpha/beta-hydrolase that can cleave SLs. Here we show via time-course analyses of SL binding and hydrolysis by Arabidopsis thaliana D14, that the level of uncleaved SL strongly correlates with the induction of the active signaling state. In addition, we show that an AtD14(D218A) catalytic mutant that lacks enzymatic activity is still able to complement the atd14 mutant phenotype in an SL-dependent manner. We conclude that the intact SL molecules trigger the D14 active signaling state, and we also describe that D14 deactivates bioactive SLs by the hydrolytic degradation after signal transmission. Together, these results reveal that D14 is a dual-functional receptor, responsible for both the perception and deactivation of bioactive SLs.
DOI:10.1038/s41586-018-0743-5URLPMID:30464344 [本文引用: 1]

The strigolactones, a class of plant hormones, regulate many aspects of plant physiology. In the inhibition of shoot branching, the alpha/beta hydrolase D14-which metabolizes strigolactone-interacts with the F-box protein D3 to ubiquitinate and degrade the transcription repressor D53. Despite the fact that multiple modes of interaction between D14 and strigolactone have recently been determined, how the hydrolase functions with D3 to mediate hormone-dependent D53 ubiquitination remains unknown. Here we show that D3 has a C-terminal alpha-helix that can switch between two conformational states. The engaged form of this alpha-helix facilitates the binding of D3 and D14 with a hydrolysed strigolactone intermediate, whereas the dislodged form can recognize unmodified D14 in an open conformation and inhibits its enzymatic activity. The D3 C-terminal alpha-helix enables D14 to recruit D53 in a strigolactone-dependent manner, which in turn activates the hydrolase. By revealing the structural plasticity of the SCF(D3-D14) ubiquitin ligase, our results suggest a mechanism by which the E3 coordinates strigolactone signalling and metabolism.
URLPMID:28809396 [本文引用: 1]
DOI:10.1104/pp.113.221259URL [本文引用: 1]

Abiotic chemical signals discovered in smoke that are known as karrikins (KARs) and the endogenous hormone strigolactone (SL) control plant growth through a shared MORE AXILLARY GROWTH2 (MAX2)-dependent pathway. A SL biosynthetic pathway and candidate KAR/SL receptors have been characterized, but signaling downstream of MAX2 is poorly defined. A screen for genetic suppressors of the enhanced seed dormancy phenotype of max2 in Arabidopsis (Arabidopsis thaliana) led to identification of a suppressor of max2 1 (smax1) mutant. smax1 restores the seed germination and seedling photomorphogenesis phenotypes of max2 but does not affect the lateral root formation, axillary shoot growth, or senescence phenotypes of max2. Expression of three transcriptional markers of KAR/SL signaling, D14-LIKE2, KAR-UP F-BOX1, and INDOLE-3-ACETIC ACID INDUCIBLE1, is rescued in smax1 max2 seedlings. SMAX1 is a member of an eight-gene family in Arabidopsis that has weak similarity to HEAT SHOCK PROTEIN 101, which encodes a caseinolytic peptidase B chaperonin required for thermotolerance. SMAX1 and the SMAX1-like (SMXL) homologs are differentially expressed in Arabidopsis tissues. SMAX1 transcripts are most abundant in dry seed, consistent with its function in seed germination control. Several SMXL genes are up-regulated in seedlings treated with the synthetic SL GR24. SMAX1 and SMXL2 transcripts are reduced in max2 seedlings, which could indicate negative feedback regulation by KAR/SL signaling. smax1 seed and seedling growth mimics the wild type treated with KAR/SL, but smax1 seedlings are still responsive to 2H-furo[2,3-c]pyran-2-one (KAR(2)) or GR24. We conclude that SMAX1 is an important component of KAR/SL signaling during seed germination and seedling growth but is not necessary for all MAX2-dependent responses. We hypothesize that one or more SMXL proteins may also act downstream of MAX2 to control the diverse developmental responses to KARs and SLs.
