 , 张泰东
, 张泰东东北林业大学生态研究中心, 哈尔滨 150040
New perspectives on forest soil carbon and nitrogen cycling processes: Roles of arbuscular mycorrhizal versus ectomycorrhizal tree species
WANGXin-Qi, WANGChuan-Kuan , ZHANGTai-Dong
, ZHANGTai-Dong通讯作者:
版权声明:2017植物生态学报编辑部本文是遵循CCAL协议的开放存取期刊,引用请务必标明出处。
基金资助:
展开
摘要
关键词:
Abstract
Keywords:
-->0
PDF (362KB)元数据多维度评价相关文章收藏文章
本文引用格式导出EndNoteRisBibtex收藏本文-->
菌根是土壤真菌与植物根系形成的共生体, 存在于94%的维管植物根系中(Brundrett, 2009)。几乎所有树木的根系都能与丛枝菌根(arbuscular mycorrhiza, AM)或外生菌根(ectomycorrhiza, EM)真菌形成共生关系; 在这种共生关系中, 菌根真菌将养分及水分提供给植物, 以换取用于其生长所需的碳水化合物(Soudzilovskaia et al., 2015; van der Heijden et al., 2015; 王茜等, 2015)。菌根真菌不仅对植物的存活和生长具有重要作用, 而且其与土壤自由微生物的相互作用在森林生态系统的生态过程(尤其是土壤碳(C)、氮(N)循环)中也扮演着重要角色(Orwin et al., 2011; Veresoglou et al., 2012; Phillips et al., 2013; Bardgett et al., 2014; McCormack et al., 2015; Laliberté, 2016)。由于森林土壤固定的C、N库容巨大(Lal et al., 2015), 森林生态系统AM、EM树种相对丰度随全球变化而发生的改变很可能会导致陆地生态系统生物地球化学循环过程的显著变化(Phillips et al., 2012; 石兆勇等, 2012b; Terrer et al., 2016, 2017)。阐明不同菌根类型森林土壤C、N循环的差异及其影响机制, 对于提高森林生产力、预测森林生态系统对全球变化的响应等具有重要意义(Averill et al., 2014; Midgley et al., 2015; Soudzilovskaia et al., 2015)。
与树种的叶习性和谱系分类(例如针叶裸子植物与阔叶被子植物)相比, 按菌根类型进行树种分类能更好地解释森林生态系统土壤C、N循环的变异性乃至森林生产力对全球变化的响应(Phillips et al., 2013; Midgley & Phillips, 2014; Terrer et al., 2016, 2017)。不同菌根树种可通过地上(凋落物)及地下(根系及菌根真菌)特性直接或间接地影响森林土壤C、N的输入、稳定及输出等过程, 从而造成不同菌根类型森林土壤C、N循环的差异(Austin & Zanne, 2015; Brzostek et al., 2015; Midgley et al., 2015; Moore et al., 2015; Soudzilovskaia et al., 2015)。然而, 由于试验方法、研究尺度等限制, 不同菌根类型树种对森林土壤C、N循环过程的影响机制仍存在较大的不确定性(Moore et al., 2015)。为此, 本文综述了AM和EM森林土壤C、N循环的差异, 并基于森林土壤C、N输入、稳定和输出等3个过程剖析了AM和EM树种对土壤C、N循环的影响机制, 然后比较了不同菌根类型森林土壤C、N循环过程对全球CO2浓度升高和N沉降增加的响应, 最后指出了该研究领域所面临的主要挑战。
1 丛枝菌根与外生菌根森林土壤C、N循环的差异
1.1 土壤C循环的差异
广义的森林土壤由凋落物层和矿质土层共同组成, 但多数研究并未同时报道不同菌根类型森林对这两个土层C储量的差异, 因此很难总结出整个森林土壤剖面C储量差异的普适性结论(Vesterdal et al., 2013; Lin et al., 2016)。对AM和EM森林凋落物层C储量差异的研究结果较为一致, 均表现为AM小于EM (图1, Vesterdal et al., 2013; Lin et al., 2016)。两个菌根类型树种凋落物C输入量基本相同; 而由于AM树种凋落物质量较高(主要因其C或木质素浓度与N浓度的比值均显著小于EM树种), 质量损失较快, 从而使AM森林凋落物层C输出量高于EM森林(Cornelissen et al., 2001; Lin et al., 2016; Taylor et al., 2016)。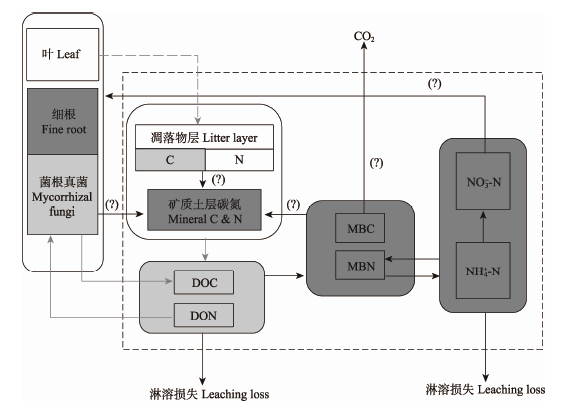 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图1丛枝菌根(AM)与外生菌根(EM)森林土壤碳氮循环的比较示意图。DOC, 溶解性有机碳; DON, 溶解性有机氮; MBC, 微生物生物量碳; MBN, 微生物生物量氮。深灰方框代表AM森林土壤储量较高; 浅灰方框代表EM森林土壤储量较高; 白色方框代表两者储量差异不显著。黑色箭头代表AM森林土壤碳氮通量较高; 灰色箭头代表EM森林土壤碳氮通量较高; 灰色虚线箭头代表两者通量没有显著差异。?代表该通量尚存争议。
-->Fig. 1Comparative diagram of soil carbon and nitrogen cycles between arbuscular mycorrhizal (AM) and ectomycorrhizal (EM) forests. DOC, dissolved organic carbon; DON, dissolved organic nitrogen; MBC, microbial biomass carbon; MBN, microbial biomass nitrogen. The dark grey boxes represent greater pools in AM than in EM forests, the light ones represent greater ones in EM than in AM forests, and the white ones represent insignificant differences between them. The black arrows represent greater fluxes in AM than in EM forest, the grey ones represent greater ones in EM than in AM forests, and the grey dashed ones represent insignificant differences between them. ? indicates inconsistent measurements of the fluxes.
-->
关于矿质土层C循环相关过程的差异尚存分歧, 并且大多数研究并未分析两个菌根树种对矿质土层C输入过程的影响, 主要比较了二者C输出过程的差异(土壤呼吸、异养呼吸)。在全球尺度上, AM森林矿质土层C含量比EM高8.0% (Lin et al., 2016), 但部分温带地区的研究结果显示两者差异不显著(图1, Hagen-Thorn et al., 2004; Mueller et al., 2012, 2015)。Vesterdal等(2012)和Taylor等(2016)的研究均表明, AM森林矿质土层异养呼吸速率显著高于EM; 而Lin等(2016)整合分析发现: 在全球尺度上, AM、EM森林土壤呼吸及矿化速率均没有显著差异。这些分歧可能是由于土壤C的短期动态会随气候条件而变化, 而大尺度整合分析又夹杂了气候、土壤类型等因素的影响。
1.2 土壤N循环的差异
与C循环相比, 不同菌根类型森林土壤N循环的研究结果较一致(图1)。全球尺度上, AM森林凋落物层N储量小于EM, 但其差异不显著; 但AM森林矿质土层N比EM森林矿质土层N高22.0%, 前者矿质土层C、N之比(C:N)显著小于后者(Midgley & Phillips, 2014; Lin et al., 2016)。AM森林矿质土层无机N (铵态N、硝态N)显著高于EM森林, 前者土壤有机N和无机N之比显著小于后者, 并且前者土壤N矿化及硝化速率显著高于后者(Phillips et al., 2013; Lin et al., 2016)。这说明AM森林土壤N循环主要以无机N为主导, EM森林则以有机N为主导, 即菌根关联养分经济(mycorrhizal-associated nutrient economy, MANE)预测模型(Phillips et al., 2013)。此外, AM森林土壤有机N (Scott & Rothstein, 2017)、硝酸盐的淋溶损失(Midgley & Phillips, 2014)显著大于EM森林。由于硝酸盐的淋溶是生态系统N饱和特征之一(Chapin et al., 2011), AM森林土壤N相较于EM森林可能更倾向于饱和状态。一般认为, AM真菌因不能通过分泌胞外酶获取复杂有机质中的N, 所以对植物获取N的过程没有影响, 其主要功能是促进植物对土壤磷的摄取能力。然而, 近年来越来越多的试验发现AM真菌能够促进植物对N的吸收, 且其吸收方式及能力随土壤N水平而变(Hodge et al., 2010; Asghari & Cavagnaro, 2011; Veresoglou et al., 2012; Hodge & Storer, 2015)。在土壤N匮乏时, AM真菌吸收有机N量会增加(Hawkins et al., 2000; Whiteside et al., 2012; 李元敬等, 2013; Hodge & Storer, 2015)。由于土壤溶解性有机氮(DON)的淋溶损失量很大, AM真菌吸收有机N对于养分匮乏地区的植物生长具有重要意义(van der Heijden et al., 2015)。此外, Veresoglou等(2012)认为, AM真菌对铵态N的吸收比细根更有效, 因此在土壤铵态N发生短暂脉冲效应时, AM真菌的生态重要性更为突出。然而, 关于AM真菌促进吸收、运输N素的研究仅基于室内培养研究, 且大多数以球囊霉科为研究对象(Veresoglou et al., 2012); 至今尚未见植株及生态系统水平上的研究报道。AM真菌在森林生态系统水平上对土壤N的吸收形式及数量尚需进一步验证。
EM真菌能通过产生胞外酶促进复杂有机质分解, 从而获取土壤中的DON, 缓解自身及植物受到的养分限制(Read & Perez-Moreno, 2003; van der Heijden et al., 2015)。不同菌根真菌酶功能的差异, 不仅影响植物的养分吸收过程, 而且调节其他土壤C、N过程。
2 丛枝菌根与外生菌根树种对土壤C、N循环的作用机制
不同菌根树种的地上和地下特性差异可影响土壤C、N的输入、稳定及输出过程, 进而影响森林土壤C、N循环。2.1 对土壤C、N输入过程的影响
地上及地下凋落物输入是矿质土层C、N的主要输入过程; 不同菌根树种对这一过程影响的研究不多, 对其机理尚不清楚(Prescott, 2010; Lin et al., 2016)。虽然凋落物层C、N输入主要取决于凋落物年产量, 但因AM、EM树种凋落物产量差异不显著, 两者凋落物层的C、N含量差异并不取决于凋落物输入数量的影响(Vesterdal et al., 2013; Lin et al., 2016), 而是受凋落物质量的影响更大。有研究认为, EM植物凋落物质量较低(C/N较高), 初期凋落物分解较慢, 会导致EM森林土壤C储量较高(Cornelissen et al., 2001; Phillips et al., 2013)。然而, 土壤有机质的核磁共振、同位素等分析结果表明, 大部分土壤有机质源于微生物及其产物, 而非难分解的植物组织(Knicker, 2011; Cotrufo et al., 2013)。根据Cotrufo等(2013)提出的微生物效率-基质稳定假说(Microbial Efficiency-Matrix Stabilization, MEMS), 凋落物质量越高, 微生物底物利用效率(SUE, del Giorgio & Cole, 1998)越高, 形成的有机质前体越多, 导致有机质含量越高(Averill, 2016)。有研究表明, AM森林土壤微生物生物量C、N显著高于EM森林, 并且它们与土壤C、N含量呈显著正相关关系(Hobbie et al., 2007; 王薪琪等, 2015; Scott & Rothstein, 2017), 证明MEMS模型可能是导致两种菌根森林类型矿质土层C、N含量差异的机制之一。土壤动物活动是凋落物及矿质土层有机质输入的又一影响因子(Hobbie et al., 2006; Mueller et al., 2015)。与EM树种相比, AM树种凋落物钙含量较高, 更能吸引蚯蚓取食, 从而能把凋落物层的有机质更多地转移至矿质土层, 增加其C、N含量(Reich et al., 2005; Vesterdal et al., 2008)。虽然关于不同菌根树种凋落物质量及其分解的研究很多, 但大多数研究并未从SUE或土壤动物活动等角度关注凋落物对矿质土层有机质输入过程的影响(Prescott, 2010), 因此尚需更多研究的验证。以往以AM和EM树种凋落物为对象的研究仍然较少, 目前还总结不出两者凋落物质量差异的一般规律。例如, 尽管EM树种有很高的多样性, 但目前研究基本局限于松科、山毛榉目等。Koele等(2012)发现, 从系统发生角度看, 同源的AM和EM植物叶片的化学组成差异不显著。由此可见, 关于AM和EM树种凋落物质量的差异及其对矿质土层C、N输入过程的贡献尚需更多的研究。
