
西北农林科技大学农学院, 杨凌 712100

引用本文
贡献者
基金资助
接受日期:2016-12-5接受日期:2017-04-3网络出版日期:2017-11-1
-->Copyright
2017《植物学报》编辑部
Contributors
History
Received:Accepted:Online:
摘要:
Abstract:
Key words:
甘蓝型油菜(Brassica napus)是我国最重要的油料作物之一。菜籽油不仅是良好的食用油, 而且是制造医药品和化妆品等多种化工产品的原料。1960年, 瑞典科学家首次从人工合成的甘蓝型油菜中找到了黄籽单株。1975年, 我国科学家也发现了甘蓝型黄籽油菜(刘后利等, 1979)。该类油菜具有种皮薄、木质素和多酚含量低、油与饼粕蛋白质含量高(张子龙和李加纳, 2001)等优点, 备受全球油菜育种学家的青睐。尽管我国在甘蓝型油菜黄籽育种方面取得了重要成就, 已育成黄杂1号、华黄1号、宁油10号、湘杂油631、渝黄1号、渝黄4号、油研817和油研9号等多个代表性优良品种, 但到目前为止, 甘蓝型黄籽油菜种子含油量高的分子机制及其调控网络仍不清楚, 且该方面仍是油菜研究中的一个重要热点。
拟南芥(Arabidopsis thaliana)中含有丰富的类黄酮, 主要包括原花青素、花青素和黄酮醇3种, 它们的存在使得花、茎和种子等组织呈现不同的颜色(Koes et al., 1994; Shirley, 1996; Mol et al., 1998; Nesi et al., 2001; Lepiniec et al., 2006)。拟南芥中AtTT2 (TRANSPARENT TESTA 2)、AtTT8 (TRANSPARENT TESTA 8)以及AtTTG1 (TRANSPARENT TESTA GLABRA 1 )分别作为MYB、bHLH与WD40型转录因子通过形成转录复合体进而调控类黄酮的生物合成(Debeaujon et al., 2003; Lepiniec et al., 2006; Xu et al., 2014)。此外, AtTTG1在调控种子发育和储藏物质积累(Tsuchiya et al., 2004; Chen et al., 2015)、种皮黏液产生(Shirley et al., 1995; Western et al., 2001; Nguyen et al., 2013)、种子休眠(Debeaujon et al., 2000)、表皮毛形成(Walker et al., 1999; Chen et al., 2015)和根毛发育(Koornneef, 1981)等方面起着重要作用。
油菜是与拟南芥亲缘关系最近的油料作物之一, 两者基因组同源性很高。因此, 拟南芥的研究成果可以作为油菜研究的重要参考。对油菜的研究表明, BnTTG1-1 (NCBI编号为EF175930)在油菜种子和根中表达量较低, BnTTG1-2 (NCBI编号为EF175931)在油菜各个组织中均有表达(Lu et al., 2009)。目前, 关于BnTTG1在表皮毛形成、花青素合成、种子储藏物质积累以及非生物逆境胁迫等方面的调节作用尚未见报道。本研究从甘蓝型油菜品种秦优7号中获得了BnTTG1-1基因的全长CDS序列, 检测了该基因在油菜不同组织中的表达模式, 推测它作为转录因子发挥调节作用。同时, 在拟南芥缺失突变体ttg1-13基础上异源表达该基因能够完全恢复突变体的一系列表型, 如无表皮毛形成和花青素合成、种皮呈黄色、种子脂肪酸和储藏蛋白含量高、在种子萌发和幼苗形态建成过程中对高葡萄糖与高盐胁迫耐受力差等。本研究不仅为深入阐明BnTTG1基因在油菜中的调控机制奠定了坚实的基础, 而且有助于人们进一步了解黄籽油菜油脂积累的调控机制, 从而为油菜育种提供基因元件和理论支撑。
1 材料与方法1.1 植物材料与培养本实验所用遗传材料有甘蓝型油菜(Brassica napus L.)品种秦优7号、拟南芥(Arabidopsis thaliana L.)哥伦比亚野生型Col-0和功能缺失突变体ttg1-13 (Liu et al., 2017)。秦优7号种植于陕西杨凌西北农林科技大学南校区科研温室。拟南芥种植于人工生长箱内, 温度为22°C, 16小时光照/8小时黑暗, 光照强度为160 μmol·m-2·s-1。
1.2 总RNA提取和cDNA合成利用MiniBEST Plant RNA Extraction Kit (TaKaRa, Code No.9769)提取秦优7号不同组织和开花后不同发育时期(15、20、25、28、33和35天)种子以及拟南芥幼嫩叶片总RNA。使用NanoDrop ND-1000检测RNA的浓度和质量, 用琼脂糖凝胶电泳检测RNA的完整性。使用PrimeScript II 1st Strand cDNA Synthesis Kit (TaKaRa, Code No.6210A)完成cDNA第1链的合成。
1.3 基因表达分析利用半定量RT-PCR和荧光定量qRT-PCR技术分析基因的表达情况。按照SYBR?Premix Ex TaqTM II (TaKaRa, Cat No. DRR820A)使用说明书对本研究相关基因的表达量进行荧光定量PCR分析。PCR体系: SYBR? Premix Ex Taq 10 μL, 0.5 μmol·L-1上下游引物各1 μL, cDNA 2 μL (50 ng·μL-1), RNase Free ddH2O加至20 μL。在Bio-Rad荧光定量PCR仪上进行反应, 反应程序为: 95°C预变性60秒; 95°C20秒, 58°C20秒, 72°C45秒, 40个循环。反应结束后分析荧光值变化曲线以及溶解曲线, 采用2-ΔΔCt法分析结果(Livak and Schmittgen, 2001)。每个样品设3次重复。荧光定量PCR过程中所需的引物序列见表1。
表1
Table 1
表1
| Primer name | Primer sequence (5′-3′) | Annotation |
|---|---|---|
| AtACTIN7-F | GCCCCTGAGGAGCACCCAGTT | RT-PCR |
| AtACTIN7-R | CCGGTTGTACGACCACTGGCA | |
| BnTTG1-1-F | GCCAGTATCCGTCCTCAACA | RT-PCR |
| BnTTG1-1-R | CTCCCAGATAAGAGCCTGCG | |
| BnACTIN7-F | GGAGCTGAGAGATTCCGTTG | qRT-PCR |
| BnACTIN7-R | GAACCACCACTGAGGACGAT | |
| BnTTG1-1-F | CTGCAGTGGTCTTCTTCGTT | qRT-PCR |
| BnTTG1-1-R | GTTACAATCACATAGATGCAGAGAC | |
| BnTTG1-1-Xma1-F | TATTcccgggATGGACAACTCAGCTCCAGACTC | 35S:BnTTG1-1-GFP and 35S:BnTTG1-1 |
| BnTTG1-1-Spe1-R | GGactagtAACTCTAAGGAGCTGCATTTTGTTAGC |
表1
引物序列
Table 1
Sequences of primers
1.4 基因克隆和植物表达载体构建依据NCBI数据库中BnTTG1全长CDS序列(编号分别为EF175930、EF175931、EU192030和EU192031)设计特异引物(表1), 以秦优7号发育种子cDNA为模板, 进行PCR扩增。具体扩增程序如下: 98°C变性30秒; 98°C10秒, 58°C15秒, 72°C60秒, 32个循环; 72°C延伸5分钟。切胶回收后连接pMD18-T载体(Code No. D101A), 随机挑选6个阳性克隆进行测序。测序结果序列一致, 表明我们从秦优7号中成功获得了BnTTG1-1基因的全长CDS序列。用限制性内切酶XmaI和SpeI进行双酶切, 并与用相同限制酶酶切处理的pGreen和pGreen-GFP载体连接转化大肠杆菌DH5α, 筛选出阳性克隆, 得到该基因植物表达载体35S:BnTTG1-1和35S:BnTTG1-1-GFP。提取阳性克隆质粒, 转化农杆菌(Agrobacterium tumefacie- ns GV3101), 在含有50 μg·mL-1卡那(Kana)以及25 μg·mL-1利福平(Rif)的培养基上挑取单克隆进行PCR鉴定。使用农杆菌花序浸泡法(Clough and Bent, 1998)转染拟南芥突变体ttg1-13, 将收获的种子均匀散播在营养土上, 待真叶长出后喷施除草剂(Basta)。将抗性苗移至新的营养土中生长, 待成熟后收集种子即获得T0代, 继续筛选, 直至获得T3代纯合体种子(作为实验材料备用)。