URLPMID:18690207 [本文引用: 1]
URLPMID:30545887 [本文引用: 1]
[本文引用: 1]
DOI:10.1105/tpc.15.00605URLPMID:26546446 [本文引用: 3]

Strigolactones (SLs) are carotenoid-derived phytohormones that control many aspects of plant development, including shoot branching, leaf shape, stem secondary thickening, and lateral root growth. In rice (Oryza sativa), SL signaling requires the degradation of DWARF53 (D53), mediated by a complex including D14 and D3, but in Arabidopsis thaliana, the components and mechanism of SL signaling involving the D3 ortholog MORE AXILLARY GROWTH2 (MAX2) are unknown. Here, we show that SL-dependent regulation of shoot branching in Arabidopsis requires three D53-like proteins, SUPPRESSOR OF MORE AXILLARY GROWTH2-LIKE6 (SMXL6), SMXL7, and SMXL8. The smxl6 smxl7 smxl8 triple mutant suppresses the highly branched phenotypes of max2 and the SL-deficient mutant max3. Overexpression of a mutant form of SMXL6 that is resistant to SL-induced ubiquitination and degradation enhances shoot branching. Exogenous application of the SL analog rac-GR24 causes ubiquitination and degradation of SMXL6, 7, and 8; this requires D14 and MAX2. D53-like SMXLs form complexes with MAX2 and TOPLESS-RELATED PROTEIN2 (TPR2) and interact with D14 in a GR24-responsive manner. Furthermore, D53-like SMXLs exhibit TPR2-dependent transcriptional repression activity and repress the expression of BRANCHED1. Our findings reveal that in Arabidopsis, D53-like SMXLs act with TPR2 to repress transcription and so allow lateral bud outgrowth but that SL-induced degradation of D53-like proteins activates transcription to inhibit outgrowth.
DOI:10.1038/s41586-020-2382-xURLPMID:32528176 [本文引用: 7]

Plant hormones known as strigolactones control plant development and interactions between host plants and symbiotic fungi or parasitic weeds(1-4). In Arabidopsis thaliana and rice, the proteins DWARF14 (D14), MORE AXILLARY GROWTH 2 (MAX2), SUPPRESSOR OF MAX2-LIKE 6, 7 and 8 (SMXL6, SMXL7 and SMXL8) and their orthologues form a complex upon strigolactone perception and play a central part in strigolactone signalling(5-10). However, whether and how strigolactones activate downstream transcription remains largely unknown. Here we use a synthetic strigolactone to identify 401 strigolactone-responsive genes in Arabidopsis, and show that these plant hormones regulate shoot branching, leaf shape and anthocyanin accumulation mainly through transcriptional activation of the BRANCHED 1, TCP DOMAIN PROTEIN 1 and PRODUCTION OF ANTHOCYANIN PIGMENT 1 genes. We find that SMXL6 targets 729 genes in the Arabidopsis genome and represses the transcription of SMXL6, SMXL7 and SMXL8 by binding directly to their promoters, showing that SMXL6 serves as an autoregulated transcription factor to maintain the homeostasis of strigolactone signalling. These findings reveal an unanticipated mechanism through which a transcriptional repressor of hormone signalling can directly recognize DNA and regulate transcription in higher plants.
DOI:10.1105/tpc.20.00140URLPMID:32358074 [本文引用: 1]

Strigolactones (SLs) and karrikins (KARs) are related butenolide signaling molecules that control plant development. In Arabidopsis (Arabidopsis thaliana), they are recognized separately by two closely related receptors but use the same F-box protein MORE AXILLARY GROWTH2 (MAX2) for signal transduction, targeting different members of the SMAX1-LIKE (SMXL) family of transcriptional repressors for degradation. Both signals inhibit hypocotyl elongation in seedlings, raising the question of whether signaling is convergent or parallel. Here, we show that synthetic SL analog GR24(4DO) enhanced the interaction between the SL receptor DWARF14 (D14) and SMXL2, while the KAR surrogate GR24 (ent-5DS) induced association of the KAR receptor KARRIKIN INSENSITIVE2 (KAI2) with SMAX1 and SMXL2. Both signals trigger polyubiquitination and degradation of SMXL2, with GR24(4DO) dependent on D14 and GR24 (ent-5DS) dependent mainly on KAI2. SMXL2 is critical for hypocotyl responses to GR24(4DO) and functions redundantly with SMAX1 in hypocotyl response to GR24 (ent-5DS) Furthermore, GR24(4DO) induced response of D14-LIKE2 and KAR-UP F-BOX1 through SMXL2, whereas GR24 (ent-5DS) induced expression of these genes via both SMAX1 and SMXL2. These findings demonstrate that both SLs and KARs could trigger polyubiquitination and degradation of SMXL2, thus uncovering an unexpected but important convergent pathway in SL- and KAR-regulated gene expression and hypocotyl elongation.