AM、EM树种的根系特性也是矿质土层C、N输入量的一个重要影响因素(Cotrufo et al., 2013; Freschet et al., 2013)。但同一立地条件下不同菌根类型树种细根生物量的比较研究不多, 且结果不一致。例如, 有研究报道AM森林地下净初级生产力是EM森林的2.6倍, 而AM对细根的贡献远大于对粗根的贡献(石兆勇等, 2012a), 前者细根生物量显著高于后者(Withington et al., 2006), 且细根周转迅速, 因而可增加有机质输入量; 也有研究表明两个菌根树种细根生物量差异并不显著(Oostra et al., 2006)。此外, 根据MEMS模型(Cotrufo et al., 2013), 细根质量的差异也可能导致有机质输入量的不同。但既有研究表明AM树种细根质量显著高于EM树种(Hobbie et al., 2007; Mueller et al., 2012), 也有研究表明二者之间差异不显著(Taylor et al., 2016)。另外, 菌根周转凋落物的输入也能够通过影响土壤自由微生物SUE调节矿质土层C、N输入过程(Clemmensen et al., 2013)。通常认为EM菌丝生物量比AM高, 因此EM森林矿质土层C、N来自菌根真菌的部分可能比AM森林高(Brzostek et al., 2015), 但目前尚未有研究比较两者的相对大小。综上所述, 不同菌根类型树种根系特性对有机质输入过程的影响不一致, 这可能与测定过程中受很多不确定性因子干扰有关(Freschet et al., 2013), 细根特性对森林土壤矿质土层C、N输入过程的影响尚需更多研究验证。
2.2 对土壤C、N稳定过程的影响
土壤有机质能够通过与铁铝矿物(铁铝氧化物、铁铝离子等)结合的物理化学方式或形成团聚体的物理方式降低其生物有效性, 从而提高其稳定性(S?rensen, 1972; Lützow et al., 2006; 刘满强等, 2007; Cotrufo et al., 2013), 最终融入土壤形成稳定的有机质, 是控制和调节土壤C、N储量的关键。然而, 不同菌根类型森林土壤C、N稳定性的研究十分有限。不同菌根类型树种可通过影响铁铝矿物的有效性及其与有机质的结合过程, 继而影响矿质土层C、N的稳定过程。Mueller等(2012)提出细根N浓度差异会造成细根凋落物N矿化和硝化差异, 从而引起质子产生和土壤酸度的不同, 进而影响铁铝矿物与有机质前体反应, 形成复杂的稳定有机质。若AM树种凋落物N含量较高, 加之其土壤硝化速率较高, 则推测AM森林土壤质子量及土壤总酸度加大, 从而促进铁铝矿物的释放, 导致更多有机质前体与铁铝矿物结合, 形成更多以物理化学形式被保护的稳定有机质。此外, 细根N浓度较高通常对应较短的根寿命; 细根死亡速率的加快也会进一步增加N矿化和硝化速率(Withington et al., 2006), 产生更多的质子, 置换出更多的铁铝矿物, 从而增加有机质前体与矿物质的结合比例, 提高土壤有机质的稳定性。土壤团聚体主要通过将微生物与有机质进行空间隔离的物理方式降低土壤有机质的周转速率, 提高土壤有机质的稳定性(Tisdall & Oades, 1982; Lützow et al., 2006; 刘满强等, 2007)。土壤团聚体大小也影响土壤有机质的稳定性。大团聚体(>250 μm)中的有机质能够保存几年, 而微团聚体(<250 μm)中的有机质则可能停留一个世纪(Rillig & Mummey, 2006; 黄艺等, 2011)。土壤团聚过程受生物(根系、菌根真菌、微生物)和非生物因子(铁铝矿物、温湿度)的影响, 其中不同菌根树种主要通过如下4个过程影响土壤团聚过程及矿质土层C、N稳定性。一是根系及菌丝通过对土壤的穿插、挤压和缠绕等物理作用促进相对稳定的土壤小团聚体形成(Six et al., 2004)。若AM根系生物量显著高于EM, 则推测前者根系通过物理作用促进土壤团聚过程的程度可能更大; 而由于EM菌丝的根状菌索(rhizomorphs)及其生命周期(平均11个月)较长于AM菌丝(5-32天), EM菌丝通过缠绕、网捕等物理过程改变土壤团聚过程的效应可能更明显。二是根系及菌丝分泌物对土壤颗粒的胶结作用也能促使土壤团聚体的形成(Rillig & Mummey, 2006)。AM与EM树种根系分泌物数量及分泌时间的差异可能会造成土壤团聚过程的不同。AM生产的球囊霉素(glomalin)相关土壤蛋白(GRSP)能够作为胶结剂直接影响土壤团聚体的形成及稳定(Tisdall & Oades, 1982; Rillig, 2004; Rillig & Mummey, 2006; Leifheit et al., 2014; 金樑等, 2016)。这主要是由于GRSP作为多糖蛋白含有大量的羟基(-OH), 能够与矿物体表面的氧原子形成氢键而把土粒团聚起来。Rillig等(2002)研究表明, GRSP对团聚体形成过程的直接作用高于AM菌丝的作用。由于菌丝分泌物对小团聚体的贡献较大, 因此GRSP影响小团聚体的形成过程对于AM生态系统土壤有机质的稳定性具有重要意义。此外, 由于GRSP周转较慢(7-42年), 累积的GRSP也是土壤有机质的重要组成部分(Rillig et al., 2003; 黄艺等, 2011; Paul, 2016)。EM真菌则可通过生产疏水蛋白影响土壤团聚过程(Rillig & Mummey, 2006)。三是真菌菌丝比细菌更能缠绕土壤颗粒, 并通过分泌多糖将土壤颗粒胶结在一起, 从而促进土壤团聚体的形成(Six et al., 2004)。由于AM森林土壤真菌生物量显著小于EM森林(Lin et al., 2016; Cheeke et al., 2017), 前者土壤微生物对土壤团聚过程的贡献可能较小。四是根系及菌根的其他相关特性(如细根的比根长及比菌丝长(即细根或菌丝长度与细根或菌丝生物量的比值)、拉伸强度、密度、抗水性能等)也能不同程度地影响土壤团聚体的形成及稳定(Rillig et al., 2015)。
总之, 有关AM真菌通过影响土壤团聚过程改变土壤C、N稳定性的报道较多, 而对EM真菌的研究报道很有限(Zheng et al., 2014)。此外, 不同菌根真菌对土壤团聚过程影响的研究大多基于草本植物培养试验或树木种苗试验, 关于这些团聚过程是否会对土壤C、N长期的稳定性产生影响尚不明确。由于森林是最复杂的生态系统, 上述菌根对土壤团聚体的影响机制是否适用, 其相对重要性以及能否在生态系统水平上对森林土壤C、N长期的稳定性产生影响等问题尚需进一步探索。
2.3 对土壤C、N输出过程的影响
不同菌根类型森林凋落物质量影响其凋落物层C、N输出过程, 即AM树种凋落物质量较高, 质量损失较快, 最终导致AM凋落物层C、N储量较低(Vesterdal et al., 2012; Lin et al., 2016; Taylor et al., 2016)。因此, AM、EM森林凋落物层C、N输出的不同是导致凋落物层C、N储量差异产生的主要原因。此外, 菌根及其与自由微生物之间的相互作用对凋落物分解过程也十分重要(Zhu & Ehrenfeld, 1996; Brzostek et al., 2015; Fernandez & Kennedy, 2016), 调节着森林土壤C、N的输出过程(Moore et al., 2015; Soudzilovskaia et al., 2015; Paterson et al., 2016)。虽然土壤C、N输出过程相互影响, 并且可能同时发生, 但两者有不同的路径和影响机制, 故分别讨论之。2.3.1 对土壤C输出过程的影响
菌根及其与自由微生物之间的相互作用对土壤C输出过程的影响有增加、减少和无效3种。增加效应主要指根际激发效应, 即菌根通过分泌活性有机质提高自由微生物活性, 促进有机质分解, 从而增加C输出(Zhu & Ehrenfeld, 1996; Cheng et al., 2012; Nottingham et al., 2013)。采用挖壕、树干环割等试验方法的很多研究在EM森林发现根际激发效应(Zhu & Ehrenfeld, 1996; Subke et al., 2010; Brzostek et al., 2015), 这可能主要是由于EM真菌需要更多的碳水化合物, 其菌根分泌物相应较高, 导致激发效应更明显(Sulman et al., 2017)。然而, 挖壕切断根系后, 死亡根系也会提高微生物分解底物, 进而提高分解速率, 干扰激发效应。此外, 由于土壤空间异质性较高, 而挖壕、环割等试验的空间尺度一般较小, 上述研究结果在空间尺度上推时还需慎重。AM土壤激发效应目前仅限于草本分室培养实验(Hodge et al., 2001; Paterson et al., 2016)或CO2浓度升高条件下的AM农作物培养试验(Cheng et al., 2012); 森林生态系统野外条件下的研究尚未发现。这可能是由于: (1) AM真菌外部菌丝生物量显著低于EM真菌, 故分泌物较少(Yin et al., 2014); (2) AM森林土壤有机质更稳定(Cotrufo et al., 2013)、更有持续性(Nottingham et al., 2013; Verbruggen et al., 2016), 故对激发效应可能不太敏感。值得注意的是, 激发效应多为有机质的短期变化, 对土壤C储量长期变化的贡献尚需进一步探索。
菌根对森林土壤C输出的减少效应主要是指菌根真菌与自由微生物的相互作用对分解过程产生了消极影响, 即加吉尔效应(“Gadgil effect”, Gadgil & Gadgil, 1971, 1975)。目前广泛认为导致加吉尔效应发生的机制是养分竞争假说, 即菌根真菌与自由微生物都需要可利用养分以供应自身的生长和繁殖, 两者的竞争关系导致了自由微生物的N限制, 从而抑制了有机质的分解过程(Orwin et al., 2011; Averill, 2016)。EM森林加吉尔效应的报道较多, 认为由于EM真菌能够分泌降解有机质的酶, 其利用N的路径比传统的N矿化路径短, 获取DON的能力使土壤C:N升高, 从而加剧了自由微生物的N限制, 抑制其活性, 从而抑制了有机质分解过程(Averill et al., 2014; Phillips et al., 2014)。EM真菌为获取N而“开发”有机质的能力造成了正反馈现象, 最终可能增加EM主导的生态系统土壤C固存(McGuire et al., 2010; Averill & Hawkes, 2016)。而关于AM抑制分解过程的证据较少, 且大多数研究对象为草本植物或农作物(Leifheit et al., 2015; Verbruggen et al., 2016); 仅Brzostek等(2015)的环割试验发现AM森林凋落物分解加速, 因而推测菌根存在时菌根与自由微生物的养分竞争抑制了分解过程。然而, 通常认为AM森林土壤N含量较高、C:N较低, 似乎不会导致土壤微生物受N限制。而Brzostek等(2015)认为, 虽然AM森林土壤N含量丰富, 但并不代表所有N都可直接被菌根真菌或自由微生物利用, 大部分N可通过淋溶损失, 因此AM真菌与自由微生物仍然可能存在对可利用养分的竞争。尽管养分竞争假说被广泛用于推测解释加吉尔效应, 但直接验证该假说的野外实验很少, 多数研究只采用微观室内培养实验, 而且选用的菌根真菌常为某一特定真菌, 并不能代表野外条件下的真菌群落。例如, 微观实验采用的形成根状菌索(cord-forming)的EM真菌比能生产扩散菌丝的真菌更具有侵略性, 从而可能高估竞争效应(Fernandez & Kennedy, 2016)。
关于菌根抑制分解过程的假说, 除了养分竞争假说之外, 还有化学抑制、菌寄生、水分限制假说。化学抑制假说是指与自由微生物相比, 菌根真菌从寄主根系获取C资源, 受C限制的程度较小, 因此可能会产生更多的次级代谢产物, 以限制自由微生物的活性(Werner et al., 2002; Keller et al., 2005)。菌寄生(mycoparasitism)假说认为菌根真菌为获取养分, 可直接寄生到自由微生物上, 从而抑制后者活性(Mucha et al., 2006)。以上两种假说均在室内培养的EM真菌试验中得到证实, 但这种效应在生态系统水平上是否能够对凋落物或土壤有机质分解产生影响尚不清楚(Fernandez & Kennedy, 2016)。水分限制假说认为, 由于水分是分解过程的主要限制因子(Holden et al., 2015), 菌根真菌及其共生体根系吸收大量的土壤水分, 从而可能降低自由微生物的分解能力(Koide & Wu, 2003)。但也有研究指出, 去除菌根后水分可利用性的提升是挖壕实验干扰造成的。由此可见, 不同菌根真菌与自由微生物相互作用抑制土壤C输出的机理尚需进一步验证。