1.5 烟草叶片细胞的亚细胞定位取100 μL在-80°C超低温冰箱保存的阳性农杆菌菌株(35S::BnTTG1-GFP), 放入3 mL LB培养液(含有50 μg·mL-1卡那和20 μg·mL-1利福平)中摇菌。菌液混浊至OD600达1.8-2.0时, 在常温下1 800×g离心10分钟。弃上清液, 用重悬液(10 mmol·L-1 MES, 10 mmol·L-1 MgCl2, 100 μmol·L-1 AS)重悬菌体使OD600达0.4-1.0, 将重悬菌液静置2-3小时, 用注射器将其从烟草叶片下表皮注射入叶片内。72小时后, 用注射
器吸取适量DAPI染液(1 mol·L-1 Tris pH7.4, 5 mol·L-1 NaCl, 1 μg·L-1 DAPI)注入上述烟草(Nicotiana benthamiana)叶片。20分钟后, 剪取小块成功染色的非注射区域叶片, 用PBS Buffer洗去未染色的游离DAPI染液, 制成临时装片, 然后在共聚焦显微镜下观察并拍照。
1.6 种子脂肪酸含量的测定按照文献报道的方法(Mu et al., 2008; Chen et al., 2012a)提取并测定拟南芥种子脂肪酸含量。操作步骤如下: 称取8 mg种子放入4 mL 1 mol·L-1盐酸甲醇溶液提取液(内标浓度为25 μg·mL-1)中, 80°C水浴2小时提取脂肪酸并酯化, 避光冷却至室温后, 加入2 mL 0.9% NaCl (w/v)终止酯化反应, 再加入1 mL正己烷进行萃取, 在振荡器上充分振荡混匀后, 以250×g转速离心5分钟, 随后吸取上层的有机相700 μL至气相GC小瓶中, 4°C避光保存。利用日本岛津公司生产的气相色谱仪(GC-2014)进行脂肪酸分析, 用面积归积法计算脂肪酸含量。
1.7 种子储藏蛋白含量的测定取20粒拟南芥种子于1.5 mL离心管中, 放入液氮中研磨, 磨碎后加入200 μL的提取液(50 mmol·L-1 HEPES, 5 mmol·L-1 MgCl2, 5 mmol·L-1二硫苏糖 醇, 1 mmol·L-1苯甲基磺酰氟, 1 mmol·L-1 EDTA, 10% (v/v) pH7.5乙二醇, 少许不溶性交联PVP)。4°C、 22 000×g离心10分钟后, 吸取上清液, 依据Brad- ford (1976)的方法对上清液中的蛋白质含量进行定量分析。
1.8 非生物胁迫下种子发芽和幼苗形态建成分析同时收获在同一时间种植并在相同条件下生长的拟南芥野生型Col-0、突变体ttg1-13和纯合转基因株系ttg1-13 35S::BnTTG1的种子, 室温下放置4周至完全成熟, 放入-20°C冰箱中保存备用。用于抗性实验的种子先在4°C冰箱中预冷5天, 再用75% (v/v)的乙醇清洗种子表面30-60秒, 重复2次, 之后用ddH2O冲洗5遍, 铺种于含3% (w/v)蔗糖和100 mmol·L-1 NaCl的MS固体培养基平板上, 以无任何胁迫处理的MS平板作为对照。将胚根露出种皮定义为种子萌发, 将幼苗长出2片真叶能够独立进行光合作用定义为幼苗完成形态建成(Cernac et al., 2006)。每天统计各株系种子的发芽率, 并在铺种17天对拟南芥幼苗进行拍照。
2 结果与讨论2.1 BnTTG1-1在种子发育过程中的表达模式分析从甘蓝型油菜秦优7号中克隆得到的QINYOU.Bn- TTG1蛋白质序列与NCBI数据库中的BnTTG1-1 (编号为EF175930)蛋白质序列完全相同, 因此, QINYOU.BnTTG1在本文中命名为BnTTG1-1。BnTTG1-1基因在秦优7号不同营养组织中的表达模式与前人的研究结果一致(Lu et al., 2009)。我们发现, 在种子发育过程中, BnTTG1-1基因的表达从15天开始平稳增加, 28天时达到最大值, 随后逐步下降(图1)。
图1
Figure 1
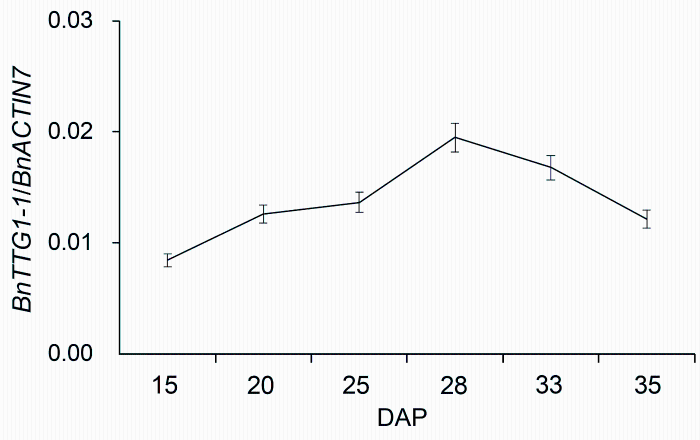
下载原图ZIP
生成PPT
图1
qRT-PCR分析BnTTG1-1基因在甘蓝型油菜秦优7号种子不同发育时期的表达模式(平均值±标准差)
DAP: 授粉后的天数。BnACTIN7为内参基因。
Figure 1
qRT-PCR analysis of BnTTG1-1 expression in de- veloping seeds at different developmental stages in Brassica napus cv. ‘QINYOU Seven’ (means±SD)
DAP: Days after pollination. The qRT-PCR result was normaliz- ed against the expression of BnACTIN7 as an internal control.
2.2 BnTTG1-1定位于烟草叶片细胞的细胞核中油菜与拟南芥同属十字花科, 是与拟南芥亲源关系最近的经济作物之一。拟南芥基因组中的每个基因在甘蓝型油菜基因组中都有2-8个同源拷贝(Osborn et al., 1997; Cavell et al., 1998)。由图2可知, BnTTG1- 1定位于烟草叶片细胞的细胞核中。拟南芥AtTTG1为WD40型转录因子, 因此可以推测BnTTG1-1也作为转录因子发挥调节作用。
图2
Figure 2
Figure 2 Subcellular localization of BnTTG1-1 protein fus- ed with GFP (35S:BnTTG1-1-GFP) in tobacco (Nicotiana ben- thamiana) leave cells
DAPI: 4’, 6-diamidino-2-phenylindole dihydrochloride; GFP: Green fluorescent protein; Merge: Merged picture of bright, DAPI, and GFP fields. Bars=5 μm'>

下载原图ZIP
生成PPT
图2
BnTTG1-1在烟草叶片细胞中的亚细胞定位
DAPI: 4', 6-二脒基-2-苯基吲哚; GFP: 绿色荧光蛋白; Merge: DAPI、GFP和亮场3个图像的合并图像。Bars=5 μm
Figure 2
Subcellular localization of BnTTG1-1 protein fus- ed with GFP (35S:BnTTG1-1-GFP) in tobacco (Nicotiana ben- thamiana) leave cells
DAPI: 4’, 6-diamidino-2-phenylindole dihydrochloride; GFP: Green fluorescent protein; Merge: Merged picture of bright, DAPI, and GFP fields. Bars=5 μm
2.3 BnTTG1-1参与调节表皮毛的形成和花青素的合成为了鉴定BnTTG1-1基因的功能, 我们构建了35S: BnTTG1-1植物表达载体, 并转入拟南芥突变体ttg1- 13中, 通过抗除草剂筛选, 成功获得了T1代转基因植株。通过对其中4个单株进行PCR鉴定, 结果显示, ttg1-13 35S:BnTTG1-1转基因植株#1、#2、#3和#4在DNA及RNA水平上均检测到该基因的存在(图3A), 说明成功获得了ttg1-13 35S:BnTTG1-1转基因植株。之后, 对T3代纯合植株进行表型鉴定(图3B), 与突变体ttg1-13相比, 转基因植株能够完全恢复突变体无表皮毛和花青素的表型, 表明BnTTG1-1具有与拟南芥AtTTG1类似的功能, 参与调节表皮毛形成和花青素合成等生物学过程。
图3
Figure 3
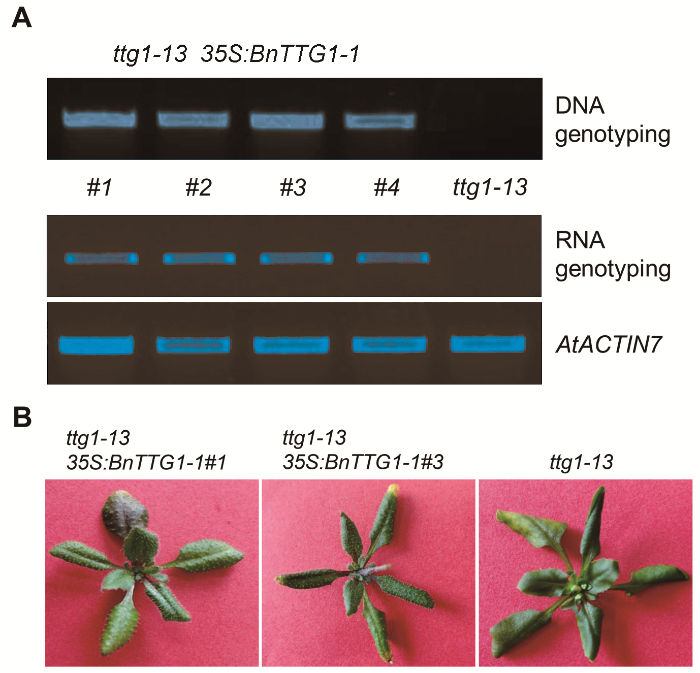
下载原图ZIP
生成PPT
图3
ttg1-13 35S:BnTTG1-1转基因植株的鉴定
(A) 在DNA和RNA水平鉴定ttg1-13 35S:BnTTG1-1转基因植株, AtACTIN7为内参基因; (B) 在突变体ttg1-13背景下异源表达BnTTG1-1能够完全恢复突变体的表型, 如无表皮毛和花青素等。
Figure 3
Identification of ttg1-13 35S:BnTTG1-1 transgenic plants
(A) PCR-based DNA and RNA genotyping of ttg1-13 35S: BnTTG1-1 transgenic plants, AtACTIN7 was regarded as an internal control; (B) Heterologous expression of BnTTG1-1 in the ttg1-13 background fully rescued no trichomes and anthocyanins phenotypes of ttg1-13.