DOI:10.1146/annurev-arplant-042916-040925URLPMID:28125281 [本文引用: 1]

Strigolactones are a structurally diverse class of plant hormones that control many aspects of shoot and root growth. Strigolactones are also exuded by plants into the rhizosphere, where they promote symbiotic interactions with arbuscular mycorrhizal fungi and germination of root parasitic plants in the Orobanchaceae family. Therefore, understanding how strigolactones are made, transported, and perceived may lead to agricultural innovations as well as a deeper knowledge of how plants function. Substantial progress has been made in these areas over the past decade. In this review, we focus on the molecular mechanisms, core developmental roles, and evolutionary history of strigolactone signaling. We also propose potential translational applications of strigolactone research to agriculture.
URLPMID:32327664 [本文引用: 1]
[本文引用: 1]
DOI:10.1038/cr.2017.3URLPMID:28059066 [本文引用: 1]
DOI:10.1093/jxb/ery014URLPMID:29365172 [本文引用: 1]

Strigolactones (SLs) act as an important class of phytohormones to regulate plant shoot branching, and also serve as rhizosphere signals to mediate interactions of host plants with soil microbes and parasitic weeds. SL receptors in dicots, such as DWARF14 in Arabidopsis (AtD14), RMS3 in pea, and ShHTL7 in Striga, serve as unconventional receptors that hydrolyze SLs into a D-ring-derived intermediate CLIM and irreversibly bind CLIM to trigger SL signal transduction. Here, we show that D14 from the monocot rice can complement Arabidopsis d14 mutant and interact with the SL signaling components in Arabidopsis. Our results further reveal that rice D14, similar to SL receptors in dicots, also serves as an unconventional hormone receptor that generates and irreversibly binds the active form of SLs. These findings uncover the conserved functions of D14 proteins in monocots and dicots.
DOI:10.1038/cr.2013.19URLPMID:23381136 [本文引用: 1]
DOI:10.1038/nature12878URL [本文引用: 1]

Strigolactones (SLs), a newly discovered class of carotenoid-derived phytohormones, are essential for developmental processes that shape plant architecture and interactions with parasitic weeds and symbiotic arbuscular mycorrhizal fungi. Despite the rapid progress in elucidating the SL biosynthetic pathway, the perception and signalling mechanisms of SL remain poorly understood. Here we show that DWARF 53 (D53) acts as a repressor of SL signalling and that SLs induce its degradation. We find that the rice (Oryza sativa) d53 mutant, which produces an exaggerated number of tillers compared to wild-type plants, is caused by a gain-of-function mutation and is insensitive to exogenous SL treatment. The D53 gene product shares predicted features with the class I Clp ATPase proteins and can form a complex with the a/b hydrolase protein DWARF 14 (D14) and the F-box protein DWARF 3 (D3), two previously identified signalling components potentially responsible for SL perception. We demonstrate that, in a D14- and D3-dependent manner, SLs induce D53 degradation by the proteasome and abrogate its activity in promoting axillary bud outgrowth. Our combined genetic and biochemical data reveal that D53 acts as a repressor of the SL signalling pathway, whose hormone-induced degradation represents a key molecular link between SL perception and responses.