菌根的存在也可能对森林土壤C输出无效应, 这就是生态位分化假说(Lindahl et al., 2007)。该假说认为, 菌根真菌与自由微生物分别占据土壤剖面的不同位置, 因此菌根真菌与微生物不存在明显的相互作用, 故不会对有机质分解过程产生显著影响(Baldrian et al., 2012)。该假说可从菌根真菌与自由微生物酶功能差异的角度进行阐释。在凋落物层, 来自于地上输入的活性底物浓度较高, 微生物需要产生降解细胞壁的水解酶对其进行利用; 而在较深土层活性底物浓度较低, 难分解的木质素和腐殖质底物较多, 因此微生物需要产生氧化酶才能加以利用(Lindahl et al., 2007)。自由微生物通常被认为主要生产水解酶且生活在凋落物层, 而EM真菌主要占据腐殖质层和矿质土层等较深土层(Lindahl & Tunlid, 2015), 分泌氧化酶是为了获取相对难分解土壤有机质中的N而非C (Phillips et al., 2014)。由此我们推测EM真菌生产氧化酶的能力可能是生态位分化的结果, 从而反证了EM真菌可能与自由微生物不存在明显的相互作用。但值得注意的是, AM真菌不具备生产获取有机N的氧化酶的能力, 因此该生态位分化假说可能不适用于AM森林生态系统。
综上所述, 菌根对土壤C输出过程的净效应取决于加吉尔效应与激发效应的相对大小。根据AM、EM森林土壤C储量的差异, 我们推测AM树种较高的凋落物质量提高了凋落物分解速率, 导致其凋落物层C储量较低, 但较高的细根生物量和凋落物(凋落叶、细根)质量通过提高微生物SUE增加了矿质土层的C储量及其稳定性; 而EM树种凋落物质量较低, 加之EM真菌能够抑制自由微生物活性从而降低凋落物分解速率, 最终造成凋落物层C储量较高, 但来自自由微生物及细根的C较少, 并且EM真菌对矿质土层存在激发效应, 可能造成了EM森林矿质土层C储量较少。当然, 森林土壤C累积是较长时间尺度(数十年或百年)的过程, 仅通过数月的研究结果进行推测须谨慎。
2.3.2 对土壤N输出过程的影响
菌根真菌功能及其共生植物凋落物差异是造成不同菌根类型森林土壤溶解性无机N (铵盐、硝酸盐)及DON淋溶损失不同的主要原因(Midgley & Phillips, 2014; Scott & Rothstein, 2017)。EM真菌能够有效地吸收DON, 一方面避免了N进一步矿化甚至以无机N的形式淋溶损失; 另一方面导致自由微生物受N限制程度加剧, 提高自由微生物对无机N的吸收效率, 进而减少无机N的淋溶损失。与AM树种相比, EM树种的凋落物更富含次级代谢产物(Kraus et al., 2003), 这些次级代谢产物将N固定在酚类化合物中或吸附更多的N, 从而可间接地抑制凋落物的硝化过程, 但这一过程还需更多试验验证。
EM森林土壤C:N高于AM森林, 可能会进一步抑制无机N的淋溶损失(Scott & Rothstein, 2017)。土壤C:N通常与矿化和硝化速率显著负相关, 因此可用于预测土壤无机N淋溶速率, 但菌根类型更容易获得, 也许能作为预测无机N淋溶的更优指标。土壤C:N与不同菌根类型树种特性相互联系, 因此很难将不同菌根树种与土壤性质对无机N淋溶的效应区别开来。
3 丛枝菌根与外生菌根森林土壤C、N循环对全球CO2浓度升高和N沉降增加的响应
植物对菌根真菌的碳分配策略及土壤养分对菌根真菌的限制与全球变化密切相关, 尤其是全球CO2浓度升高及N沉降格局的改变等全球性的C、N变化。CO2浓度升高可提高森林生产力, 从而提高植物对菌根真菌的C分配比例; 大气N沉降增加可提高土壤肥力, 减缓菌根真菌及自由微生物受养分限制的程度; 不同菌根真菌对它们响应不一(Treseder & Allen, 2000; Treseder, 2004), 进而对森林土壤C、N循环产生不同的影响。不少研究报道了AM、EM森林生产力对全球变化的响应(Thomas et al., 2010; Terrer et al., 2016), 但关于这些响应如何影响土壤C、N循环的研究(Midgley & Phillips, 2014; Midgley et al., 2015)却很少, 且有很大的不确定性。3.1 对CO2浓度升高的响应
CO2浓度升高引起AM和EM森林地上、地下生物量分配策略及其土壤有机质稳定性的差异会影响AM和EM森林土壤C、N循环(Treseder, 2004; Drake et al., 2011; Terrer et al., 2016)。美国橡树岭AM森林(枫香(Liquidambar styraciflua)林)实验表明, CO2浓度升高主要使来自细根的土壤颗粒有机质含量增加, 从而显著增加土壤有机质含量(Jastrow et al., 2005; Iversen et al., 2012); 但可能降低微生物生物量而使铵态N等速效养分未发生显著变化。这说明虽然CO2浓度升高促进地下细根生长, 但没有缓解植物受养分限制的程度, 进而影响森林生产力(Iversen et al., 2012)。虽有研究表明CO2浓度升高促进了AM农作物有机质的分解速率(Cheng et al., 2012), 但AM森林(枫香林)实验表明, CO2浓度升高对土壤呼吸速率影响并不显著, 也未出现显著的激发效应(Iversen et al., 2012)。这可能是由于AM森林矿质土壤有机质以团聚体或有机质与矿物质结合体的形态存在, 稳定性较高, 微生物难以接触利用的缘故(Jastrow et al., 2005; Iversen et al., 2012)。EM森林土壤C、N循环过程对CO2浓度升高的响应与AM不同。CO2浓度升高导致EM森林土壤有机质含量降低(Phillips et al., 2012; Talhelm et al., 2014)。这主要是由于EM森林增加地下C分配比例, 提高来自根系的土壤有机质输入量, 同时提高微生物活性、促进土壤有机质分解(Drake et al., 2011; Phillips et al., 2011, 2012; Talhelm et al., 2014), 从而减少了土壤有机质含量。
不同菌根类型森林土壤C、N循环对CO2浓度升高响应会影响植物养分限制程度, 最终造成森林生产力对气候变化的响应不同(Phillips et al., 2011; Terrer et al., 2016, 2017)。Terrer等(2016)整合分析全球AM、EM生态系统数据发现, 菌根与可利用N的相互作用解释了CO2浓度升高对不同生态系统生产力的施肥效应。CO2浓度升高显著提高了AM森林对铵态N的吸收(Zerihun & Bassirirad, 2001), 但其土壤有机质却以物理或化学形式被保护起来, 使微生物难以接触利用(Iversen et al., 2012; Mueller et al., 2012), 因此AM生态系统在土壤可利用N含量较低时可能不足以维持其生物量的增长(Terrer et al., 2016)。而EM真菌能够通过生产胞外酶获取复杂有机质中的N以满足植物的养分需求, 因此EM森林在CO2浓度升高条件下能够维持生物量的增长(Terrer et al., 2017)。但Norby等(2017)对这一结论提出质疑, 认为EM森林生物量对CO2浓度升高的积极响应是由于根系激发效应提高了N的可利用性, 缓解了植物生长的渐进式氮限制, 并不只是EM真菌的作用(Drake et al., 2011; Phillips et al., 2011; Hasegawa et al., 2016), 有些EM真菌通过多次进化后不再具备通过产生胞外酶分解复杂有机质的遗传潜力(Pellitier & Zak, 2017)。由此可见, 不同菌根类型森林对CO2施肥效应的响应机理尚不确定。深入理解不同菌根类型森林土壤C、N循环过程对CO2浓度升高的响应, 有利于评价预测森林生产力对全球变化的响应。
3.2 对N沉降增加的响应
大气N沉降改变土壤养分状况, 影响菌根真菌及自由微生物的活性及群落结构, 进而调节土壤C、N循环过程(Treseder, 2004; Midgley et al., 2015)。不同菌根类型的森林土壤养分对植物及微生物的限制程度不同, 因而会造成土壤C、N循环对N沉降响应不同。N添加实验通常加大AM森林凋落物、土壤有机质的分解速率(Midgley et al., 2015), 这可能是因为可利用N的增加提高了微生物的养分利用效率(Manzoni et al., 2012), 缓解了生产胞外酶时微生物受N限制的程度(Carreiro et al., 2000), 从而提高了土壤C、N的循环速率。此外, 由于AM森林土壤自由微生物的N限制程度比EM森林小(Phillips et al., 2013), N添加促进AM森林土壤硝酸盐淋溶的程度大于EM森林(Midgley & Phillips, 2014), 导致更多的N以淋溶方式损失。而N添加通常使EM森林土壤有机质分解速率、土壤呼吸速率减小(Janssens et al., 2010)或者影响不显著(Midgley et al., 2015)。这可能是EM真菌在N添加试验中生长和繁殖降低(Avis et al., 2003; Treseder, 2004)、酶活性受到抑制造成的(B?deker et al., 2014); 也可能是由于EM森林凋落物及有机质C:N过高, 导致其分解过程对土壤养分的变化不敏感(Midgley et al., 2015)。综上所述, 表现为无机养分经济(土壤C、N矿化速率较快)的AM森林可能更能适应N沉降增加的改变, 进而提高森林生产力; 而表现为有机养分经济(土壤C、N矿化速率较慢)的EM森林对N沉降增加的响应并不积极, 因此可能会造成树木死亡或者生产力降低(Thomas et al., 2010; Midgley & Phillips, 2016)。值得注意的是, N沉降增加对土壤C、N循环过程的影响存在短期应激反应和长期适应的过程, 这些过程对不同时间尺度的N添加如何响应, 尚需长期试验数据的支持。
4 展望
全面理解不同菌根类型森林土壤C、N循环的差异及其作用机制, 是森林生态学领域值得关注的重要命题。将菌根真菌类型作为树木的重要功能特性, 可以加深对生物地球化学循环中地上与地下过程关系的理解, 并对全球变化背景下树种分布发生改变时可能对生物地球化学循环产生的影响进行合理预测。该领域亟待解决的主要挑战有以下4个方面:一是全面比较研究不同菌根类型森林土壤C、N循环及其相关联的生态系统结构和功能特征, 为提高森林生产力、发挥生态系统服务功能提供理论基础和数据。以往室内培养试验大多仅比较单一菌根真菌存在和不存在的情况对土壤C、N循环的影响, 而未对AM、EM真菌分别存在时的状况进行比对, 因此难以得出不同菌根真菌对土壤C、N循环影响机制的普适性结论。在人工林中, 杨树、松树等EM树种作为速生树种得到大面积种植, 对其人工林的生产力及土壤C、N循环研究较多; 而AM人工林较少,研究也很有限。在AM和EM树种共存的天然林生态系统中, 探究不同菌根树种相对丰度对土壤C、N循环的作用, 对于评价预测森林生态系统生物地球化学循环过程乃至生态功能具有重要的理论和实践意义, 但如何分离、量化不同菌根相互作用对单一菌根作用的干扰仍有难度。
二是深入认知不同菌根树种地上凋落物及地下菌根与自由微生物间相互作用对土壤C、N循环的影响, 以阐明不同菌根类型森林土壤C、N循环的潜在机制。虽然不同菌根类型凋落物质量及其分解的研究很多, 但大多数研究并未从微生物底物利用效率角度关注凋落物对土壤有机质输入过程的影响, 基于微生物底物利用效率的有机质形成假说尚需进一步验证。此外, 虽然越来越多的研究关注菌根-自由微生物相互作用对土壤C、N循环的影响, 但该相互作用对土壤C、N储量的效应方向及强度说法不一, 其中的影响机制尚需系统探究。
三是改进研究方法, 应用新研究手段, 充分考虑时空尺度效应, 以便能用小尺度的研究结果合理地解释和预测生态系统C、N循环。目前有关不同菌根类型树种对土壤C、N循环的研究方法存在一定的干扰效应。随着稳定同位素探针技术、有机质组分分组技术、核磁共振、光谱分析技术等土壤原位和非破坏性分析技术的应用, 能够有效地去除或降低干扰效应, 补充完善不同菌根类型土壤C、N的运输途径, 从不同时间和空间尺度阐明不同菌根类型森林土壤C、N代谢和循环过程。
四是加强不同菌根类型森林土壤C、N稳定性差异的研究, 以准确评价森林生态系统结构和功能对全球变化的响应。AM森林土壤有机质的稳定性主要取决于物理化学或物理保护机制, 而EM森林土壤有机质的稳定性可归因于菌根真菌对自由微生物的抑制作用, 二者不同的稳定机制对全球CO2浓度升高及N沉降增加的响应不同, 对全球气温升高、降水格局改变的响应也可能存在差异, 从而可能对森林生态系统结构和功能产生不同的影响, 因此亟需对不同菌根类型土壤有机质稳定性的定量认知。
The authors have declared that no competing interests exist.
作者声明没有竞争性利益冲突.