2.4 BnTTG1-1对种皮颜色具有调节作用在突变体基础上异源表达BnTTG1-1基因能够完全恢复ttg1-13突变体黄色种皮至野生型水平(图4A)。然而, 对种子大小和重量无明显影响(图4B), 表明BnTTG1- 1与AtTTG1均不影响种子的大小和重量, 但可以通过调节花青素的合成影响种皮的颜色。
图4
Figure 4
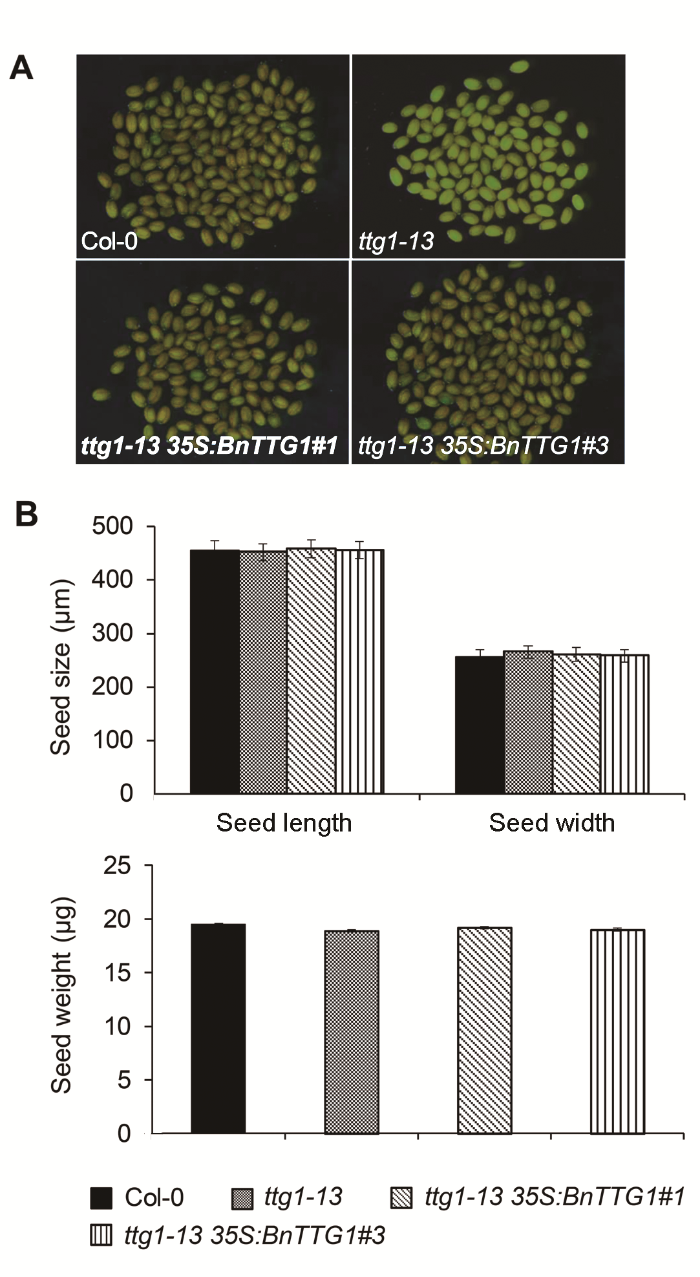
下载原图ZIP
生成PPT
图4
比较拟南芥野生型(Col-0)、突变体ttg1-13和转基因植株ttg1-13 35S:BnTTG1-1的种皮颜色、种子大小和重量(平均值±标准差)
(A) 成熟种子的显微观察; (B) 成熟种子的大小和重量比较
Figure 4
Comparison of seed coat color, seed size and se- ed weight among the wild-type (Col-0), ttg1-13, and ttg1-13 35S:BnTTG1-1 transgenic plants of Arabidopsis (means±SD)
(A) Microscopic observation of mature seeds; (B) Comparison of seed size and weight of mature seeds
2.5 BnTTG1-1调节种子储藏物质的积累由图5可知, 与野生型Col-0相比, 突变体ttg1-13种子脂肪酸和蛋白质的含量都显著增加, 而ttg1-13 35S: BnTTG1-1转基因植株种子的储藏蛋白和脂肪酸含量与野生型相比无显著差异, 表明BnTTG1-1参与调节种子储藏物质的积累, 并与AtTTG1具有相似的调节作用。
图5
Figure 5
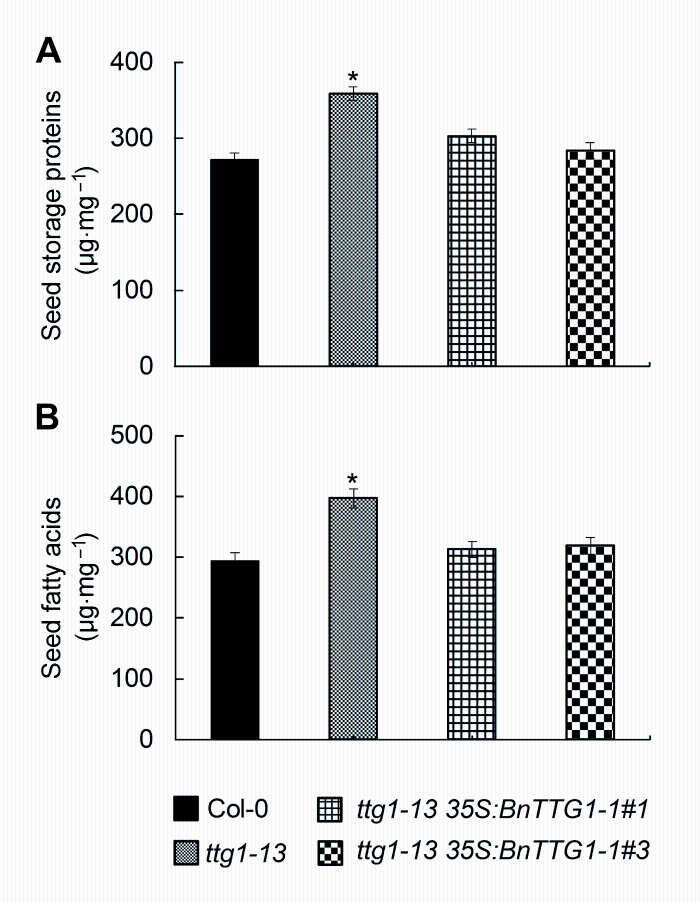
下载原图ZIP
生成PPT
图5
比较拟南芥野生型(Col-0)、突变体ttg1-13和转基因植株ttg1-13 35S:BnTTG1-1种子的储藏蛋白与脂肪酸含量(平均值±标准差)
(A) 种子储藏蛋白含量; (B) 种子脂肪酸含量。*表示在P<0.05水平上差异显著。
Figure 5
Comparison of seed storage compounds among the wild-type (Col-0), ttg1-13, and ttg1-13 35S:BnTTG1-1 trans- genic plants of Arabidopsis (means±SD)
(A) The content of seed storage proteins in different lines; (B) The content of seed fatty acids in different lines. Asterisks de- note statistically signi?cant differences between the wild-type and ttg1-13 mutant (Student’s t test, P<0.05).
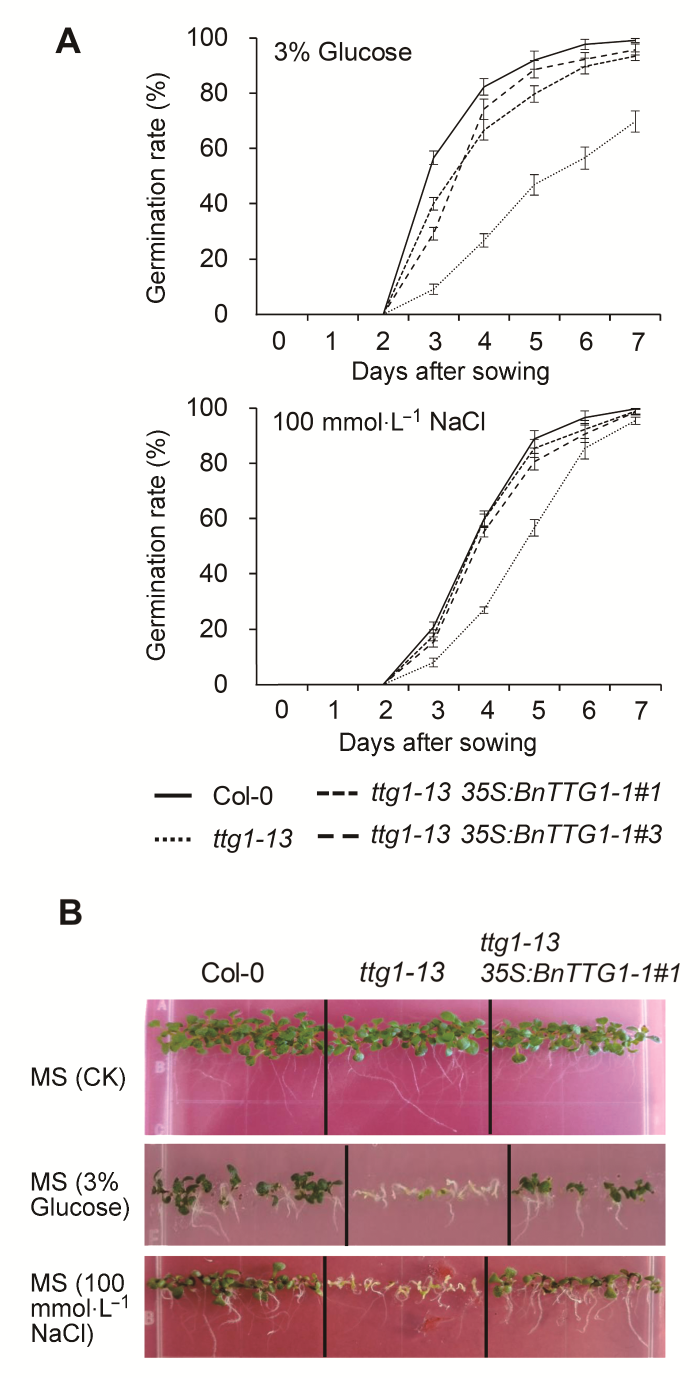
2.6 非生物胁迫下BnTTG1-1对种子萌发和幼苗形态建成的影响在无胁迫处理的MS培养基上, 野生型、突变体ttg1- 13和转基因植株ttg1-13 35S:BnTTG1-1的种子萌发
与幼苗形态建成无明显差异(图6)。在含3% (w/v)葡萄糖的MS培养基上, ttg1-13突变体的发芽率明显低于野生型。7天时, 野生型种子的发芽率接近100%, 而突变体ttg1-13的发芽率仅有70%左右(图6A)。17天时, 野生型已完成幼苗的形态建成, 而突变体大部分种子仍未长出真叶(图6B)。同样, 在含有100 mmol·
L-1 NaCl的MS培养基上, ttg1-13突变体的发芽率明显低于野生型, 但在7天时, 两者发芽率无明显差异, 均接近100% (图6A)。17天时, 野生型完成了幼苗的形态建成, 而ttg1-13突变体幼苗未完成形态建成, 并且根的生长明显受到抑制(图6B)。在两种非生物逆境胁迫下, ttg1-13 35S:BnTTG1-1转基因植株的种子发芽率、幼苗的形态建成和根长等表型均明显强于ttg1- 13突变体, 并能够完全或者部分恢复至野生型水平
(图6)。这些结果表明, AtTTG1基因功能缺失导致种子萌发和幼苗形态建成对高浓度葡萄糖及盐分敏感, BnTTG1-1参与植株应对非生物逆境胁迫过程, Bn- TTG1-1与AtTTG1在种子萌发以及幼苗形态建成过程中具有类似的功能。
2.7 讨论越来越多的证据表明, 拟南芥AtTTG1参与调控表皮毛的形成(Walker et al., 1999; Chen et al., 2015)、花青素的合成(Debeaujon et al., 2003; Lepiniec et al., 2006; Xu et al., 2014)、种子储藏物质的积累(Tsuchiya et al., 2004; Chen et al., 2015)和非生物逆境胁迫响应(Liu et al., 2017)等多个生物学过程。油菜是与拟南芥亲缘关系最近的油料作物之一。尽管早在2006和2007年, 我国(编号为EF175930和EF- 175931)和法国(编号为EU192030和EU192031)科学家已分别向NCBI数据库提交了甘蓝型油菜TTG1的全长CDS序列, 但该基因在表皮毛形成、花青素合成、种子储藏物质积累以及非生物逆境胁迫响应等方面的调控作用尚未见报道。
我们根据NCBI数据库中已有的甘蓝型油菜TTG1基因全长CDS序列, 从甘蓝型油菜品种秦优7号中成功克隆了BnTTG1-1基因的全长CDS序列。BnTTG1-1在秦优7号的时空表达模式显示其可能在油菜生长发育的多个生物学过程中发挥作用(图1)。烟草叶片细胞的亚细胞定位结果显示, BnTTG1-1定位于细胞核, 推测其作为转录因子发挥调节作用(图2)。拟南芥ttg1-13突变体表现出无表皮毛和花青素、黄种皮以及种子脂肪酸和储藏蛋白含量显著升高等表型 (图3-图5)。拟南芥AtTTG1作为重要转录因子能够独立地发挥功能或者与其它转录因子互作, 进而参与调控表皮毛形成和花青素合成的多个重要结构基因的表达来影响它们的形成与合成(Walker et al., 1999; Debeaujon et al., 2003; Lepiniec et al., 2006; Xu et al., 2014; Chen et al., 2015)。拟南芥中几个与种皮发育相关的重要转录因子(AtTT2、AtTT8和AtGL2)相对应的突变体均表现出种子脂肪酸含量升高和种皮黏液合成受阻(Chen et al., 2012b, 2014; Shi et al., 2012)。拟南芥AtTTG1也具有类似的功能, 其相对应的突变体ttg1种子的脂肪酸含量升高, 种皮黏液合成减少(Koornneef, 1981; Walker et al., 1999; Chen et al., 2015; Liu et al., 2017)。Zhang和Rock (2004)与Wang等(2014)的研究表明, 花青素可能从种皮渗入种胚, 通过抑制编码脂肪酸碳链延伸的重要酶基因的表达进而抑制种胚中脂肪酸的积累。此外, 种子脂肪酸和种皮黏液的生物合成均需要利用光合作用的C源。我们的研究结果表明, AtTTG1在拟南芥种子发育过程中既可通过间接抑制多个脂肪酸和蛋白合成途径上重要基因的表达, 也可通过母体效应影响种子脂肪酸和储藏蛋白的积累(Chen et al., 2015)。拟南芥突变体ttg1-13种子脂肪酸含量升高可能是以上多个因素共同作用的结果。在该突变体基础上异源表达BnTTG1-1能够完全恢复突变体表皮毛、花青素、种皮颜色以及种子脂肪酸和储藏蛋白含量等方面的表型(图3-图5), 表明从秦优7号中获得的Bn- TTG1-1在调控以上多个生物学过程中与AtTTG1具有类似的功能。
前人的研究表明, 类黄酮作为次级代谢产物不仅在种子休眠和种子活力方面起着重要作用, 而且参与调控植物逆境胁迫响应过程(Winkel-Shirley, 2002; Peer and Murphy, 2007; Petrussa et al., 2013; Nakabayashi et al., 2014)。表皮毛也有助于提高植物对非生物逆境的抵抗能力(Szymanski et al., 2000)。因此, 我们研究了AtTTG1在非生物逆境胁迫中的作用, 结果表明, ttg1-13突变体在种子萌发和幼苗形态建成过程中对高盐和高葡萄糖等非生物逆境胁迫较野生型更敏感(图6)。高盐和高葡萄糖非生物胁迫均依赖于脱落酸合成和转导途径(Gibson, 2001; Finkelstein et al., 2002; Cutler et al., 2010)。在高盐和高葡萄糖胁迫条件下, 参与脱落酸合成和转导以及对逆境胁迫响应的多个基因在ttg1-13突变体幼苗中均被显著下调, 这可能导致该突变体对非生物逆境胁迫敏感(Liu et al., 2017)。Hong等(2008)和Mu等(2008)的研究表明, 脂肪酸作为信号分子参与非生物逆境胁迫响应过程。此外, 由种皮花青素合成缺失所引起的ttg1-13种皮变薄可能使得逆境胁迫分子对种胚和胚乳的伤害更大。这些因素可能通过协同作用的方式影响拟南芥突变体ttg1-13种子萌发和幼苗形态建成。转基因植株ttg1-13 35S:BnTTG1-1能够恢复突变体在高盐以及高葡萄糖条件下的表型(图6), 这表明BnTTG1-1在响应非生物逆境胁迫方面具有与AtTTG1类似的功能。最近的研究表明, 禾本科单子叶植物谷子(Setaria italica) SiTTG1基因在调控植物生长发育方面也与拟南芥AtTTG1功能类似(Liu et al., 2017)。可见, TTG1基因的功能在双子叶和单子叶植株中高度保守。
图6
Figure 6
下载原图ZIP
生成PPT
图6
非生物胁迫条件下(含有3%葡萄糖和100 mmol·L-1 NaCl)拟南芥野生型Col-0、突变体ttg1-13和转基因植株ttg1-13 35S: BnTTG1-1的发芽率和幼苗形态建成
(A) 种子发芽率; (B) 幼苗的形态建成。数据为3个生物学重复的平均值±标准差, 每个生物学重复统计100粒种子。
Figure 6
Seed germination rate and seedling establishment on MS agar medium containing 3% (w/v) Glucose and containing 100 mmol·L-1 NaCl among the wild-type (Col-0), ttg1- 13, and ttg1-13 35S:BnTTG1-1 transgenic plants of Arabidopsis
(A) Seed germination rate; (B) Seedling establishment. Values are the means±SD from three independent experiments evaluating 100 seeds.
综上所述, 甘蓝型油菜BnTTG1-1调控表皮毛形成、花青素合成、种子储藏物质积累以及非生物胁迫响应等多个生物学过程。该研究结果为深入揭示油菜BnTTG1-1作用机制奠定了坚实的基础。
参考文献
文献选项
原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
| [1] | URL正 1975年夏季,我们从现有推广品种华油3号进行株系繁殖的一个株系中,在我国第一次发现甘蓝型黄籽油菜,1976—1978年各年都相继发现了一批甘蓝型黄籽油菜。其来源主要是:(1)来自种间杂种,尤以白菜型父本是黄籽油菜的,一般在晚期世代可能出现黄籽,如363×七星剑(黄籽)、1368×501(黄籽)和日本油菜×金华红籽(部分自然杂 [本文引用: 1] |
| [2] | DOI:10.3969/j.issn.1001-7283.2001.06.016URL本文概述了甘蓝型黄籽油菜的主要特点,综述了20多年来国内外对甘蓝型黄籽油菜粒色遗传及其育种的研究进展,并指出了今后的研究重点和方向. [本文引用: 1] |
| [3] | [本文引用: 1] |
| [4] | [本文引用: 1] |
| [5] | [本文引用: 1] |
| [6] | DOI:10.1104/pp.106.079574URLStorage compound accumulation during seed development prepares the next generation of plants for survival. Therefore, processes involved in the regulation and synthesis of storage compound accumulation during seed development bear relevance to germination and seedling establishment. The wrinkled1 (wri1) mutant of Arabidopsis (Arabidopsis thaliana) is impaired in seed oil accumulation. The WRI1 gene encodes an APETALA2/ethylene-responsive element-binding protein transcription factor involved in the control of metabolism, particularly glycolysis, in the developing seeds. Here we investigate the role of this regulatory factor in seed germination and seedling establishment by comparing the wri1-1 mutant, transgenic lines expressing the WRI1 wild-type cDNA in the wri1-1 mutant background, and the wild type. Plants altered in the expression of the WRI1 gene showed different germination responses to the growth factor abscisic acid (ABA), sugars, and fatty acids provided in the medium. Germination of the mutant was more sensitive to ABA, sugars, and osmolites, an effect that was alleviated by increased WRI1 expression in transgenic lines. The expression of ABA-responsive genes AtEM6 and ABA-insensitive 3 (ABI3) was increased in the wri1-1 mutant. Double-mutant analysis between abi3-3 and wri1-1 suggested that WRI1 and ABI3, a transcription factor mediating ABA responses in seeds, act in parallel pathways. Addition of 2-deoxyglucose inhibited seed germination, but did so less in lines overexpressing WRI1. Seedling establishment was decreased in the wri1-1 mutant but could be alleviated by sucrose. Apart from a possible signaling role in germination, sugars in the medium were required as building blocks and energy supply during wri1-1 seedling establishment. |