DOI:10.1093/jxb/erx487URLPMID:29385517 [本文引用: 1]

The development and growth of plants are regulated by interplay of a plethora of complex chemical reactions in which plant hormones play a pivotal role. In recent years, a group of new plant hormones, namely strigolactones (SLs), was discovered and identified. The first SL, strigol, was isolated in 1966, but it took almost 20 years before the details of its structure were fully elucidated. At present, two families of SLs are known, one having the stereochemistry of (+)-strigol and the other that of (-)-orobanchol, the most abundant naturally occurring SL. The most well-known bioproperty of SLs is the germination of seeds of the parasitic weeds Striga and Orobanche. It is evident that SLs are going to play a prominent role in modern molecular botany. In this review, relevant molecular and bioproperties of SLs are discussed. Items of importance are the effect of stereochemistry, structure-activity relationships, design and synthesis of analogues with a simple structure, but with retention of bioactivity, introduction of fluorescent labels into SLs, biosynthetic origin of SLs, mode of action in plants, application in agriculture for the control of parasitic weeds, stimulation of the branching of arbuscular mycorrhizal (AM) fungi, and the control of plant architecture. The future potential of SLs in molecular botany is highlighted.
新中国成立70年来植物激素研究进展
1
2019
... 植物的正常生长发育需要适宜的光照、温度、水分和各种营养元素.在整个生命周期中, 植物还需不断地应对各种环境变化.植物激素是植物自身产生的、微量浓度就能引起植物生理效应的信号分子, 在调节植物生长发育、应对环境变化和防御病虫害等方面具有重要作用, 影响植(作)物的生存、产量和品质.迄今研究得较为深入的植物激素包括生长素、赤霉素、乙烯、细胞分裂素、脱落酸、油菜素内酯、水杨酸、茉莉素和独脚金内酯共9类小分子激素及一些重要的多肽类激素; 同时, 一氧化氮和多胺类生长调节物质也受到高度关注(
The many models of strigolactone signaling
1
2020
... SL如何发挥生物学功能一直是植物领域的重要科学问题.得益于拟南芥(Arabidopsis thaliana)、水稻(Oryza sativa)、豌豆(Pisum sativum)和矮牵牛(Petunia hybrida)等模式植物完备的遗传研究体系, SL的生物合成、转运及信号传导机理研究取得了一系列重要进展(
A histidine covalent receptor and butenolide complex mediates strigolactone perception
1
2016
... SL如何发挥生物学功能一直是植物领域的重要科学问题.得益于拟南芥(Arabidopsis thaliana)、水稻(Oryza sativa)、豌豆(Pisum sativum)和矮牵牛(Petunia hybrida)等模式植物完备的遗传研究体系, SL的生物合成、转运及信号传导机理研究取得了一系列重要进展(
Strigolactone promotes cytokinin degradation through transcriptional activation of CYTOKININ OXIDASE/DEHYDROGENASE 9 in rice
1
2019
... 李家洋团队和中国农业科学院作物科学研究所万建民团队在2013年分别发现了水稻SL信号通路的抑制子D53 (
Strigolactones and brassinosteroids antagonistically regulate the stability of the D53-OsBZR1 complex to determine FC1 expression in rice tillering
1
2020
... 此前的研究表明, SMXL6,7,8招募TPL/TPR共抑制子(
Strigolactone inhibition of shoot branching
1
2008