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
| [1] | Arbuscular mycorrhizal fungi (AMF) can increase plant growth and nutrition. However, their capacity to reduce the leaching of nutrients through the soil profile is less well understood. Here we present results of an experiment in which the effects of forming arbuscular mycorrhizas (AM) on plant growth and nutrition, nutrient depletion from soil, and nutrient leaching, were investigated in microcosms containing the grass Phalaris aquatica L. Mycorrhizal and non-mycorrhizal plants were grown in a mixture of riparian soil and sand under glasshouse conditions. The formation of AM by P. aquatica significantly increased plant growth and nutrient uptake. Lower levels of NO6163, NH6262 and plant available P in both soil and leachate were observed in columns containing mycorrhizal root systems. These differences in nutrient interception were proportionally greater than the increase in root biomass of the mycorrhizal plants, compared with their non-mycorrhizal counterparts. Taken together, these data indicate that mycorrhizal root systems have an important, but previously little considered, role to play reducing the net loss of nutrients via leaching. |
| [2] | Plants have numerous impacts on biogeochemical cycling across both aquatic and terrestrial ecosystems. These effects extend well beyond the critical role of carbon (C) fixation through photosynthesis that provides the basis for ecosystem energy flow. While foliar and root traits of senescent plant material (litter) have been explored in detail in terrestrial ecosystems, there is a resurgence of interest in how plants modulate biogeochemical cycling in ways other than litter quality effects on C and nutrient mineralization. This Special Feature represents a collection of resh perspectives on how plants alone, or in interaction with other organisms, have important and lasting impacts on biogeochemical cycles of C and nutrients in a range of terrestrial and aquatic environments. We begin in the open ocean and then peer from the forest edge before moving into forest understoreys and grasslands to examine the control by live terrestrial plants on ecosystem C and nutrient cycling. Plants directly affect biogeochemical cycling while living through their diversity and composition, nutrient capture and strategies for assimilating C, and by altering the microclimate for decomposition. In addition, how they construct their tissues and alter the abiotic environment has large impacts on the turnover of C and nutrients once plants have senesced or died. From the direct impact of plants, we move onto the influence of plant nsect interactions, which effectively determine changes in plant stoichiometry in grasslands of varying diversity. Finally, looking directly in the soil, it is clear that plant ycorrhizae interactions are important in modulating the response of litter decomposition to nutrient addition and the nature of C metabolism in the soil. Synthesis. The papers here highlight careful matching between how plants live and their biotic and abiotic contexts. Taken together, it appears that the dynamic, rather than passive, nature of plant responses to variable environments is key in affecting ecosystem level processes of C and nutrient turnover. This Special Feature highlights a diversity of connections between plants and their environment and demonstrates that in both life and death, how plants respond to these changes differs among plant lineages and this diversity will play a central role in determining biogeochemical cycling in the future in aquatic and terrestrial ecosystems. |
| [3] | Ecosystems dominated by plants in symbiosis with ectomycorrhizal fungi store more carbon in soils. There is increasing evidence that this may be due to competition between primary producers and microbial decomposers for soil nitrogen, mediated by ectomycorrhizal fungi. This competitive interaction inhibits decomposition and increases soil carbon storage. However, other work suggests elevated carbon storage is due to recalcitrant plant tissue chemistry in ectomycorrhizal ecosystems, rather than ectomycorrhizal competition for soil nitrogen. These two frameworks make similar predictions for soil carbon storage, making them difficult to distinguish empirically. Here I argue that the ectomycorrhizal-recalcitrance hypothesis is not well supported by recent developments in the understanding of soil carbon chemistry, or evolutionary relationships among ectomycorrhizal plants. Therefore, differences in input chemistry are not sufficient to discount alternative mechanisms of carbon stabilization in ectomycorrhizal ecosystems. Future work on EM-specific stabilization of soil C should focus on alternative mechanisms including competition for N, direct antagonistic interactions, and other microbial community driven mechanisms. |
| [4] | Abstract Respiration of soil organic carbon is one of the largest fluxes of CO2 on earth. Understanding the processes that regulate soil respiration is critical for predicting future climate. Recent work has suggested that soil carbon respiration may be reduced by competition for nitrogen between symbiotic ectomycorrhizal fungi that associate with plant roots and free-living microbial decomposers, which is consistent with increased soil carbon storage in ectomycorrhizal ecosystems globally. However, experimental tests of the mycorrhizal competition hypothesis are lacking. Here we show that ectomycorrhizal roots and hyphae decrease soil carbon respiration rates by up to 67% under field conditions in two separate field exclusion experiments, and this likely occurs via competition for soil nitrogen, an effect larger than 2 掳C soil warming. These findings support mycorrhizal competition for nitrogen as an independent driver of soil carbon balance and demonstrate the need to understand microbial community interactions to predict ecosystem feedbacks to global climate. |
| [5] | Soil contains more carbon than the atmosphere and vegetation combined. Understanding the mechanisms controlling the accumulation and stability of soil carbon is critical to predicting the Earth's future climate. Recent studies suggest that decomposition of soil organic matter is often limited by nitrogen availability to microbes and that plants, via their fungal symbionts, compete directly with free-living decomposers for nitrogen. Ectomycorrhizal and ericoid mycorrhizal (EEM) fungi produce nitrogen-degrading enzymes, allowing them greater access to organic nitrogen sources than arbuscular mycorrhizal (AM) fungi. This leads to the theoretical prediction that soil carbon storage is greater in ecosystems dominated by EEM fungi than in those dominated by AM fungi. Using global data sets, we show that soil in ecosystems dominated by EEM-associated plants contains 70% more carbon per unit nitrogen than soil in ecosystems dominated by AM-associated plants. The effect of mycorrhizal type on soil carbon is independent of, and of far larger consequence than, the effects of net primary production, temperature, precipitation and soil clay content. Hence the effect of mycorrhizal type on soil carbon content holds at the global scale. This finding links the functional traits of mycorrhizal fungi to carbon storage at ecosystem-to-global scales, suggesting that plant-decomposer competition for nutrients exerts a fundamental control over the terrestrial carbon cycle. |
| [6] | Summary 6168 Here we examine the effects of increased nitrogen (N) supply on the ectomycorrhizal fungal communities of a temperate oak savanna. 6168 In a 16-yr N-addition experiment in which replicate 1000m 2 plots received 0, 5.4 or 17gNm 612 yr 611 , ectomycorrhizal sporocarp production was measured in the 14th, 15th and 16th year of fertilization. Ectomycorrhizal fungi (EMF) colonizing roots were examined by morphotyping-PCR-RFLP and sequence analysis in the 14th and 15th year of fertilization. 6168 Total sporocarp richness was reduced by >50% in both fertilization treatments in all 3yrs, whereas Russula spp. produced approx. five times more sporocarps with 17gNm 612 yr 611 . Below-ground, treatment-scale species richness and species area curves were lower with 17gNm 612 yr 611 but richness, diversity indices and evenness at smaller spatial scales were not. Dominant fungi colonizing roots included Cenococcum geophilum , common in all treatments, Cortinarius spp., dominant in unfertilized plots, and Russula spp., dominant with 17gNm 612 yr 611 . 6168 Communities of EMF in this temperate deciduous ecosystem responded to N addition similarly to those of coniferous ecosystems in that increased N supply altered EMF diversity and community composition but differently in that dominance of Russula spp. increased. |
| [7] | Abstract Soils of coniferous forest ecosystems are important for the global carbon cycle, and the identification of active microbial decomposers is essential for understanding organic matter transformation in these ecosystems. By the independent analysis of DNA and RNA, whole communities of bacteria and fungi and its active members were compared in topsoil of a Picea abies forest during a period of organic matter decomposition. Fungi quantitatively dominate the microbial community in the litter horizon, while the organic horizon shows comparable amount of fungal and bacterial biomasses. Active microbial populations obtained by RNA analysis exhibit similar diversity as DNA-derived populations, but significantly differ in the composition of microbial taxa. Several highly active taxa, especially fungal ones, show low abundance or even absence in the DNA pool. Bacteria and especially fungi are often distinctly associated with a particular soil horizon. Fungal communities are less even than bacterial ones and show higher relative abundances of dominant species. While dominant bacterial species are distributed across the studied ecosystem, distribution of dominant fungi is often spatially restricted as they are only recovered at some locations. The sequences of cbhI gene encoding for cellobiohydrolase (exocellulase), an essential enzyme for cellulose decomposition, were compared in soil metagenome and metatranscriptome and assigned to their producers. Litter horizon exhibits higher diversity and higher proportion of expressed sequences than organic horizon. Cellulose decomposition is mediated by highly diverse fungal populations largely distinct between soil horizons. The results indicate that low-abundance species make an important contribution to decomposition processes in soils. |
| [8] | Ecologists are increasingly adopting trait-based approaches to understand how community change influences ecosystem processes. However, most of this research has focussed on aboveground plant traits, whereas it is becoming clear that root traits are important drivers of many ecosystem processes, such as carbon (C) and nutrient cycling, and the formation and structural stability of soil. Here, we synthesise emerging evidence that illustrates how root traits impact ecosystem processes, and propose a pathway to unravel the complex roles of root traits in driving ecosystem processes and their response to global change. Finally, we identify research challenges and novel technologies to address them. |
| [9] | |
| [10] | |
| [11] | Summary Although it is increasingly being recognized that roots play a key role in soil carbon (C) dynamics, the magnitude and direction of these effects are unknown. Roots can accelerate soil C losses by provisioning microbes with energy to decompose organic matter or impede soil C losses by enhancing microbial competition for nutrients. We experimentally reduced belowground C supply to soils via tree girdling, and contrasted responses in control and girdled plots for three consecutive growing seasons. We hypothesized that decreases in belowground C supply would have stronger effects in plots dominated by ectomycorrhizal (ECM) trees rather than arbuscular mycorrhizal (AM) trees. In ECM-dominated plots, girdling decreased the activity of enzymes that break down soil organic matter (SOM) by c . 40%, indicating that, in control plots, C supply from ECM roots primes microbial decomposition. In AM-dominated plots, girdling had little effect on SOM-degrading enzymes, but increased the decomposition of AM leaf litter by c . 43%, suggesting that, in control plots, AM roots may intensify microbial competition for nutrients. Our findings indicate that root-induced changes in soil processes depend on forest composition, and that shifts in the distribution of AM and ECM trees owing to climate change may determine soil C gains and losses. |
| [12] | |
| [13] | , |
| [14] | Abstract While it is well established that plants associating with arbuscular mycorrhizal (AM) and ectomycorrhizal (ECM) fungi cycle carbon (C) and nutrients in distinct ways, we have a limited understanding of whether varying abundance of ECM and AM plants in a stand can provide integrative proxies for key biogeochemical processes. We explored linkages between the relative abundance of AM and ECM trees and microbial functioning in three hardwood forests in southern Indiana, USA. Across each site's ycorrhizal gradient , we measured fungal biomass, fungal : bacterial (F : B) ratios, extracellular enzyme activities, soil carbon : nitrogen ratio, and soil pH over a growing season. We show that the percentage of AM or ECM trees in a plot promotes microbial communities that both reflect and determine the C to nutrient balance in soil. Soils dominated by ECM trees had higher F : B ratios and more standing fungal biomass than AM stands. Enzyme stoichiometry in ECM soils shifted to higher investment in extracellular enzymes needed for nitrogen and phosphorus acquisition than in C-acquisition enzymes, relative to AM soils. Our results suggest that knowledge of mycorrhizal dominance at the stand or landscape scale may provide a unifying framework for linking plant and microbial community dynamics, and predicting their effects on ecological function. |
| [15] | The extent to which terrestrial ecosystems can sequester carbon to mitigate climate change is a matter of debate. The stimulation of arbuscular mycorrhizal fungi (AMF) by elevated atmospheric carbon dioxide (CO(2)) has been assumed to be a major mechanism facilitating soil carbon sequestration by increasing carbon inputs to soil and by protecting organic carbon from decomposition via aggregation. We present evidence from four independent microcosm and field experiments demonstrating that CO(2) enhancement of AMF results in considerable soil carbon losses. Our findings challenge the assumption that AMF protect against degradation of organic carbon in soil and raise questions about the current prediction of terrestrial ecosystem carbon balance under future climate-change scenarios. |
| [16] | Boreal forest soils function as a terrestrial net sink in the global carbon cycle. The prevailing dogma has focused on aboveground plant litter as a principal source of soil organic matter. Using C-14 bomb-carbon modeling, we show that 50 to 70% of stored carbon in a chronosequence of boreal forested islands derives from roots and root-associated microorganisms. Fungal biomarkers indicate impaired degradation and preservation of fungal residues in late successional forests. Furthermore, 454 pyrosequencing of molecular barcodes, in conjunction with stable isotope analyses, highlights root-associated fungi as important regulators of ecosystem carbon dynamics. Our results suggest an alternative mechanism for the accumulation of organic matter in boreal forests during succession in the long-term absence of disturbance. |