| [7] | DOI:10.1111/j.1365-3040.2012.02546.xPMID:22632271URLPrevious studies based on microarray analysis have found that DELLAs down-regulate several GDSL genes in unopened flowers and/or imbibed seeds. This suggests the role of DELLAs in seed fatty acid (FA) metabolism. In the present study, enhancement of gibberellin (GA) signalling through DELLA mutation or exogenous gibberellin acid A3 (GA3) resulted in the up-regulated expression of transcription factors for embryogenesis and seed development, genes involved in the FA biosynthesis pathway, and five GDSL-type Seed Fatty Acid Reducer (SFAR) genes. SFAR overexpression reduced the total seed FA content and led to a particular pattern of seed FA composition. This FAR footprint can also be found in plants with enhanced GA3 signalling. By contrast, the loss of SFAR function dramatically increases the seed FA content. The transgenic lines that overexpress SFAR were less sensitive to stressful environments, reflected by a higher germination rate and better seedling establishment compared with the wild type (WT) plants. The GDSL-type hydrolyzer is a family of proteins largely uncharacterized in Arabidopsis. Their biological function remains poorly understood. SFAR reduces seed FA storage and acts downstream of the GA signalling pathway. We provide the first evidence that some GDSL proteins are somehow involved in FA degradation in Arabidopsis seeds. [本文引用: 1] |
| [8] | [本文引用: 2] |
| [9] | DOI:10.1104/pp.114.235507PMID:24722549URLAbstract Fatty acids (FAs) and FA-derived complex lipids play important roles in plant growth and vegetative development and are a class of prominent metabolites stored in mature seeds. The factors and regulatory networks that control FA accumulation in plant seeds remain largely unknown. The role of TRANSPARENT TESTA8 (TT8) in the regulation of flavonoid biosynthesis and the formation of seed coat color is extensively studied; however, its function in affecting seed FA biosynthesis is poorly understood. In this article, we show that Arabidopsis (Arabidopsis thaliana) TT8 acts maternally to affect seed FA biosynthesis and inhibits seed FA accumulation by down-regulating a group of genes either critical to embryonic development or important in the FA biosynthesis pathway. Moreover, the tt8 mutation resulted in reduced deposition of protein in seeds during maturation. Posttranslational activation of a TT8-GLUCOCORTICOID RECEPTOR fusion protein and chromatin immunoprecipitation assays demonstrated that TT8 represses the activities of LEAFY COTYLEDON1, LEAFY COTYLEDON2, and FUSCA3, the critical transcriptional factors important for seed development, as well as CYTIDINEDIPHOSPHATE DIACYLGLYCEROL SYNTHASE2, which mediates glycerolipid biosynthesis. These results help us to understand the entire function of TT8 and increase our knowledge of the complicated networks regulating the formation of FA-derived complex lipids in plant seeds. 2014 American Society of Plant Biologists. All Rights Reserved. [本文引用: 7] |
| [10] | [本文引用: 1] |
| [11] | [本文引用: 1] |
| [12] | DOI:10.1146/annurev-arplant-042809-112122PMID:20192755URLAbstract Abscisic acid (ABA) regulates numerous developmental processes and adaptive stress responses in plants. Many ABA signaling components have been identified, but their interconnections and a consensus on the structure of the ABA signaling network have eluded researchers. Recently, several advances have led to the identification of ABA receptors and their three-dimensional structures, and an understanding of how key regulatory phosphatase and kinase activities are controlled by ABA. A new model for ABA action has been proposed and validated, in which the soluble PYR/PYL/RCAR receptors function at the apex of a negative regulatory pathway to directly regulate PP2C phosphatases, which in turn directly regulate SnRK2 kinases. This model unifies many previously defined signaling components and highlights the importance of future work focused on defining the direct targets of SnRK2s and PP2Cs, dissecting the mechanisms of hormone interactions (i.e., cross talk) and defining connections between this new negative regulatory pathway and other factors implicated in ABA signaling. [本文引用: 1] |
| [13] | DOI:10.1104/pp.122.2.403URLThe testa of higher plant seeds protects the embryo against adverse environmental conditions. Its role is assumed mainly by controlling germination through dormancy imposition and by limiting the detrimental activity of physical and biological agents during seed storage. To analyze the function of the testa in the model plant Arabidopsis, we compared mutants affected in testa pigmentation and/or structure for dormancy, germination, and storability. The seeds of most mutants exhibited reduced dormancy. Moreover, unlike wild-type testas, mutant testas were permeable to tetrazolium salts. These altered dormancy and tetrazolium uptake properties were related to defects in the pigmentation of the endothelium and its neighboring crushed parenchymatic layers, as determined by vanillin staining and microscopic observations. Structural aberrations such as missing layers or a modified epidermal layer in specific mutants also affected dormancy levels and permeability to tetrazolium. Both structural and pigmentation mutants deteriorated faster than the wild types during natural aging at room temperature, with structural mutants being the most strongly affected. [本文引用: 3] |
| [14] | DOI:10.1105/tpc.014043PMID:14555692URLAnthocyanidin reductase encoded by the BANYULS (BAN) gene is the core enzyme in proanthocyanidin (PA) biosynthesis. Here, we analyzed the developmental mechanisms that regulate the spatiotemporal expression of BAN in the developing Arabidopsis seed coat. PA-accumulating cells were localized histochemically in the inner integument (seed body and micropyle) and pigment strand (chalaza). BAN promoter activity was detected specifically in these cells. Gain-of-function experiments showed that an 86-bp promoter fragment functioned as an enhancer specific for PA-accumulating cells. Mutations in regulatory genes of PA biosynthesis abolished BAN promoter activity (transparent testa2 [tt2], tt8, and transparent testa glabra1 [ttg1]), modified its spatial pattern (tt1 and tt16), or had no influence (ttg2), thus revealing complex regulatory interactions at several developmental levels. Genetic ablation of PA-accumulating cells targeted by the BAN promoter fused to BARNASE led to the formation of normal plants that produced viable yellow seeds. Importantly, these seeds had no obvious defects in endosperm and embryo development. [本文引用: 1] |