... 独脚金内酯(strigolactone, SL)是一类新近被鉴定为植物激素的萜类小分子化合物(
DAD2 is an α/β hydrolase likely to be involved in the perception of the plant branching hormone, strigolactone
1
2012
... SL如何发挥生物学功能一直是植物领域的重要科学问题.得益于拟南芥(Arabidopsis thaliana)、水稻(Oryza sativa)、豌豆(Pisum sativum)和矮牵牛(Petunia hybrida)等模式植物完备的遗传研究体系, SL的生物合成、转运及信号传导机理研究取得了一系列重要进展(
BES1 functions as the co-regulator of D53-like SMXLs to inhibit BRC1 expression in strigolactone-regulated shoot branching in Arabidopsis
1
2020
... 此前的研究表明, SMXL6,7,8招募TPL/TPR共抑制子(
DWARF 53 acts as a repressor of strigolactone signaling in rice
3
2013
... 李家洋团队和中国农业科学院作物科学研究所万建民团队在2013年分别发现了水稻SL信号通路的抑制子D53 (
... 对SL不敏感, 呈现矮化多分蘖表型.SL可促进D14与D53及F-box蛋白D3互作, 诱导D53泛素化修饰和降解, 从而抑制植物分枝.D53中含有EAR基序, 李家洋团队发现D53能够招募TPL共抑制子(co-repressor), 进而结合转录因子IPA1蛋白并抑制其转录激活功能(
... 此前的研究表明, SMXL6,7,8招募TPL/TPR共抑制子(
A D53 repression motif induces oligomerization of TOPLESS corepressors and promotes assembly of a corepressor-nucleosome complex
1
2017
... 李家洋团队和中国农业科学院作物科学研究所万建民团队在2013年分别发现了水稻SL信号通路的抑制子D53 (
Strigolactone perception and deactivation by a hydrolase receptor DWARF14
1
2019
... SL如何发挥生物学功能一直是植物领域的重要科学问题.得益于拟南芥(Arabidopsis thaliana)、水稻(Oryza sativa)、豌豆(Pisum sativum)和矮牵牛(Petunia hybrida)等模式植物完备的遗传研究体系, SL的生物合成、转运及信号传导机理研究取得了一系列重要进展(
Structural plasticity of D3-D14 ubiquitin ligase in strigolactone signaling
1
2018
... SL如何发挥生物学功能一直是植物领域的重要科学问题.得益于拟南芥(Arabidopsis thaliana)、水稻(Oryza sativa)、豌豆(Pisum sativum)和矮牵牛(Petunia hybrida)等模式植物完备的遗传研究体系, SL的生物合成、转运及信号传导机理研究取得了一系列重要进展(
IPA1 functions as a downstream transcription factor repressed by D53 in strigolactone signaling in rice
1
2017
... 李家洋团队和中国农业科学院作物科学研究所万建民团队在2013年分别发现了水稻SL信号通路的抑制子D53 (
SUPPRESSOR OF MORE AXILLARY GROWTH2 1 controls seed germination and seedling development in Arabidopsis
1
2013
... 此前的研究表明, SMXL6,7,8招募TPL/TPR共抑制子(
Inhibition of shoot branching by new terpenoid plant hormones
1
2008
... 独脚金内酯(strigolactone, SL)是一类新近被鉴定为植物激素的萜类小分子化合物(
A femtomolar-range suicide germination stimulant for the parasitic plant Striga hermonthica
1
2018
... SL如何发挥生物学功能一直是植物领域的重要科学问题.得益于拟南芥(Arabidopsis thaliana)、水稻(Oryza sativa)、豌豆(Pisum sativum)和矮牵牛(Petunia hybrida)等模式植物完备的遗传研究体系, SL的生物合成、转运及信号传导机理研究取得了一系列重要进展(
Strigolactones. In: Li JY, Li CY, Smith SM, eds. Hormone Metabolism and Signaling in Plants
1
... 作为一种新型植物激素, SL在调控植物生长发育及环境适应性的多个方面具有重要功能, 包括调控植物分枝、茎秆粗细和叶片形态等地上部分的株型, 以及主根长度、侧根和根毛密度等地下部分的株型, 还能介导植物对灰霉菌、干旱及营养匮乏等生物/非生物逆境胁迫的抗性(
Strigolactone signaling in Arabidopsis regulates shoot development by targeting D53-like SMXL repressor proteins for ubiquitination and degradation
3
2015