| [17] | Ecosystem carbon cycling depends strongly on the productivity of plant species and the decomposition rates of the litter they produce. We tested the hypothesis that classifying plant functional types according to mycorrhizal association explains important interspecific variation in plant carbon cycling traits, particularly in those traits that feature in a hypothesized feedback between vegetation productivity and litter turnover. We compared data from standardized 'screening' tests on inherent potential seedling relative growth rate (RGR), foliar nutrient concentrations, and leaf litter decomposability among 83 British plant species of known mycorrhizal type. There was important variation in these parameters between mycorrhizal plant types. Plant species with ericoid mycorrhiza showed consistently low inherent RGR, low foliar N and P concentrations, and poor litter decomposability; plant species with ectomycorrhiza had an intermediate RGR, higher foliar N and P, and intermediate to poor litter decomposability; plant species with arbuscular-mycorrhiza showed comparatively high RGR, high foliar N and P, and fast litter decomposition. Within the woody species subset, differentiation in RGR between mycorrhizal types was mostly confounded with deciduous versus evergreen habit, but the overall differentiation in litter mass loss between mycorrhizal types remained strong within each leaf habit. These results indicate that, within a representative subset of a temperate flora, ericoid and ectomycorrhizal strategies are linked with low and arbuscular-mycorrhizal species with high ecosystem carbon turnover. The incorporation of mycorrhizal association into current functional type classifications is a valuable tool in the assessment of plant-mediated controls on carbon and nutrient cycling. |
| [18] | |
| [19] | |
| [20] | |
| [21] | Abstract In forest ecosystems, ectomycorrhizal and saprotrophic fungi play a central role in the breakdown of soil organic matter (SOM). Competition between these two fungal guilds has long been hypothesized to lead to suppression of decomposition rates, a phenomenon known as the 'Gadgil effect'. In this review, we examine the documentation, generality, and potential mechanisms involved in the 'Gadgil effect'. We find that the influence of ectomycorrhizal fungi on litter and SOM decomposition is much more variable than previously recognized. To explain the inconsistency in size and direction of the 'Gadgil effect', we argue that a better understanding of underlying mechanisms is required. We discuss the strengths and weaknesses of each of the primary mechanisms proposed to date and how using different experimental methods (trenching, girdling, microcosms), as well as considering different temporal and spatial scales, could influence the conclusions drawn about this phenomenon. Finally, we suggest that combining new research tools such as high-throughput sequencing with experiments utilizing natural environmental gradients will significantly deepen our understanding of the 'Gadgil effect' and its consequences on forest soil carbon and nutrient cycling. 2015 The Authors. New Phytologist 2015 New Phytologist Trust. |
| [22] | Conceptual frameworks relating plant traits to ecosystem processes such as organic matter dynamics are progressively moving from a leaf-centred to a whole-plant perspective. Through the use of meta-analysis and global literature data, we quantified the relative roles of litters from above- and below-ground plant organs in ecosystem labile organic matter dynamics.We found that decomposition rates of leaves, fine roots and fine stems were coordinated across species worldwide although less strongly within ecosystems. We also show that fine roots and stems had lower decomposition rates relative to leaves, with large differences between woody and herbaceous species. Further, we estimated that on average below-ground litter represents approximately 33 and 48% of annual litter inputs in grasslands and forests, respectively.These results suggest a major role for below-ground litter as a driver of ecosystem organic matter dynamics. We also suggest that, given that fine stem and fine root litters decompose approximately 1.5 and 2.8 times slower, respectively, than leaf litter derived from the same species, cycling of labile organic matter is likely to be much slower than predicted by data from leaf litter decomposition only.Synthesis. Our results provide evidence that within ecosystems, the relative inputs of above- versus below-ground litter strongly control the overall quality of the litter entering the decomposition system. This in turn determines soil labile organic matter dynamics and associated nutrient release in the ecosystem, which potentially feeds back to the mineral nutrition of plants and therefore plant trait values and plant community composition. |
| [23] | react-text: 515 Ecosystems dominated by plants in symbiosis with ectomycorrhizal fungi store more carbon in soils. There is increasing evidence that this may be due to competition between primary producers and microbial decomposers for soil nitrogen, mediated by ectomycorrhizal fungi. This competitive interaction inhibits decomposition and increases soil carbon storage. However, other work suggests elevated... /react-text react-text: 516 /react-text [Show full abstract] |
| [24] | |
| [25] | |
| [26] | Abstract Free-air CO2 enrichment (FACE) experiments have demonstrated increased plant productivity in response to elevated (e)CO2, with the magnitude of responses related to soil nutrient status. Whilst understanding nutrient constraints on productivity responses to eCO2 is crucial for predicting carbon uptake and storage, very little is known about how eCO2 affects nutrient cycling in phosphorus (P)-limited ecosystems. Our study investigates eCO2 effects on soil N and P dynamics at the EucFACE experiment in Western Sydney over an 18-month period. Three ambient and three eCO2 (+150 ppm) FACE rings were installed in a P-limited, mature Cumberland Plain Eucalyptus woodland. Levels of plant accessible nutrients, evaluated using ion exchange resins, were increased under eCO2, compared to ambient, for nitrate (+93%), ammonium (+12%) and phosphate (+54%). There was a strong seasonality to responses, particularly for phosphate, resulting in a relatively greater stimulation in available P, compared to N, under eCO2 in spring and summer. eCO2 was also associated with faster nutrient turnover rates in the first six months of the experiment, with higher N (+175%) and P (+211%) mineralization rates compared to ambient rings, although this difference did not persist. Seasonally dependant effects of eCO2 were seen for concentrations of dissolved organic carbon in soil solution (+31%), and there was also a reduction in bulk soil pH (-0.18 units) observed under eCO2. These results demonstrate that CO2 fertilization increases nutrient availability particularly for phosphate in P-limited soils, likely via increased plant belowground investment in labile carbon and associated enhancement of microbial turnover of organic matter and mobilization of chemically bound P. Early evidence suggests that there is the potential for the observed increases in P availability to support increased ecosystem C-accumulation under future predicted CO2 concentrations. |
| [27] | |
| [28] | We studied the influence of tree species on soil carbon and nitrogen (N) dynamics in a common garden of replicated monocultures of fourteen angiosperm and gymnosperm, broadleaf and needleleaf species in southwestern Poland. We hypothesized that species would influence soil organic matter (SOM) decomposition primarily via effects on biogeochemical recalcitrance, with species having tissues with high lignin concentrations retarding rates of decomposition in the O and A horizons. Additionally, because prior work demonstrated substantial divergence in foliar and soil base cation concentrations and soil pH among species, we hypothesized that species would influence chemical stabilization of SOM via cation bridging to mineral surfaces in the A-horizon. Our hypotheses were only partially supported: SOM decomposition and microbial biomass were unrelated to plant tissue lignin concentrations, but in the mineral horizon, were significantly negatively related to the percentage of the cation exchange complex (CEC) occupied by polyvalent acidic (hydrolyzing) cations (Al and Fe), likely because these cations stabilize SOM via cation bridging and flocculation and/or because of inhibitory effects of Al or low pH on decomposers. Percent CEC occupied by exchangeable Al and Fe was in turn related to both soil clay content (a parent material characteristic) and root Ca concentrations (a species characteristic). In contrast, species influenced soil N dynamics largely via variation in tissue N concentration. In both laboratory and in situ assays, species having high-N roots exhibited faster rates of net N mineralization and nitrification. Nitrification:mineralization ratios were greater, though, under species with high exchangeable soil Ca2+. Our results indicate that tree species contribute to variation in SOM dynamics, even in the mineral soil horizons. To our knowledge the influence of tree species on SOM decomposition via cation biogeochemistry has not been demonstrated previously, but could be important in other poorly buffered systems dominated by tree species that differ in cation nutrition or that are influenced by acidic deposition. |
| [29] | |
| [30] | Abstract Arbuscular mycorrhizal fungi (order Glomales), which form mycorrhizal symbioses with two out of three of all plant species, are believed to be obligate biotrophs that are wholly dependent on the plant partner for their carbon supply. It is thought that they possess no degradative capability and that they are unable to decompose complex organic molecules, the form in which most soil nutrients occur. Earlier suggestions that they could exist saprotrophically were based on observation of hyphal proliferation on organic materials. In contrast, other mycorrhizal types have been shown to acquire nitrogen directly from organic sources. Here we show that the arbuscular mycorrhizal symbiosis can both enhance decomposition of and increase nitrogen capture from complex organic material (grass leaves) in soil. Hyphal growth of the fungal partner was increased in the presence of the organic material, independently of the host plant. |
| [31] | Despite their large role in ecosystems and plant nutrition, our knowledge of the nutritional ecology of the fungi involved in the arbuscular mycorrhizal symbiosis, the Glomeromycota, is poor. We briefly describe the mechanisms that underlie the fluxes of the three major elements (C, N and P) and outline a model for the interchange of these between the partners. This model is consistent with data from physiological, ecological and taxonomic studies and allows a new and necessary focus on the nutritional requirements of the fungus itself, separately from its role in the symbiosis. There is an urgent need for new studies to identify the sources of nutrients such as N and P that AM fungi (AMF) use for their own growth and to elucidate the mechanisms that control the transfer of these to the plant in relation to fungal demand. |
| [32] | Arbuscular mycorrhizal fungi (AMF) form mutualistic symbioses with c. two-thirds of all land plants. Traditionally, it was thought that they played no role in nitrogen (N) acquisition for their host, |
| [33] | Climate warming is projected to increase the frequency and severity of wildfires in boreal forests, and increased wildfire activity may alter the large soil carbon (C) stocks in boreal forests. Changes in boreal soil C stocks that result from increased wildfire activity will be regulated in part by the response of microbial decomposition to fire, but post-fire changes in microbial decomposition are poorly understood. Here, we investigate the response of microbial decomposition to a boreal forest fire in interior Alaska and test the mechanisms that control post-fire changes in microbial decomposition. We used a reciprocal transplant between a recently burned boreal forest stand and a late successional boreal forest stand to test how post-fire changes in abiotic conditions, soil organic matter (SOM) composition, and soil microbial communities influence microbial decomposition. We found that SOM decomposing at the burned site lost 30.9% less mass over two years than SOM decomposing at the unburned site, indicating that post-fire changes in abiotic conditions suppress microbial decomposition. Our results suggest that moisture availability is one abiotic factor that constrains microbial decomposition in recently burned forests. In addition, we observed that burned SOM decomposed more slowly than unburned SOM, but the exact nature of SOM changes in the recently burned stand are unclear. Finally, we found no evidence that post-fire changes in soil microbial community composition significantly affect decomposition. Taken together, our study has demonstrated that boreal forest fires can suppress microbial decomposition due to post-fire changes in abiotic factors and the composition of SOM. Models that predict the consequences of increased wildfires for C storage in boreal forests may increase their predictive power by incorporating the observed negative response of microbial decomposition to boreal wildfires. |
| [34] | . 球囊霉素(glomalin)是丛枝菌根真菌产生的一种含有金属 离子的耐热糖蛋白,能够改善土壤结构,固定土壤中的重金属,近期被更名为球囊霉素相关土壤蛋白(glomalin-related soil protein).该文从球囊霉素的定义、性质与环境功能等方面对相关文献进行了综述,认为目前对球囊霉素的共识仍停留在理论假设蛋白的程度上,包 括:1)该蛋白可能是热激蛋白60 (HSP60)的同系物;2)该蛋白所携带的阳离子可能随着土壤性质的改变而不同.目前还没有清楚确切地定义球囊霉素的真实分子结构与理化性质.今后需从 分子层面对球囊霉素予以深入研究.同时,需要不断改进球囊霉素的提取和测定方法,以便进一步探讨球囊霉素固定重金属离子的机理,提高植物的重金属抗性. |
| [35] | Increased partitioning of carbon (C) to fine roots under elevated [CO2], especially deep in the soil profile, could alter soil C and nitrogen (N) cycling in forests. After more than 11 years of free-air CO2 enrichment in a Liquidambar styraciflua L. (sweetgum) plantation in Oak Ridge, TN, USA, greater inputs of fine roots resulted in the incorporation of new C (i.e., C with a depleted 13C) into root-derived particulate organic matter (POM) pools to 90-cm depth. Even though production in the sweetgum stand was limited by soil N availability, soil C and N contents were greater throughout the soil profile under elevated [CO2] at the conclusion of the experiment. Greater C inputs from fine-root detritus under elevated [CO2] did not result in increased net N immobilization or C mineralization rates in long-term laboratory incubations, possibly because microbial biomass was lower in the CO2-enriched plots. Furthermore, the 13CO2 of the C mineralized from the incubated soil closely tracked the 13C of the labile POM pool in the elevated [CO2] treatment, especially in shallower soil, and did not indicate significant priming of the decomposition of pre-experiment soil organic matter (SOM). Although potential C mineralization rates were positively and linearly related to total SOM C content in the top 30 cm of soil, this relationship did not hold in deeper soil. Taken together with an increased mean residence time of C in deeper soil pools, these findings indicate that C inputs from relatively deep roots under elevated [CO2] may increase the potential for long-term soil C storage. However, C in deeper soil is likely to take many years to accrue to a significant fraction of total soil C given relatively smaller root inputs at depth. Expanded representation of biogeochemical cycling throughout the soil profile may improve model projections of future forest responses to rising atmospheric [CO2]. |