| [15] | DOI:10.1105/tpc.010441PMID:12045268URLAbscisic acid (ABA) regulates many agronomically important aspects of plant development, including the synthesis of seed storage proteins and lipids, the promotion of seed desiccation tolerance and dormancy, and the inhibition of the phase transitions from embryonic to germinative growth and from vegetative to reproductive growth (reviewed by Leung and Giraudat, 1998; Rock, 2000; Rohde et al., 2000b). In addition, ABA mediates some aspects of physiological responses to environmental stresses such as drought- or osmotica-induced stomatal closure, the induction of tolerance of water, salt, hypoxic, and cold stress, and wound or pathogen response (Leung and Giraudat, 1998; Rock, 2000; Shinozaki and Yamaguchi-Shinozaki, 2000). A traditional distinction among these responses has been that of speed: the stomatal responses are relatively fast, occurring within minutes and involving changes in the activity of various signaling molecules and ion channels, whereas the rest are slower and require changes in gene expression. However, these sets of responses clearly require the action of common signaling elements, because several individual mutants (e.g., the Arabidopsis ABA-insensitive abi1 and abi2 mutants and the ABA-hypersensitive era1 mutant) affect subsets of both types of responses. Furthermore, cell biological studies have implicated common classes of secondary messengers or components of phosphorylation cascades in both fast and slow responses to ABA. [本文引用: 1] |
| [16] | DOI:10.1104/pp.124.4.1532PMID:11115871URLThe characterization of the pathways by which plants respond to sugars as signalling molecules are reviewed. The approaches or methods used and the obtained results are discussed. |
| [17] | DOI:10.1007/s00425-007-0637-5PMID:17929052URLGDSL-type lipase is a hydrolytic enzyme whose amino acid sequence contains a pentapeptide motif (Gly-X-Ser-X-Gly) with active serine (Ser). Pepper GDSL-type lipase (CaGLIP1) gene was isolated and functionally characterized from pepper leaf tissues infected by Xanthomonas campestris pv. vesicatoria (Xcv). The CaGLIP1 protein was located in the vascular tissues of Arabidopsis root. The CaGLIP1 gene was preferentially expressed in pepper leaves during the compatible interaction with Xcv. Treatment with salicylic acid, ethylene and methyl jasmonate induced CaGLIP1 gene expression in pepper leaves. Sodium nitroprusside, methyl viologen, high salt, mannitol-mediated dehydration and wounding also induced early and transient CaGLIP1 expression in pepper leaf tissues. Virus-induced gene silencing of CaGLIP1 in pepper conferred enhanced resistance to Xcv, accompanied by the suppressed expression of basic PR1 (CaBPR1) and defensin (CaDEF1) genes. The CaGLIP1 lipase produced in Escherichia coli hydrolyzed the substrates of short and long chain nitrophenyl esters. The CaGLIP1-overexpressing Arabidopsis exhibited enhanced hydrolytic activity toward short and long chain nitrophenyl ester, as well as enhanced susceptibility to the bacterial pathogen Pseudomonas syringae pv. tomato and the biotrophic oomycete Hyaloperonospora parasitica. SA-induced expression of AtPR1 and AtGST1, also was delayed in CaGLIP1-overexpressing plants by SA application. During seed germination and plant growth, the CaGLIP1 transgenic plants showed drought tolerance and differential expression of drought- and abscisic acid (ABA)-inducible genes AtRD29A, AtADH and AtRab18. ABA treatment differentially regulated seed germination and gene expression in wild-type and CaGLIP1 transgenic Arabidopsis. Overexpression of CaGLIP1 also regulated glucose- and oxidative stress signaling. Together, these results indicate that CaGLIP1 modulates disease susceptibility and abiotic stress tolerance. [本文引用: 1] |
| [18] | DOI:10.1002/bies.950160209URLAbstract Flavonoids are a class of low molecular weight phenolic compounds that is widely distributed in the plant kingdom. They exhibit a diverse spectrum of biological functions and play an important role in the interaction between plants and their environment. Flavonoids not only protect the plant from the harmful effects of UV irradiation but also play a crucial role in the sexual reproduction process. A special class of flavonoid polymers, the tannins, plays a structural role in the plant. Yet other classes of flavonoids, flavonols and anthocyanins, have been implicated in the attraction of pollinators. Certain flavonoids participate in the interaction between plants and other organisms such as symbiotic bacteria and parasites. This raises the intriguing question as to how these different compounds arose and evolved. Based on taxonomy and molecular analysis of gene expression patterns it is possible to deduce a putative sequence of acquisition of the different branches of the biosynthetic pathway and their regulators. [本文引用: 2] |
| [19] | URL [本文引用: 4] |
| [20] | DOI:10.1146/annurev.arplant.57.032905.105252PMID:16669768URLAbstract Flavonoids are secondary metabolites that accumulate in most plant seeds and are involved in physiological functions such as dormancy or viability. This review presents a current view of the genetic and biochemical control of flavonoid metabolism during seed development. It focuses mainly on proanthocyanidin accumulation in Arabidopsis, with comparisons to other related metabolic and regulatory pathways. These intricate networks and their fine-tuned regulation, once they are determined, should contribute to a better understanding of seed coat development and the control of PA and flavonol metabolism. In addition, flavonoids provide an interesting model to study various biological processes and metabolic and regulatory networks. [本文引用: 5] |
| [21] | DOI:10.1016/j.plantsci.2016.10.010PMID:27964785URLTRANSPARENT TESTA GLABRA 1 ofArabidopsis thaliana(AtTTG1) is a WD40 repeat transcription factor that plays multiple roles in plant growth and development, particularly in seed metabolite production. In the present study, to determine whether SiTTG1 of the phylogenetically distant monocot foxtail millet (Setaria italica)has similar functions, we used transgenic Arabidopsis andNicotianasystems to explore its activities. We found that SiTTG1 functions as a transcription factor. Overexpression of theSiTTG1gene rescued many of the mutant phenotypes in Arabidopsisttg1-13plants. Additionally,SiTTG1overexpression fully corrected the reduced expression of mucilage biosynthetic genes, and the induced expression of genes involved in accumulation of seed fatty acids and storage proteins in developing seeds ofttg1-13plants. Ectopic expression ofSiTTG1restored the sensitivity of thettg1-13mutant to salinity and high glucose stresses during germination and seedling establishment, and restored altered expression levels of some stress-responsive genes inttg1-13seedlings to the wild type level under salinity and glucose stresses. Our results provide information that will be valuable for understanding the function ofTTG1from monocot to dicot species and identifying a promising target for genetic manipulation of foxtail millet to improve the amount of seed metabolites. [本文引用: 1] |
| [22] | DOI:10.1006/meth.2001.1262URL [本文引用: 2] |