... 李家洋团队和中国农业科学院作物科学研究所万建民团队在2013年分别发现了水稻SL信号通路的抑制子D53 (
... 此前的研究表明, SMXL6,7,8招募TPL/TPR共抑制子(
... ;
Transcriptional regulation of strigolactone signaling in Arabidopsis
7
2020
... 李家洋团队近期的研究发现SMXL6,7,8是具有抑制子和转录因子双重功能的新型抑制子, 初步阐明了SL调控植物分枝、叶片伸长和花色素苷积累的转录调控机理(
... 的表达调控植物抗旱性(
... 的表达, 提高ABA含量, 从而抑制植物分枝(
... 此外, 该研究发现调节叶片发育的TCP1基因以依赖D14的方式受到GR244DO的诱导, 进而通过一系列遗传表型和基因表达分析证明SMXL6,7,8通过抑制TCP1表达而抑制叶片伸长, 且该抑制作用依赖其EAR基序, 从而建立了SL控制叶片发育的转录调控途径(
... 抑制子SMXL6,7,8也是转录因子, 可通过直接结合DNA调控基因的表达(
... 李家洋团队最新的研究发现抑制子SMXL6,7,8也是转录因子, 揭示了SL诱导SMXL6,7,8自身基因表达的调控机制, 并初步阐明了SL调控植物分枝、叶片伸长和花色素苷积累的转录调控机理(
... 赤霉素、茉莉素和生长素信号途径中的抑制子(DELLA、JAZ和AUX/IAA)可招募共抑制子并结合下游转录因子, 但这些抑制子是否具有转录因子的功能尚不清楚(
Strigolactone and karrikin signaling pathways elicit ubiquitination and proteolysis of SMXL2 to regulate hypocotyl elongation in Arabidopsis thaliana
1
2020
... 李家洋团队和中国农业科学院作物科学研究所万建民团队在2013年分别发现了水稻SL信号通路的抑制子D53 (
Strigolactone signaling and evolution
1
2017
... 独脚金内酯(strigolactone, SL)是一类新近被鉴定为植物激素的萜类小分子化合物(
Arabidopsis FHY3 and FAR1 integrate light and strigolactone signaling to regulate branching
1
2020
... 此前的研究表明, SMXL6,7,8招募TPL/TPR共抑制子(
DWARF14 is a non-canonical hormone receptor for strigolactone
1
2016
... SL如何发挥生物学功能一直是植物领域的重要科学问题.得益于拟南芥(Arabidopsis thaliana)、水稻(Oryza sativa)、豌豆(Pisum sativum)和矮牵牛(Petunia hybrida)等模式植物完备的遗传研究体系, SL的生物合成、转运及信号传导机理研究取得了一系列重要进展(
ShHTL7 is a non-canonical receptor for strigolactones in root parasitic weeds
1
2017
... SL如何发挥生物学功能一直是植物领域的重要科学问题.得益于拟南芥(Arabidopsis thaliana)、水稻(Oryza sativa)、豌豆(Pisum sativum)和矮牵牛(Petunia hybrida)等模式植物完备的遗传研究体系, SL的生物合成、转运及信号传导机理研究取得了一系列重要进展(
Rice DWARF14 acts as an unconventional hormone receptor for strigolactone
1
2018
... SL如何发挥生物学功能一直是植物领域的重要科学问题.得益于拟南芥(Arabidopsis thaliana)、水稻(Oryza sativa)、豌豆(Pisum sativum)和矮牵牛(Petunia hybrida)等模式植物完备的遗传研究体系, SL的生物合成、转运及信号传导机理研究取得了一系列重要进展(
Crystal structures of two phytohormone signal-transducing α/β hydrolases: karrikin-signaling KAI2 and strigolactone-signaling DWARF14
1
2013
... SL如何发挥生物学功能一直是植物领域的重要科学问题.得益于拟南芥(Arabidopsis thaliana)、水稻(Oryza sativa)、豌豆(Pisum sativum)和矮牵牛(Petunia hybrida)等模式植物完备的遗传研究体系, SL的生物合成、转运及信号传导机理研究取得了一系列重要进展(
D14-SCFD3-dependent degradation of D53 regulates strigolactone signaling
1
2013
... 李家洋团队和中国农业科学院作物科学研究所万建民团队在2013年分别发现了水稻SL信号通路的抑制子D53 (
Strigolactones: new plant hormones in the spotlight
1
2018
... 作为一种新型植物激素, SL在调控植物生长发育及环境适应性的多个方面具有重要功能, 包括调控植物分枝、茎秆粗细和叶片形态等地上部分的株型, 以及主根长度、侧根和根毛密度等地下部分的株型, 还能介导植物对灰霉菌、干旱及营养匮乏等生物/非生物逆境胁迫的抗性(
备案号: 京ICP备16067583号-21
版权所有 © 2021 《植物学报》编辑部
地址:北京香山南辛村20号 邮编:100093
电话:010-62836135 010-62836131 E-mail:cbb@ibcas.ac.cn
本系统由北京玛格泰克科技发展有限公司设计开发