| [36] | The use of fossil fuels and fertilizers has increased the amount of biologically reactive nitrogen in the atmosphere over the past century. As a consequence, forests in industrialized regions have experienced greater rates of nitrogen deposition in recent decades. This unintended fertilization has stimulated forest growth, but has also affected soil microbial activity, and thus the recycling of soil carbon and nutrients. A meta-analysis suggests that nitrogen deposition impedes organic matter decomposition, and thus stimulates carbon sequestration, in temperate forest soils where nitrogen is not limiting microbial growth. The concomitant reduction in soil carbon emissions is substantial, and equivalent in magnitude to the amount of carbon taken up by trees owing to nitrogen fertilization. As atmospheric nitrogen levels continue to rise, increased nitrogen deposition could spread to older, more weathered soils, as found in the tropics; however, soil carbon cycling in tropical forests cannot yet be assessed. |
| [37] | The general lack of significant changes in mineral soil C stocks during CO 2 -enrichment experiments has cast doubt on predictions that increased soil C can partially offset rising atmospheric CO 2 concentrations. Here, we show, through meta-analysis techniques, that these experiments collectively exhibited a 5.6% increase in soil C over 2–9 years, at a median rate of 19 g C m 612 yr 611 . We also measured C accrual in deciduous forest and grassland soils, at rates exceeding 40 g C m 612 yr 611 for 5–8 years, because both systems responded to CO 2 enrichment with large increases in root production. Even though native C stocks were relatively large, over half of the accrued C at both sites was incorporated into microaggregates, which protect C and increase its longevity. Our data, in combination with the meta-analysis, demonstrate the potential for mineral soils in diverse temperate ecosystems to store additional C in response to CO 2 enrichment. |
| [38] | . |
| [39] | Much of natural product chemistry concerns a group of compounds known as secondary metabolites. These low-molecular-weight metabolites often have potent physiological activities. Digitalis, morphine and quinine are plant secondary metabolites, whereas penicillin, cephalosporin, ergotrate and the statins are equally well known fungal secondary metabolites. Although chemically diverse, all secondary metabolites are produced by a few common biosynthetic pathways, often in conjunction with morphological development. Recent advances in molecular biology, bioinformatics and comparative genomics have revealed that the genes encoding specific fungal secondary metabolites are clustered and often located near telomeres. In this review, we address some important questions, including which evolutionary pressures led to gene clustering, why closely related species produce different profiles of secondary metabolites, and whether fungal genomics will accelerate the discovery of new pharmacologically active natural products. |
| [40] | The availability of Soil Organic Nitrogen (SON) determines soil fertility and biomass production to a great extent. SON also affects the amounts and turnover rates of the soil organic carbon (SOC) pools. Although there is increasing awareness of the impact of the nitrogen (N) cycle on the carbon (C) cycle, the extent of this interaction and the implications for soil organic matter (SOM) dynamics are still under debate. Therefore, present knowledge about the inter-relationships of the soil cycles of C and N as well as current ideas about SON stabilization are summarized in this paper in order to develop an advanced concept of the role of N on C sequestration. Modeling global C-cycling, it was already recognized that SON and SOC are closely coupled via biomass production and degradation. However, the narrow C/N ratio of mature soil organic matter (SOM) shows further that the impact of SON on the refractory SOM is beyond that of determining the size of the active cycling entities. It affects the quantity of the slow cycling pool and as a major contributor it also determines its chemical composition. Although the chemical nature of SON is still not very well understood, both improved classical wet chemical analyses and modern spectroscopic techniques provide increasing evidence that almost the entire organic N in fire-unaffected soils is bound in peptide-like compounds and to a lesser extent in amino sugars. This clearly points to the conclusion, that such compounds have greater importance for SOM formation than previously assumed. Based on published papers, I suggest that peptides even have a key function in the C-sequestration process. Although the mechanisms involved in their medium and long-term stabilization are far from understood, the immobilization of these biomolecules seems to determine the chemistry and functionality of the slow cycling SOM fraction and even the potential of a soil to act as a C sink. Pyrogenic organic N, which derives mostly from incomplete combustion of plant and litter peptides is another under-rated player in soil organic matter preservation. In fire-prone regions, its formation represents a major N stabilization mechanism, leading to the accumulation of heterocyclic aromatic N, the stability of which is still not elaborated. The concept of peptide-like compounds as a key in SOM-sequestration implies that for an improved evaluation of the potential of soils as C-sinks our research focus as to be directed to a better understanding of their chemistry and of the mechanisms which are responsible for their resistance against biochemical degradation in soils. |
| [41] | The concept that ectomycorrhizal plants have a particular foliar trait suite characterized by low foliar nutrients and high leaf mass per unit area (LMA) is widely accepted, but whether this trait suite can be generalized to all ectomycorrhizal clades is unclear.We identified 19 evolutionary clades of ectomycorrhizal plants and used a global leaf traits dataset comprising 11 466 samples across c. 3000 species to test whether there were consistent shifts in leaf nutrients or LMA with the evolution of ectomycorrhiza.There were no consistent effects of ectomycorrhizal status on foliar nutrients or LMA in the 17 ectomycorrhizal/non-ectomycorrhizal pairs for which we had sufficient data, with some ectomycorrhizal groups having higher and other groups lower nutrient status than non-ectomycorrhizal contrasts. Controlling for the woodiness of host species did not alter the results.Our findings suggest that the concepts of ectomycorrhizal plant trait suites should be re-examined to ensure that they are broadly reflective of mycorrhizal status across all evolutionary clades, rather than reflecting the traits of a few commonly studied groups, such as the Pinaceae and Fagales. |
| [42] | |
| [43] | Tannins make up a significant portion of forest carbon pools and foliage and bark may contain up to 40% tannin. Like many other plant secondary compounds, tannins were believed to function primarily as herbivore deterrents. However, recent evidence casts doubts on their universal effectiveness against herbivory. Alternatively, tannins may play an important role in plant lant and plant itter oil interactions. The convergent evolution of tanninrich plant communities on highly acidic and infertile soils throughout the world, and the intraspecific variation in tannin concentrations along edaphic gradients suggests that tannins can affect nutrient cycles. This paper reviews nutrient dynamics in forest ecosystems in relation to tannins. Tannins comprise a complex class of organic compounds whose concentration and chemistry differ greatly both among and within plant species. Because the function and reactivity of tannins are strongly controlled by their chemical structure, the effects of tannins on forest ecosystem processes are expected to vary widely. Tannins can affect nutrient cycling by hindering decomposition rates, complexing proteins, inducing toxicity to microbial populations and inhibiting enzyme activities. As a result, tannins may reduce nutrient losses in infertile ecosystems and may alter N cycling to enhance the level of organic versus mineral N forms. The ecological consequences of elevated tannin levels may include allelopathic responses, changes in soil quality and reduced ecosystem productivity. These effects may alter or control successional pathways. While a great deal of research has addressed tannins and their role in nutrient dynamics, there are many facets of tannin biogeochemistry that are not known. This lack of information hinders a complete synthesis of tannin effects on forest ecosystem processes and nutrient cycling. Areas of study that would help clarify the role of tannins in forest ecosystems include improved characterization and quantification techniques, enhanced understanding of structure-activity relationships, investigation of the fate of tannins in soil, further determination of the influence of environmental factors on plant tannin production and decomposition, and additional information on the effects of tannins on soil organisms. |
| [44] | |
| [45] | Abstract Contents 1597 I. 1597 II. 1597 III. 1598 IV. 1598 V. 1600 VI. 1601 VII. 1601 VIII. 1601 1602 References 1602 SUMMARY: Trait-based approaches have led to significant advances in plant ecology, but are currently biased toward above-ground traits. It is becoming clear that a stronger emphasis on below-ground traits is needed to better predict future changes in plant biodiversity and their consequences for ecosystem functioning. Here I propose six 'below-ground frontiers' in trait-based plant ecology, with an emphasis on traits governing soil nutrient acquisition: redefining fine roots; quantifying root trait dimensionality; integrating mycorrhizas; broadening the suite of root traits; determining linkages between root traits and abiotic and biotic factors; and understanding ecosystem-level consequences of root traits. Focusing research efforts along these frontiers should help to fulfil the promise of trait-based ecology: enhanced predictive capacity across ecological scales. 0008 2016 The Authors. New Phytologist 0008 2016 New Phytologist Trust. |
| [46] | The decomposition of plant organic matter and the stability of soil aggregates are important components of soil carbon cycling, and the relationship between decomposition rate and arbuscular mycorrhizal fungi (AMF) has recently received considerable attention. The interaction of AMF with their associated microorganisms and the consequences for litter decomposition and soil aggregation still remain fairly unclear. In a laboratory pot experiment we simultaneously tested the single and combined effects of one AMF species ( Rhizophagus irregularis ) and a natural non-AMF microbial community on the decomposition of small wooden sticks and on soil aggregation. To disentangle effects of hyphae and roots we placed mesh bags as root exclusion compartments in the soil. The decomposition of the wooden sticks in this compartment was significantly reduced in the presence of AMF, but not with the non-AMF microbial community only, compared to the control, while aggregation was increased in all treatments compared to the control. We suggest that AMF directly (via localized nutrient removal or altered moisture conditions) or indirectly (by providing an alternative carbon source) inhibited the activity of decomposers, leading to different levels of plant litter degradation under our experimental settings. Reduced decomposition of woody litter in presence of AMF can be important for nutrient cycling in AMF-dominated forests and in the case of woody plants and perennials that develop lignified roots in grasslands. |
| [47] | Soil aggregation is a crucial aspect of ecosystem functioning in terrestrial ecosystems. Arbuscular mycorrhizal fungi (AMF) play a key role in soil aggregate formation and stabilization. Here we quant |
| [48] | . 丛枝菌根真菌能与80%的陆生维管植物形成互惠共生关系,共生体的存在对促进植物营养吸收和提高抗逆性具有重要意义.丛枝菌根真菌从宿主植物获取其光合产物碳水化合物的同时,通过外生菌丝吸收各种氮源,有效增强了宿主植物对氮素的吸收,以及氮在植物居群和群落水平上的交流,改善了植物营养代谢,增强了植物应对外界环境胁迫的能力.而共生体对氮的吸收、转运,以及氮从真菌到宿主植物的传输、代谢机制至今仍有许多问题亟待解决.本文综述了当前丛枝菌根共生体中氮传输代谢的主要机制,以及碳、磷对共生体氮传输代谢的影响;从群落和生态系统水平,简要阐述了丛枝菌根真菌在植物中氮分配的作用和对宿主植物的生态学意义,并提出共生体中氮代谢的一些需要深入研究的问题. |
| [49] | Abstract Compared with ectomycorrhizal (ECM) forests, arbuscular mycorrhizal (AM) forests are hypothesized to have higher carbon (C) cycling rates and a more open nitrogen (N) cycle. To test this hypothesis, we synthesized 645 observations, including 22 variables related to below-ground C and N dynamics from 100 sites, where AM and ECM forests co-occurred at the same site. Leaf litter quality was lower in ECM than in AM trees, leading to greater forest floor C stocks in ECM forests. By contrast, AM forests had significantly higher mineral soil C concentrations, and this result was strongly mediated by plant traits and climate. No significant differences were found between AM and ECM forests in C fluxes and labile C concentrations. Furthermore, inorganic N concentrations, net N mineralization and nitrification rates were all higher in AM than in ECM forests, indicating ‘mineral’ N economy in AM but ‘organic’ N economy in ECM trees. AM and ECM forests show systematic differences in mineral vs organic N cycling, and thus mycorrhizal type may be useful in predicting how different tree species respond to multiple environmental change factors. By contrast, mycorrhizal type alone cannot reliably predict below-ground C dynamics without considering plant traits and climate. |
| [50] | Summary 6168 Our understanding of how saprotrophic and mycorrhizal fungi interact to re-circulate carbon and nutrients from plant litter and soil organic matter is limited by poor understanding of their spatiotemporal dynamics. 6168 In order to investigate how different functional groups of fungi contribute to carbon and nitrogen cycling at different stages of decomposition, we studied changes in fungal community composition along vertical profiles through a Pinus sylvestris forest soil. We combined molecular identification methods with 14 C dating of the organic matter, analyses of carbon:nitrogen (C:N) ratios and 15 N natural abundance measurements. 6168 Saprotrophic fungi were primarily confined to relatively recently (<4yr) shed litter components on the surface of the forest floor, where organic carbon was mineralized while nitrogen was retained. Mycorrhizal fungi dominated in the underlying, more decomposed litter and humus, where they apparently mobilized N and made it available to their host plants. 6168 Our observations show that the degrading and nutrient-mobilizing components of the fungal community are spatially separated. This has important implications for biogeochemical studies of boreal forest ecosystems. |