| [23] | DOI:10.1007/BF03191146URLEncoding a WD40 protein, Arabidopsis thaliana TRANSPARENT TESTA GLABRA1 (AtTTG1) regulates trichome and root hair differentiation as well as flavonoids and seed mucilage deposition in plants. Here, two Brassica napus TTG1 (BnTTG1) genes were isolated, and Southern hybridization also generated only two bands. The 1511-bp BnTTG1-1 and the 1555-bp BnTTG1-2 both have one intron, and show alternative sites for transcription start, polyadenylation and intron right border splicing. EST and GSS tags suggested that BnTTG1-1 was derived from B. rapa, while BnTTG1-2 from B. oleracea. Evidence implies that TTG1 was possibly triplicated in Brassiceae, but some triplicated members were lost soon, which might involve fragmental rearrangements. BnTTG1-1 shares 88.7% genomic and 95.7% mRNA identities with BnTTG1-2 . Deduced BnTTG1-1 and BnTTG1-2 proteins both are 337 aa, differed only by substitution of a similar residue. They resemble AtTTG1 in WD40 domain and all conserved motifs. TTG1 / AN11-type WD40 proteins are extremely conserved even across kingdoms. Homological and structural characterizations identified BnTTG1-1 and BnTTG1-2 to be orthologs of AtTTG1. Several non-coding motifs are conserved between AtTTG1 and BnTTG1. BnTTG1 coding regions tend to evolve high GC contents through T/A /G substitutions especially T transition, but AtTTG1 shows opposite base preference. BnTTG1 genes also evolve a GA-stretch in the leader sequence. RT-PCR detected complementation in organ-specificity between BnTTG1-1 and BnTTG1-2 . BnTTG1-2 is more like AtTTG1 and is expressed in all major organs. BnTTG1-1 is more organ-specific with lower expression in seed and root, possibly withdrawing from regulating seed coat pigment /mucilage deposition and root hair formation. [本文引用: 1] |
| [24] | DOI:10.1016/S1360-1385(98)01242-4URLMutant analyses have given insight into the various parameters that contribute to flower colour and pattern, which is so important for pollination. One important factor is the accumulation of orange, red and purple anthocyanin pigments in the cell vacuole-patterns arise by cell-specific expression of combinations of regulatory proteins. The overall colour perceived is also influenced by vacuolar pH, co-pigmentation and the shape of the petal cells. Although understanding of the biochemistry and genetics of anthocyanin and flavonol biosynthesis is well developed, this is not the case for pH and cell-shape control. [本文引用: 1] |
| [25] | URL [本文引用: 1] |
| [26] | DOI:10.1111/tpj.12388PMID:4282528URLThe notion that plants use specialized metabolism to protect against environmental stresses needs to be experimentally proven by addressing the question of whether stress tolerance by specialized metabolism is directly due to metabolites such as flavonoids. We report that flavonoids with radical scavenging activity mitigate against oxidative and drought stress in Arabidopsis thaliana. Metabolome and transcriptome profiling and experiments with oxidative and drought stress in wild-type, single overexpressors of MYB12/PFG1 (PRODUCTION OF FLAVONOL GLYCOSIDES1) or MYB75/PAP1 (PRODUCTION OF ANTHOCYANIN PIGMENT1), double overexpressors of MYB12 and PAP1, transparent testa4 (tt4) as a flavonoid-deficient mutant, and flavonoid-deficient MYB12 or PAP1 overexpressing lines (obtained by crossing tt4 and the individual MYB overexpressor) demonstrated that flavonoid overaccumulation was key to enhanced tolerance to such stresses. Antioxidative activity assays using 2,2-diphenyl-1-picrylhydrazyl, methyl viologen, and 3,3鈥-diaminobenzidine clearly showed that anthocyanin overaccumulation with strong in vitro antioxidative activity mitigated the accumulation of reactive oxygen species in vivo under oxidative and drought stress. These data confirm the usefulness of flavonoids for enhancing both biotic and abiotic stress tolerance in crops. [本文引用: 1] |
| [27] | DOI:10.1105/tpc.13.9.2099PMID:11549766URLIn Arabidopsis, proanthocyanidins specifically accumulate in the endothelium during early seed development. At least three TRANSPARENT TESTA (TT) genes, TT2, TT8, and TTG1, are necessary for the normal expression of several flavonoid structural genes in immature seed, such as DIHYDROFLAVONOL-4-REDUCTASE and BANYULS (BAN). TT8 and TTG1 were characterized recently and found to code for a basic helix-loop-helix domain transcription factor and a WD-repeat-containing protein, respectively. Here the molecular cloning of the TT2 gene was achieved by T-DNA tagging. TT2 encoded an R2R3 MYB domain protein with high similarity to the rice OsMYB3 protein and the maize COLORLESS1 factor. A TT2-green fluorescent protein fusion protein was located mostly in the nucleus, in agreement with the regulatory function of the native TT2 protein. TT2 expression was restricted to the seed during early embryogenesis, consistent with BAN expression and the proanthocyanidin deposition profile. Finally, in gain-of-function experiments, TT2 was able to induce ectopic expression of BAN in young seedlings and roots in the presence of a functional TT8 protein. Therefore, our results strongly suggest that stringent spatial and temporal BAN expression, and thus proanthocyanidin accumulation, are determined at least partially by TT2. [本文引用: 1] |
| [28] | DOI:10.1007/s00299-012-1382-1PMID:23408189URLOur results demonstrate that the flavonoids biosynthetic pathway can be effectively manipulated to confer enhanced plant root growth under water-stress conditions.Abscisic acid (ABA) is one of most important phytohormones. It functions in various processes during the plant lifecycle. Previous studies indicate that ABA has a negative effect on root growth and branching. Auxin is another key plant growth regulator that plays an essential role in plant growth and development. In contrast to ABA, auxin is a positive regulator of root growth and development at low concentrations. This study was performed to help understand whether flavonoids can suppress the effect of ABA on lateral root growth. The recessive TRANSPARENT TESTA GLABRA 1 (ttg1) mutant was characterized on ABA and sucrose treatments. It was determined that auxin mobilization could be altered by modifying flavonoids biosynthesis, which resulted in alterations of root architecture in response to ABA treatment. Moreover, transgenic TTG1-overexpression (TTG1-OX) seedlings exhibited enhanced root length and lateral root number compared to wild-type seedlings grown under normal or stress conditions. Genetic manipulation of the flavonoids biosynthetic pathway could therefore be employed successfully for the improvement of plant root systems by overcoming the inhibition of ABA and some abiotic stresses. [本文引用: 1] |
| [29] | [本文引用: 1] |
| [30] | DOI:10.1016/j.tplants.2007.10.003PMID:18198522URLFlavonoids are polyphenolic compounds found in all vascular and non-vascular plants. Although nonessential for plant growth and development, flavonoids have species-specific roles in nodulation, fertility, defense and UV protection. Flavonoids have been shown to modulate transport of the phytohormone auxin in addition to auxin-dependent tropic responses. However, flavonoids are not essential regulators of these processes because transport and tropic responses occur in their absence. Flavonoids modulate the activity of auxin-transporting P-glycoproteins and seem to modulate the activity of regulatory proteins such as phosphatases and kinases. Phylogenetic analysis suggests that auxin transport mechanisms evolved in the presence of flavonoid compounds produced for the scavenging of reactive oxygen species and defense from herbivores and pathogens. [本文引用: 1] |
| [31] | DOI:10.3390/ijms140714950URL [本文引用: 1] |