| [51] | Abstract Although hypothesized for many years, the involvement of ectomycorrhizal fungi in decomposition of soil organic matter remains controversial and has not yet been fully acknowledged as an important factor in the regulation of soil carbon (C) storage. Here, we review recent findings, which support the view that some ectomycorrhizal fungi have the capacity to oxidize organic matter, either by 'brown-rot' Fenton chemistry or using 'white-rot' peroxidases. We propose that ectomycorrhizal fungi benefit from organic matter decomposition primarily through increased nitrogen mobilization rather than through release of metabolic C and question the view that ectomycorrhizal fungi may act as facultative saprotrophs. Finally, we discuss how mycorrhizal decomposition may influence organic matter storage in soils and mediate responses of ecosystem C sequestration to environmental changes. 0008 2014 The Authors. New Phytologist 0008 2014 New Phytologist Trust. |
| [52] | . 土壤有机碳的增加不仅有助于农业可持续发展,而且对缓解温室气体增加和全球气候变化等也具有重要意义。土壤有机碳的稳定机制决定着土壤固定和储备有机碳的能力,对有机碳稳定机制的研究,将为政府制定有效的温室气体减排措施提供依据。土壤有机碳的稳定机制主要包括:(1)有机碳的难降解性;(2)金属氧化物和粘土矿物与有机碳的相互作用;(3)土壤团聚体的物理保护导致的生物与有机碳空间隔离;(4)土壤生物学机制,主要指土壤生物自身对有机碳稳定性的直接贡献。至今,有机碳稳定性的主导机制尚不清楚,但影响因素与生态系统类型、土壤类型、土层深度、土壤管理措施、土壤生物活性及群落组成等有关。作者建议今后研究有机碳稳定性机制时,应同时考虑上述4种机制的综合作用,并加强探索土壤生物的贡献。 |
| [53] | Mechanisms for C stabilization in soils have received much interest recently due to their relevance in the global C cycle. Here we review the mechanisms that are currently, but often contradictorily or inconsistently, considered to contribute to organic matter (OM) protection against decomposition in temperate soils: (i) selective preservation due to recalcitrance of OM, including plant litter, rhizodeposits, microbial products, humic polymers, and charred OM; (ii) spatial inaccessibility of OM against decomposer organisms due to occlusion, intercalation, hydrophobicity and encapsulation; and (iii) stabilization by interaction with mineral surfaces (Fe-, Al-, Mn-oxides, phyllosilicates) and metal ions. Our goal is to assess the relevance of these mechanisms to the formation of soil OM during different stages of decomposition and under different soil conditions. The view that OM stabilization is dominated by the selective preservation of recalcitrant organic components that accumulate in proportion to their chemical properties can no longer be accepted. In contrast, our analysis of mechanisms shows that: (i) the soil biotic community is able to disintegrate any OM of natural origin; (ii) molecular recalcitrance of OM is relative, rather than absolute; (iii) recalcitrance is only important during early decomposition and in active surface soils; while (iv) during late decomposition and in the subsoil, the relevance of spatial inaccessibility and organo-mineral interactions for SOM stabilization increases. We conclude that major difficulties in the understanding and prediction of SOM dynamics originate from the simultaneous operation of several mechanisms. We discuss knowledge gaps and promising directions of future research. |
| [54] | Summary Carbon (C) metabolism is at the core of ecosystem function. Decomposers play a critical role in this metabolism as they drive soil C cycle by mineralizing organic matter to CO 2 . Their growth depends on the carbon-use efficiency (CUE), defined as the ratio of growth over C uptake. By definition, high CUE promotes growth and possibly C stabilization in soils, while low CUE favors respiration. Despite the importance of this variable, flexibility in CUE for terrestrial decomposers is still poorly characterized and is not represented in most biogeochemical models. Here, we synthesize the theoretical and empirical basis of changes in CUE across aquatic and terrestrial ecosystems, highlighting common patterns and hypothesizing changes in CUE under future climates. Both theoretical considerations and empirical evidence from aquatic organisms indicate that CUE decreases as temperature increases and nutrient availability decreases. More limited evidence shows a similar sensitivity of CUE to temperature and nutrient availability in terrestrial decomposers. Increasing CUE with improved nutrient availability might explain observed declines in respiration from fertilized stands, while decreased CUE with increasing temperature and plant C:N ratios might decrease soil C storage. Current biogeochemical models could be improved by accounting for these CUE responses along environmental and stoichiometric gradients. |
| [55] | Fine roots acquire essential soil resources and mediate biogeochemical cycling in terrestrial ecosystems. Estimates of carbon and nutrient allocation to build and maintain these structures remain uncertain due to challenges in consistent measurement and interpretation of fine-root systems. We define fine roots as all roots less than or equal to 2 mm in diameter, yet it is now recognized that this approach fails to capture the diversity of form and function observed among fine-root orders. We demonstrate how order-based and functional classification frameworks improve our understanding of dynamic root processes in ecosystems dominated by perennial plants. In these frameworks, fine roots are separated into either individual root orders or functionally defined into a shorter-lived absorptive pool and a longer-lived transport fine root pool. Furthermore, using these frameworks, we estimate that fine-root production and turnover represent 22% of terrestrial net primary production globally a ca. 30% reduction from previous estimates assuming a single fine-root pool. In the future we hope to develop tools to rapidly differentiate functional fine-root classes, explicit incorporation of mycorrhizal fungi in fine-root studies, and wider adoption of a two-pool approach to model fine roots provide opportunities to better understand belowground processes in the terrestrial biosphere. |
| [56] | Bacteria and fungi drive the cycling of plant litter in forests, but little is known about their role in tropical rain forest nutrient cycling, despite the high rates of litter decay observed in these ecosystems. However, litter decay rates are not uniform across tropical rain forests. For example, decomposition can differ dramatically over small spatial scales between low-diversity, monodominant rain forests, and species-rich, mixed forests. Because the climatic patterns and soil parent material are identical in co-occurring mixed and monodominant forests, differences in forest floor accumulation, litter production, and decomposition between these forests may be biotically mediated. To test this hypothesis, we conducted field and laboratory studies in a monodominant rain forest in which the ectomycorrhizal tree Dicymbe corymbosa forms > 80% of the canopy, and a diverse, mixed forest dominated by arbuscular mycorrhizal trees. After 2 years, decomposition was significantly slower in the monodominant forest (P < 0.001), but litter production was significantly greater in the mixed forest (P < 0.001). In the laboratory, we found microbial community biomass was greater in the mixed forest (P = 0.02), and the composition of fungal communities was distinct between the two rain forest types (P = 0.001). Sequencing of fungal rDNA revealed a significantly lower richness of saprotrophic fungi in the monodominant forest (19 species) relative to the speciesrich forest (84 species); moreover, only 4% percent of fungal sequences occurred in both forests. These results show that nutrient cycling patterns in tropical forests can vary dramatically over small spatial scales, and that changes in microbial community structure likely drive the observed differences in decomposition. |
| [57] | Summary While it is well established that leaf litter decomposition is controlled by climate and substrate quality at broad spatial scales, conceptual frameworks that consider how local-scale factors affect litter decay in heterogeneous landscapes are generally lacking. A critical challenge in disentangling the relative impacts of and interactions among local-scale factors is that these factors frequently covary due to feedbacks between plant and soil communities. For example, forest plots dominated by trees that associate with ectomycorrhizal (ECM) fungi often differ from those dominated by trees that associate with arbuscular mycorrhizal (AM) fungi in terms of their litter quality, microbial community structure and inorganic nutrient availability. Here, we evaluate the extent to which such factors alter leaf litter decomposition rates. To characterize variations in decomposition rates, we compared decay rates of high-quality litter (maple; AM) and low-quality litter (oak; ECM) across forest plots representing a gradient in litter matrix quality and nitrogen (N) availability driven by the relative proportions of AM and ECM trees in each plot. In experiment two, we added litter from two AM and three ECM tree species to forest plots with either a high-quality litter matrix and high N availability (i.e. AM-dominated plots) or a low-quality litter matrix and low N availability (i.e. ECM-dominated plots). In both experiments, we found that AM litter decomposed more rapidly than ECM litter, and this effect was enhanced in AM-dominated plots. Then, to separate the contributions of litter matrix effects from N availability effects, we added N fertilizer to a subset of plots from experiment two. Nitrogen addition increased decay rates of high-quality litter across all sites, but had no effect on low-quality litter, suggesting that low N availability, not litter matrix quality, constrains decomposition of high-quality litters. Hence, N availability appears to alter litter decomposition patterns independently of litter matrix properties. Synthesis . Our results indicate that shifts in the relative abundance of ECM- and AM-associated trees in a plot or stand have the potential to affect litter decay rates through both changes in litter quality as well as through alterations of the local-scale soil environment. |
| [58] | Temperate forests receive some of the highest rates of nitrogen (N) deposition in the world. While numerous studies have investigated the effects of N enrichment on forests, there is little consensus on why some forests become N saturated while others do not. To investigate this, we used a multi-factor meta-analysis to simultaneously estimate the relative importance of several environmental, experimental, and anthropogenic variables on nitrate (NO 3 61 ) leaching in response to experimental N addition. Given that overstory tree species composition and soil C:N ratio influence forest responses to N, we hypothesized that forests dominated by arbuscular mycorrhizal (AM) trees would respond differently than forests dominated by ectomycorrhizal (ECM) trees in the context of forest susceptibility to NO 3 61 leaching. We found that mycorrhizal association is an important predictor of NO 3 61 leaching, and AM-dominated forests leach more NO 3 61 in response to N deposition than ECM forests. Additionally, we found that the amount of total N added, ambient N deposition rates, and the form of N added influenced the magnitude of the NO 3 61 leaching response. Given that the mycorrhizal associations of most temperate trees are known, our results suggest that this functional grouping may be useful in identifying forests that are most susceptible to NO 3 61 leaching. |
| [59] | Abstract Ecosystems often show differential sensitivity to chronic nitrogen (N) deposition; hence, a critical challenge is to improve our understanding of how and why site-specific factors mediate biogeochemical responses to N enrichment. We examined the extent to which N impacts on soil carbon (C) and N dynamics depend on microbial resource stoichiometry. We added N to forest plots dominated by ectomycorrhizal (ECM) trees, which have litter and soil pools rich in organic N and relatively wide C:N ratios, and adjacent forest plots dominated by arbuscular mycorrhizal (AM) trees, which have litter and soil pools rich in inorganic N and relatively narrow C:N ratios. While microbes in both plot types exhibited fairly strict biomass homeostasis, microbes in AM-and ECM-dominated plots differed in their physiological responses to N addition. Microbes in ECM plots responded to N enrichment by decreasing their investment in N-acquisition enzymes (relative to C-acquisition enzymes) and increasing N mineralization rates (relative to C mineralization rates), suggesting that N addition alleviated microbial N demand. In contrast, heterotrophic microbial activities in AM plots were unaffected by N addition, most likely as a result of N-induced increases in net nitrification (60% increase relative to control plots) and nitrate mobilization (e.g., sixfold increases in mobilization relative to control plots). Combined, our findings suggest the stoichiometric differences between AM and ECM soils are the primary drivers of the observed responses. Plant and microbial communities characterized by wide C:N are more susceptible to N-induced changes in decomposition and soil C dynamics, whereas communities characterized by narrow C:N are more susceptible to N-induced nitrate leaching losses. Hence, the biogeochemical consequences of N deposition in temperate forests may be driven by the stoichiometry of the dominant trees and their associated microbes. |
| [60] | Summary Plant roots, their associated microbial community and free-living soil microbes interact to regulate the movement of carbon from the soil to the atmosphere, one of the most important and least understood fluxes of terrestrial carbon. Our inadequate understanding of how plant–microbial interactions alter soil carbon decomposition may lead to poor model predictions of terrestrial carbon feedbacks to the atmosphere. Roots, mycorrhizal fungi and free-living soil microbes can alter soil carbon decomposition through exudation of carbon into soil. Exudates of simple carbon compounds can increase microbial activity because microbes are typically carbon limited. When both roots and mycorrhizal fungi are present in the soil, they may additively increase carbon decomposition. However, when mycorrhizas are isolated from roots, they may limit soil carbon decomposition by competing with free-living decomposers for resources. We manipulated the access of roots and mycorrhizal fungi to soil in02situ in a temperate mixed deciduous forest. We added 13C-labelled substrate to trace metabolized carbon in respiration and measured carbon-degrading microbial extracellular enzyme activity and soil carbon pools. We used our data in a mechanistic soil carbon decomposition model to simulate and compare the effects of root and mycorrhizal fungal presence on soil carbon dynamics over longer time periods. Contrary to what we predicted, root and mycorrhizal biomass did not interact to additively increase microbial activity and soil carbon degradation. The metabolism of 13C-labelled starch was highest when root biomass was high and mycorrhizal biomass was low. These results suggest that mycorrhizas may negatively interact with the free-living microbial community to influence soil carbon dynamics, a hypothesis supported by our enzyme results. Our steady-state model simulations suggested that root presence increased mineral-associated and particulate organic carbon pools, while mycorrhizal fungal presence had a greater influence on particulate than mineral-associated organic carbon pools. Synthesis . Our results suggest that the activity of enzymes involved in organic matter decomposition was contingent upon root–mycorrhizal–microbial interactions. Using our experimental data in a decomposition simulation model, we show that root–mycorrhizal–microbial interactions may have longer-term legacy effects on soil carbon sequestration. Overall, our study suggests that roots stimulate microbial activity in the short term, but contribute to soil carbon storage over longer periods of time. |