| [32] | DOI:10.1111/j.1365-313X.2011.04768.xPMID:21883555URLSummary Seed oil, one of the major seed storage compounds in plants, is of great economic importance for human consumption, as an industrial raw material and as a source of biofuels. Thus, improving the seed oil yield in crops is an important objective. The GLABRA2 ( GL2 ) gene in Arabidopsis thaliana encodes a transcription factor that is required for the proper differentiation of several epidermal cell types. GL2 has also been shown to regulate seed oil levels, as a loss-of-function mutation in the GL2 gene results in plants with a higher seed oil content than wild-type. We have extended this observation by showing that loss-of-function mutations in several positive regulators of GL2 also result in a high seed oil phenotype. The GL2 gene is expressed in both the seed coat and embryo, but the embryo is the main site of seed oil accumulation. Surprisingly, our results indicate that it is loss of GL2 activity in the seed coat, not the embryo, that contributes to the high seed oil phenotype. One target of GL2 in the seed coat is the gene MUCILAGE MODIFIED 4 ( MUM4 ), which encodes a rhamnose synthase that is required for seed mucilage biosynthesis. We found that mum4 mutant seeds, like those of gl2 mutants, have an increased seed oil content in comparison with wild-type. Therefore, GL2 regulates seed oil production at least partly through its influence on MUM4 expression in the seed coat. We propose that gl2 mutant seeds produce more oil due to increased carbon allocation to the embryo in the absence of seed coat mucilage biosynthesis. [本文引用: 1] |
| [33] | DOI:10.1016/S1360-1385(96)80312-8URLFlavonoids are important plant secondary metabolites that have been extensively studied using genetic, biochemical and molecular approaches. Recent analyses have focused on our understanding of the role of flavonoids in such well-established processes as plant-microbe interactions and protection against ultraviolet (UV) light, and have also uncovered a previously unsuspected role in male fertility. These studies demonstrate the utility of flavonoid structures for a broad range of activities, and also highlight the value of the flavonoid pathway as a paradigm for studying the evolution of plant metabolism. [本文引用: 1] |
| [34] | DOI:10.1046/j.1365-313X.1995.08050659.xPMID:8528278URLEleven loci that play a role in the synthesis of flavonoids in Arabidopsis are described. Mutations at these loci, collectively named transparent testa (tt) , disrupt the synthesis of brown pigments in the seed coat (testa). Several of these loci ( tt3, tt4, tt5 and ttg ) are also required for the accumulation of purple anthocyanins in leaves and stems and one locus ( ttg ) plays additional roles in trichome and root hair development. Specific functions were previously assigned to tt1–7 and ttg . Here, the results of additional genetic, biochemical and molecular analyses of these mutants are described. Genetic map positions were determined for tt8, tt9 and tt10 . Thin‐layer chromatography identified tissue‐ and locus‐specific differences in the flavonols and anthocyanidins synthesized by mutant and wild‐type plants. It was found that UV light reveals distinct differences in the floral tissues of tt3, tt4, tt5, tt6 and ttg , even though these tissues are indistinguishable under visible light. Evidence was also uncovered that tt8 and ttg specifically affect dihydroflavonol reductase gene expression. A summary of these and previously published results are incorporated into an overview of the genetics of flavonoid biosynthesis in Arabidopsis . [本文引用: 1] |
| [35] | DOI:10.1016/S1360-1385(00)01597-1PMID:10785667URLArabidopsis trichomes are large unicellular structures that develop on the surface of most shoot-derived organs. In leaves, the number, spacing and shape of trichomes is tightly regulated, and this process has been used as an experimental system to study the control of cell fate and pattern formation. The control of trichome initiation is complex: both the potential of a cell to adopt the trichome cell fate and an intricate signaling pathway determine the pattern of trichome initiation events. Several important new results suggest that trichome initiation and morphogenesis are redundantly regulated by both positive and negative factors. A testable model for the control of trichome initiation is presented. [本文引用: 2] |
| [36] | DOI:10.1046/j.1365-313X.2003.01939.xPMID:14675433URLLoss-of-function mutations in the FUSCA3 ( FUS3 ) gene of Arabidopsis result in alterations in cotyledon identity, inability to complete late seed maturation processes, and the premature activation of apical and root embryonic meristems, which indicates that this transcription factor is an essential regulator of embryogenesis. Although FUS3 shows a complex pattern of expression in the embryo, this gene is only required in the protoderm to carry out its functions. Moreover, the epidermal morphogenesis regulator TRANSPARENT TESTA GLABRA1 ( TTG1 ) is negatively regulated by FUS3 in the embryo. When a loss-of-function ttg1 mutation is introduced into a fus3 mutant, a number of fus3 -related phenotypes are rescued, indicating a functional TTG1 gene is required to manifest the fus3 mutant phenotype. It therefore appears that one of the functions of FUS3 is to restrict the domain of expression of TTG1 during embryogenesis. The FUS3 TG1 interaction is both maternal and zygotic, suggesting a complex relationship is required between these gene products to allow correct seed development. [本文引用: 4] |
| [37] | |
| [38] | [本文引用: 1] |
| [39] | DOI:10.1104/pp.127.3.998URLIn , induces the epidermal cells of the outer ovule integument to differentiate into a specialized seed coat cell type producing pectinaceous mucilage and a volcano-shaped . Differentiation involves a regulated series of cytological events including growth, cytoplasmic rearrangement, mucilage synthesis, and production. We have tested the potential of seed coat epidermal cells as a model system for the genetic analysis of these processes. A screen for mutants defective in seed mucilage identified five novel genes (MUCILAGE-MODIFIED []1-5). The of these mutants, and that of three previously identified ones (TRANSPARENT TESTA GLABRA1, GLABRA2, and APETALA2) were characterized. Our results show that the genes identified define several events in seed coat differentiation. Although APETALA2 is needed for differentiation of both outer layers of the seed coat, TRANSPARENT TESTA GLABRA1, GLABRA2, and are required for complete mucilage synthesis and cytoplasmic rearrangement. and MUM5 may be involved in the regulation of mucilage composition, whereas and appear to play novel roles in post-synthesis necessary for mucilage extrusion. [本文引用: 1] |
| [40] | DOI:10.1016/S1369-5266(02)00256-XPMID:11960739URLThe accumulation of red or purple flavonoids is a hallmark of plant stress. Mounting evidence points to diverse physiological functions for these compounds in the stress response. Advances are also being made toward understanding how plants control the types and amounts of flavonoids that are produced in response to different cues. [本文引用: 3] |
| [41] | DOI:10.1111/nph.12620PMID:24299194URLIn Arabidopsis thaliana, proanthocyanidins (PAs) accumulate in the innermost cell layer of the seed coat (i.e. endothelium, chalaza and micropyle). The expression of the biosynthetic genes involved relies on the transcriptional activity of R2R3-MYB and basic helix-loop-helix (bHLH) proteins which form ternary complexes (090004MBW090005) with TRANSPARENT TESTA GLABRA1 (TTG1) (WD repeat protein). The identification of the direct targets and the determination of the nature and spatio-temporal activity of these MBW complexes are essential steps towards a comprehensive understanding of the transcriptional mechanisms that control flavonoid biosynthesis.In this study, various molecular, genetic and biochemical approaches were used.Here, we have demonstrated that, of the 12 studied genes of the pathway, only dihydroflavonol-4-reductase (DFR), leucoanthocyanidin dioxygenase (LDOX), BANYULS (BAN), TRANSPARENT TESTA 19 (TT19), TT12 and H+-ATPase isoform 10 (AHA10) are direct targets of the MBW complexes. Interestingly, although the TT2090009TT8090009TTG1 complex plays the major role in developing seeds, three additional MBW complexes (i.e. MYB5090009TT8090009TTG1, TT2090009EGL3090009TTG1 and TT2090009GL3090009TTG1) were also shown to be involved, in a tissue-specific manner. Finally, a minimal promoter was identified for each of the target genes of the MBW complexes.Altogether, by answering fundamental questions and by demonstrating or invalidating previously made hypotheses, this study provides a new and comprehensive view of the transcriptional regulatory mechanisms controlling PA and anthocyanin biosynthesis in Arabidopsis. |
| [42] | DOI:10.1074/jbc.M403697200URL |
甘蓝型黄籽油菜的发现及其遗传行为的初步研究
1
1979
... 甘蓝型油菜(Brassica napus)是我国最重要的油料作物之一.菜籽油不仅是良好的食用油, 而且是制造医药品和化妆品等多种化工产品的原料.1960年, 瑞典科学家首次从人工合成的甘蓝型油菜中找到了黄籽单株.1975年, 我国科学家也发现了甘蓝型黄籽油菜(
甘蓝型黄籽油菜粒色遗传及其育种研究进展
1
2001
... 甘蓝型油菜(Brassica napus)是我国最重要的油料作物之一.菜籽油不仅是良好的食用油, 而且是制造医药品和化妆品等多种化工产品的原料.1960年, 瑞典科学家首次从人工合成的甘蓝型油菜中找到了黄籽单株.1975年, 我国科学家也发现了甘蓝型黄籽油菜(
1
1976
... 取20粒拟南芥种子于1.5 mL离心管中, 放入液氮中研磨, 磨碎后加入200 μL的提取液(50 mmol·L-1 HEPES, 5 mmol·L-1 MgCl2, 5 mmol·L-1二硫苏糖 醇, 1 mmol·L-1苯甲基磺酰氟, 1 mmol·L-1 EDTA, 10% (v/v) pH7.5乙二醇, 少许不溶性交联PVP).4°C、 22 000×g离心10分钟后, 吸取上清液, 依据
1
... 油菜与拟南芥同属十字花科, 是与拟南芥亲源关系最近的经济作物之一.拟南芥基因组中的每个基因在甘蓝型油菜基因组中都有2-8个同源拷贝(
1
1998
... 同时收获在同一时间种植并在相同条件下生长的拟南芥野生型Col-0、突变体ttg1-13和纯合转基因株系ttg1-13 35S::BnTTG1的种子, 室温下放置4周至完全成熟, 放入-20°C冰箱中保存备用.用于抗性实验的种子先在4°C冰箱中预冷5天, 再用75% (v/v)的乙醇清洗种子表面30-60秒, 重复2次, 之后用ddH2O冲洗5遍, 铺种于含3% (w/v)蔗糖和100 mmol·L-1 NaCl的MS固体培养基平板上, 以无任何胁迫处理的MS平板作为对照.将胚根露出种皮定义为种子萌发, 将幼苗长出2片真叶能够独立进行光合作用定义为幼苗完成形态建成(
2006
1
2012
... 按照文献报道的方法(
2
2012
... 我们根据NCBI数据库中已有的甘蓝型油菜TTG1基因全长CDS序列, 从甘蓝型油菜品种秦优7号中成功克隆了BnTTG1-1基因的全长CDS序列.BnTTG1-1在秦优7号的时空表达模式显示其可能在油菜生长发育的多个生物学过程中发挥作用(
... ,
7
2014
... 拟南芥(Arabidopsis thaliana)中含有丰富的类黄酮, 主要包括原花青素、花青素和黄酮醇3种, 它们的存在使得花、茎和种子等组织呈现不同的颜色(
... ;
... 越来越多的证据表明, 拟南芥AtTTG1参与调控表皮毛的形成(
... ;
... 我们根据NCBI数据库中已有的甘蓝型油菜TTG1基因全长CDS序列, 从甘蓝型油菜品种秦优7号中成功克隆了BnTTG1-1基因的全长CDS序列.BnTTG1-1在秦优7号的时空表达模式显示其可能在油菜生长发育的多个生物学过程中发挥作用(
... ;
... ).Zhang和Rock (2004)与Wang等(2014)的研究表明, 花青素可能从种皮渗入种胚, 通过抑制编码脂肪酸碳链延伸的重要酶基因的表达进而抑制种胚中脂肪酸的积累.此外, 种子脂肪酸和种皮黏液的生物合成均需要利用光合作用的C源.我们的研究结果表明, AtTTG1在拟南芥种子发育过程中既可通过间接抑制多个脂肪酸和蛋白合成途径上重要基因的表达, 也可通过母体效应影响种子脂肪酸和储藏蛋白的积累(
1
2015
... 依据NCBI数据库中BnTTG1全长CDS序列(编号分别为EF175930、EF175931、EU192030和EU192031)设计特异引物(
1
1998
... 前人的研究表明, 类黄酮作为次级代谢产物不仅在种子休眠和种子活力方面起着重要作用, 而且参与调控植物逆境胁迫响应过程(
1
2010
... 拟南芥(Arabidopsis thaliana)中含有丰富的类黄酮, 主要包括原花青素、花青素和黄酮醇3种, 它们的存在使得花、茎和种子等组织呈现不同的颜色(
3
2000
... 拟南芥(Arabidopsis thaliana)中含有丰富的类黄酮, 主要包括原花青素、花青素和黄酮醇3种, 它们的存在使得花、茎和种子等组织呈现不同的颜色(
... 越来越多的证据表明, 拟南芥AtTTG1参与调控表皮毛的形成(
... 我们根据NCBI数据库中已有的甘蓝型油菜TTG1基因全长CDS序列, 从甘蓝型油菜品种秦优7号中成功克隆了BnTTG1-1基因的全长CDS序列.BnTTG1-1在秦优7号的时空表达模式显示其可能在油菜生长发育的多个生物学过程中发挥作用(
1
2003
... 前人的研究表明, 类黄酮作为次级代谢产物不仅在种子休眠和种子活力方面起着重要作用, 而且参与调控植物逆境胁迫响应过程(
1
2002
... 前人的研究表明, 类黄酮作为次级代谢产物不仅在种子休眠和种子活力方面起着重要作用, 而且参与调控植物逆境胁迫响应过程(
2001
1
2008
... 拟南芥(Arabidopsis thaliana)中含有丰富的类黄酮, 主要包括原花青素、花青素和黄酮醇3种, 它们的存在使得花、茎和种子等组织呈现不同的颜色(
2
1994
... 拟南芥(Arabidopsis thaliana)中含有丰富的类黄酮, 主要包括原花青素、花青素和黄酮醇3种, 它们的存在使得花、茎和种子等组织呈现不同的颜色(
... 我们根据NCBI数据库中已有的甘蓝型油菜TTG1基因全长CDS序列, 从甘蓝型油菜品种秦优7号中成功克隆了BnTTG1-1基因的全长CDS序列.BnTTG1-1在秦优7号的时空表达模式显示其可能在油菜生长发育的多个生物学过程中发挥作用(
4
1981
... 拟南芥(Arabidopsis thaliana)中含有丰富的类黄酮, 主要包括原花青素、花青素和黄酮醇3种, 它们的存在使得花、茎和种子等组织呈现不同的颜色(
... ;
... 越来越多的证据表明, 拟南芥AtTTG1参与调控表皮毛的形成(
... 我们根据NCBI数据库中已有的甘蓝型油菜TTG1基因全长CDS序列, 从甘蓝型油菜品种秦优7号中成功克隆了BnTTG1-1基因的全长CDS序列.BnTTG1-1在秦优7号的时空表达模式显示其可能在油菜生长发育的多个生物学过程中发挥作用(
5
2006
... 本实验所用遗传材料有甘蓝型油菜(Brassica napus L.)品种秦优7号、拟南芥(Arabidopsis thaliana L.)哥伦比亚野生型Col-0和功能缺失突变体ttg1-13 (
... 越来越多的证据表明, 拟南芥AtTTG1参与调控表皮毛的形成(
... 我们根据NCBI数据库中已有的甘蓝型油菜TTG1基因全长CDS序列, 从甘蓝型油菜品种秦优7号中成功克隆了BnTTG1-1基因的全长CDS序列.BnTTG1-1在秦优7号的时空表达模式显示其可能在油菜生长发育的多个生物学过程中发挥作用(
... 前人的研究表明, 类黄酮作为次级代谢产物不仅在种子休眠和种子活力方面起着重要作用, 而且参与调控植物逆境胁迫响应过程(
... 功能类似(
1
2017
... 利用半定量RT-PCR和荧光定量qRT-PCR技术分析基因的表达情况.按照SYBR?Premix Ex TaqTM II (TaKaRa, Cat No. DRR820A)使用说明书对本研究相关基因的表达量进行荧光定量PCR分析.PCR体系: SYBR? Premix Ex Taq 10 μL, 0.5 μmol·L-1上下游引物各1 μL, cDNA 2 μL (50 ng·μL-1), RNase Free ddH2O加至20 μL.在Bio-Rad荧光定量PCR仪上进行反应, 反应程序为: 95°C预变性60秒; 95°C20秒, 58°C20秒, 72°C45秒, 40个循环.反应结束后分析荧光值变化曲线以及溶解曲线, 采用2-ΔΔCt法分析结果(
2
2001
... 油菜是与拟南芥亲缘关系最近的油料作物之一, 两者基因组同源性很高.因此, 拟南芥的研究成果可以作为油菜研究的重要参考.对油菜的研究表明, BnTTG1-1 (NCBI编号为EF175930)在油菜种子和根中表达量较低, BnTTG1-2 (NCBI编号为EF175931)在油菜各个组织中均有表达(
... 从甘蓝型油菜秦优7号中克隆得到的QINYOU.Bn- TTG1蛋白质序列与NCBI数据库中的BnTTG1-1 (编号为EF175930)蛋白质序列完全相同, 因此, QINYOU.BnTTG1在本文中命名为BnTTG1-1.BnTTG1-1基因在秦优7号不同营养组织中的表达模式与前人的研究结果一致(
1
2009
... 拟南芥(Arabidopsis thaliana)中含有丰富的类黄酮, 主要包括原花青素、花青素和黄酮醇3种, 它们的存在使得花、茎和种子等组织呈现不同的颜色(
1
1998
... 按照文献报道的方法(
1
2008
... 前人的研究表明, 类黄酮作为次级代谢产物不仅在种子休眠和种子活力方面起着重要作用, 而且参与调控植物逆境胁迫响应过程(
1
2014
... 拟南芥(Arabidopsis thaliana)中含有丰富的类黄酮, 主要包括原花青素、花青素和黄酮醇3种, 它们的存在使得花、茎和种子等组织呈现不同的颜色(
1
2001
... 拟南芥(Arabidopsis thaliana)中含有丰富的类黄酮, 主要包括原花青素、花青素和黄酮醇3种, 它们的存在使得花、茎和种子等组织呈现不同的颜色(
1
2013
... 油菜与拟南芥同属十字花科, 是与拟南芥亲源关系最近的经济作物之一.拟南芥基因组中的每个基因在甘蓝型油菜基因组中都有2-8个同源拷贝(
1
1997
... 前人的研究表明, 类黄酮作为次级代谢产物不仅在种子休眠和种子活力方面起着重要作用, 而且参与调控植物逆境胁迫响应过程(
1
2007
... 前人的研究表明, 类黄酮作为次级代谢产物不仅在种子休眠和种子活力方面起着重要作用, 而且参与调控植物逆境胁迫响应过程(
1
2013
... 我们根据NCBI数据库中已有的甘蓝型油菜TTG1基因全长CDS序列, 从甘蓝型油菜品种秦优7号中成功克隆了BnTTG1-1基因的全长CDS序列.BnTTG1-1在秦优7号的时空表达模式显示其可能在油菜生长发育的多个生物学过程中发挥作用(
1
2012
... 拟南芥(Arabidopsis thaliana)中含有丰富的类黄酮, 主要包括原花青素、花青素和黄酮醇3种, 它们的存在使得花、茎和种子等组织呈现不同的颜色(
1
1996
... 拟南芥(Arabidopsis thaliana)中含有丰富的类黄酮, 主要包括原花青素、花青素和黄酮醇3种, 它们的存在使得花、茎和种子等组织呈现不同的颜色(
1
1995
... 前人的研究表明, 类黄酮作为次级代谢产物不仅在种子休眠和种子活力方面起着重要作用, 而且参与调控植物逆境胁迫响应过程(
2
2000
... 拟南芥(Arabidopsis thaliana)中含有丰富的类黄酮, 主要包括原花青素、花青素和黄酮醇3种, 它们的存在使得花、茎和种子等组织呈现不同的颜色(
... 越来越多的证据表明, 拟南芥AtTTG1参与调控表皮毛的形成(
4
2004
... 拟南芥(Arabidopsis thaliana)中含有丰富的类黄酮, 主要包括原花青素、花青素和黄酮醇3种, 它们的存在使得花、茎和种子等组织呈现不同的颜色(
... 越来越多的证据表明, 拟南芥AtTTG1参与调控表皮毛的形成(
... 我们根据NCBI数据库中已有的甘蓝型油菜TTG1基因全长CDS序列, 从甘蓝型油菜品种秦优7号中成功克隆了BnTTG1-1基因的全长CDS序列.BnTTG1-1在秦优7号的时空表达模式显示其可能在油菜生长发育的多个生物学过程中发挥作用(
... ;
1999
1
2014
... 拟南芥(Arabidopsis thaliana)中含有丰富的类黄酮, 主要包括原花青素、花青素和黄酮醇3种, 它们的存在使得花、茎和种子等组织呈现不同的颜色(
1
2001
... 前人的研究表明, 类黄酮作为次级代谢产物不仅在种子休眠和种子活力方面起着重要作用, 而且参与调控植物逆境胁迫响应过程(
3
2002
... 拟南芥(Arabidopsis thaliana)中含有丰富的类黄酮, 主要包括原花青素、花青素和黄酮醇3种, 它们的存在使得花、茎和种子等组织呈现不同的颜色(
... 越来越多的证据表明, 拟南芥AtTTG1参与调控表皮毛的形成(
... 我们根据NCBI数据库中已有的甘蓝型油菜TTG1基因全长CDS序列, 从甘蓝型油菜品种秦优7号中成功克隆了BnTTG1-1基因的全长CDS序列.BnTTG1-1在秦优7号的时空表达模式显示其可能在油菜生长发育的多个生物学过程中发挥作用(
2014
2004