| [61] | The production of enzymes involved in mycoparasitism by several strains of ectomycorrhizal fungi: Amanita muscaria (16-3), Laccaria laccata (9-12), L. laccata (9-1), Suillus bovinus (15-4), S. bovinus (15-3), S. luteus (14-7) on different substrates such as colloidal chitin, mycelia of Trichoderma harzianum , T. virens and Mucor hiemalis was examined. Chitinases and β-1,3-glucanases were assayed spectrophotometrically by measuring the amount of reducing sugars releasing from suitable substrate by means of Miller’s method. β -glucosidases were determined by measuring the amount of p -nitrophenol released from p -nitrophenyl- β -D-glucopyranoside. It was observed that A. muscaria (16-3) and L. laccata (9-12) biosynthesized the highest activity of enzymes in contrast to the strains of S. bovinus and S. luteus . The mycelium of T. harzianum turned out to be the best substrate for the induction of β-1,3-glucanases and β-glucosidases for both strains of L. laccata , although the difference in the induction of chitinases in the presence of mycelia of different species of Trichoderma was not indicated. |
| [62] | Forest biogeochemical cycles are shaped by effects of dominant tree species on soils, but the underlying mechanisms are not well understood. We investigated effects of temperate tree species on interactions among carbon (C), nitrogen (N), and acidity in mineral soils from an experiment with replicated monocultures of 14 tree species. To identify how trees affected these soil properties, we evaluated correlations among species-level characteristics (e.g. nutrient concentrations in leaf litter, wood, and roots), stand-level properties (e.g. nutrient fluxes through leaf litterfall, nutrient pools in stemwood), and components of soil C, N, and cation cycles. Total extractable acidity (aciditytot) was correlated positively with mineral soil C stocks (R2 = 0.72, P < 0.001), such that a nearly two-fold increase in aciditytot was associated with a more than two-fold increase of organic C. We attribute this correlation to effects of tree species on soil acidification and subsequent mineral weathering reactions, which make hydrolyzing cations available for stabilization of soil organic matter. The effects of tree species on soil acidity were better understood by measuring multiple components of soil acidity, including pH, the abundance of hydrolyzing cations in soil solutions and on cation exchange sites, and aciditytot. Soil pH and aciditytot were correlated with proton-producing components of the soil N cycle (e.g. nitrification), which were positively correlated with species-level variability in fine root N concentrations. Soluble components of soil acidity, such as aluminum in saturated paste extracts, were more strongly related to plant traits associated with calcium cycling, including leaf and root calcium concentrations. Our results suggest conceptual models of plant impacts on soil biogeochemistry should be revised to account for underappreciated plant traits and biogeochemical processes. |
| [63] | Tree species interact with soil biota to impact soil organic carbon (C) pools, but it is unclear how this interaction is shaped by various ecological factors. We used multiple regression to describe how ~100 variables were related to soil organic C pools in a common garden experiment with 14 temperate tree species. Potential predictor variables included: (i) the abundance, chemical composition, and decomposition rates of leaf litter and fine roots, (ii) species richness and abundance of bacteria, fungi, and invertebrate animals in soil, and (iii) measures of soil acidity and texture. The amount of organic C in the organic horizon and upper 20 cm of mineral soil (i.e. the combined C pool) was strongly negatively correlated with earthworm abundance and strongly positively correlated with the abundance of aluminum, iron, and protons in mineral soils. After accounting for these factors, we identified additional correlations with soil biota and with litter traits. Rates of leaf litter decomposition, measured as litter mass loss, were negatively correlated with size of the combined soil organic C pool. Somewhat paradoxically, the combined soil organic C pool was also negatively related to the ratio of recalcitrant compounds to nitrogen in leaf litter. These apparent effects of litter traits probably arose because two independent components of litter uality were controlling different aspects of decomposition. Leaf litter mass loss rates were positively related with leaf litter calcium concentrations, reflecting greater utilization and depolymerization of calcium-rich leaf litter by earthworms and other soil biota, which presumably led to greater proportional losses of litter C as CO 2 or dissolved organic C. The fraction of depolymerized and metabolized litter that is ultimately lost as CO 2 is an inverse function of microbial C use efficiency, which increases with litter nutrient concentrations but decreases with concentrations of recalcitrant compounds (e.g. lignin); thus, high ratios of recalcitrant compounds to nitrogen in leaf litter likely caused a greater fraction of depolymerized litter to be lost as CO 2 . Existing conceptual models of soil C stabilization need to reconcile the effects of litter quality on these two potentially counteracting factors: rates of litter depolymerization and microbial C use efficiency. |
| [64] | |
| [65] | Tropical forests have high rates of soil carbon cycling, but little information is available on how roots, arbuscular mycorrhizal fungi (AMF), and free‐living microorganisms interact and influence organic matter mineralization in these ecosystems. We used mesh ingrowth cores and isotopic tracers in phospholipid fatty acid biomarkers to investigate the effects of roots and AMF mycelia on (1) microbial community composition, microbial carbon utilization, and hydrolytic enzyme activities for large, potted tropical trees and (2) enzyme activities and litter mass loss in a lowland tropical forest. Under the tropical tree, plant‐derived carbon was incorporated predominantly into bacterial groups in both rhizosphere and AMF‐only soils. Gram‐positive bacteria incorporated additional soil‐derived carbon in rhizosphere soils, which also contained the highest microbial biomass. For hydrolytic enzymes, β‐glucosidase and ‐acetyl β‐glucosaminidase activities were highest in rhizosphere soils, while phosphomonoesterase activity was highest in AMF‐only soil. In the forest, leaf litter mass loss was increased by the presence of roots, but not by the presence of AMF mycelia only. Root–microbial interactions influenced organic matter cycling, with evidence for rhizosphere priming and accelerated leaf litter decomposition in the presence of roots. Although AMF mycelia alone did not stimulate organic matter mineralization, they were a conduit of carbon to other soil microorganisms. |
| [66] | |
| [67] | Understanding the factors that drive soil carbon (C) accumulation is of fundamental importance given their potential to mitigate climate change. Much research has focused on the relationship between plant traits and C sequestration, but no studies to date have quantitatively considered traits of their mycorrhizal symbionts. Here, we use a modelling approach to assess the contribution of an important mycorrhizal fungal trait, organic nutrient uptake, to soil C accumulation. We show that organic nutrient uptake can significantly increase soil C storage, and that it has a greater effect under nutrient-limited conditions. The main mechanism behind this was an increase in plant C fixation and subsequent increased C inputs to soil through mycorrhizal fungi. Reduced decomposition due to increased nutrient limitation of saprotrophs also played a role. Our results indicate that direct uptake of nutrients from organic pools by mycorrhizal fungi could have a significant effect on ecosystem C cycling and storage. |
| [68] | Background and aims: Arbuscular mycorrhizal (AM) hyphae represent an important route for input of plant-derived C to soil, but impacts of these inputs on microbial communities and processes are poorly understood. In this study we characterised pathways of C-flow through microbial communities associated with AM hyphae and quantified impacts on mineralisation of native SOM. Methods: Continuous, steady-state CO labelling was applied throughout the growth period (60 d) of Lolium perenne. Exclusion meshes were used to control access of roots and AM hyphae to soil, and plant-derived C was quantified within microbial PLFA and NLFA, and soil CO efflux was partitioned into plant- and soil organic matter (SOM) derived components. Results: Pathways of C-flow through hyphosphere and mycorrhizosphere communities were distinct, as was the fate of plant-derived C from AM hyphae accessing soil through 37 and 1 脦录m meshes. Mineralisation of native SOM was increased in all treatments, relative to unplanted controls, and this priming effect was largest for AM hyphae accessing soil through the 1 脦录m mesh size. Conclusions: We demonstrated that AM hyphae can strongly increase mineralisation of native SOM and identified distinct pathways of C-flow through hyphosphere communities. Our results suggest that, in addition to affecting rates of litter decomposition, AM hyphae may have a significant influence on turnover of native SOM. |
| [69] | This review covers historical perspectives, the role of plant inputs, and the nature and dynamics of soil organic matter (SOM), often known as humus. Information on turnover of organic matter components, the role of microbial products, and modeling of SOM, and tracer research should help us to anticipate what future research may answer today's challenges. Our globe's most important natural resource is best studied relative to its chemistry, dynamics, matrix interactions, and microbial transformations. Humus has similar, worldwide characteristics, but varies with abiotic controls, soil type, vegetation inputs and composition, and the soil biota. It contains carbohydrates, proteins, lipids, phenol-aromatics, protein-derived and cyclic nitrogenous compounds, and some still unknown compounds. Protection of transformed plant residues and microbial products occurs through spatial inaccessibility-resource availability, aggregation of mineral and organic constituents, and interactions with sesquioxides, cations, silts, and clays. Tracers that became available in the mid-20th century made the study of SOM dynamics possible. Carbon dating identified resistant, often mineral-associated, materials to be thousands of years old, especially at depth in the profile. The 13 C associated with C 3 C 4 plant switches characterized slow turnover pools with ages ranging from dozens to hundreds of years. Added tracers, in conjunction with compound-specific product analysis and incubation, identified active pools with fast turnover rates. Physical fractionations of the intra- and inter-aggregate materials, and those associated with silt and clay, showed that all pools contain both old and young materials. Charcoal is old but not inert. The C:N ratio changes from 25 to 70:1 for plant residues to 6 to 9:1 for soil biota and microbial products associated with soil minerals. Active, slow and passive (resistant) pool concepts have been well used in biogeochemical models. The concepts discussed herein have implications for today's challenges in nutrient cycling, biogeochemistry, soil ecosystem functioning, pollution control, feeding the expanding global population and global change. |
| [70] | Abstract Contents 'Summary' I. 'Introduction' II. 'Have ECM fungi retained genes with lignocellulolytic potential from saprotrophic ancestors?' III. 'Are genes with saprotrophic function expressed by ECM fungi when in symbiosis?' IV. 'Do transcribed enzymes operate to obtain N from SOM?' V. 'Is the organic N derived from SOM transferred to the plant host?' VI. 'Concluding remarks' 'Acknowledgements' References The view that ectomycorrhizal (ECM) fungi commonly participate in the enzymatic liberation of nitrogen (N) from soil organic matter (SOM) has recently been invoked as a key mechanism governing the biogeochemical cycles of forest ecosystems. Here, we provide evidence that not all evolutionary lineages of ECM have retained the genetic potential to produce extracellular enzymes that degrade SOM, calling into question the ubiquity of the proposed mechanism. Further, we discuss several untested conditions that must be empirically validated before it is certain that any lineage of ECM fungi actively expresses extracellular enzymes in order to degrade SOM and transfer N contained therein to its host plant. |
| [71] | Abstract Soils of northern temperate and boreal forests represent a large terrestrial carbon (C) sink. The fate of this C under elevated atmospheric CO2 and climate change is still uncertain. A fundamental knowledge gap is the extent to which ectomycorrhizal fungi (EMF) and saprotrophic fungi contribute to C cycling in the systems by soil organic matter (SOM) decomposition. In this study, we used a novel approach to generate and compare enzymatically active EMF hyphae-dominated and saprotrophic hyphae-enriched communities under field conditions. Fermentation-humus (FH)-filled mesh bags, surrounded by a sand barrier, effectively trapped EMF hyphae with a community structure comparable to that found in the surrounding FH layer, at both trophic and taxonomic levels. In contrast, over half the sequences from mesh bags with no sand barrier were identified as belonging to saprotrophic fungi. The EMF hyphae-dominated systems exhibited levels of hydrolytic and oxidative enzyme activities that were comparable to or higher than saprotroph-enriched systems. The enzymes assayed included those associated with both labile and recalcitrant SOM degradation. Our study shows that EMF hyphae are likely important contributors to current SOM turnover in sub-boreal systems. Our results also suggest that any increased EMF biomass that might result from higher below-ground C allocation by trees would not suppress C fluxes from sub-boreal soils. |
| [72] | Understanding the context dependence of ecosystem responses to global changes requires the development of new conceptual frameworks. Here we propose a framework for considering how tree species and their mycorrhizal associates differentially couple carbon (C) and nutrient cycles in temperate forests. Given that tree species predominantly associate with a single type of mycorrhizal fungi (arbuscular mycorrhizal (AM) fungi or ectomycorrhizal (ECM) fungi), and that the two types of fungi differ in their modes of nutrient acquisition, we hypothesize that the abundance of AM and ECM trees in a plot, stand, or region may provide an integrated index of biogeochemical transformations relevant to C cycling and nutrient retention. First, we describe how forest plots dominated by AM tree species have nutrient economies that differ in their C utrient couplings from those in plots dominated by ECM trees. Secondly, we demonstrate how the relative abundance of AM and ECM trees can be used to estimate nutrient dynamics across the landscape. Finally, we describe how our framework can be used to generate testable hypotheses about forest responses to global change factors, and how these dynamics can be used to develop better representations of plant oil feedbacks and nutrient constraints on productivity in ecosystem and earth system models. |
| [73] | The degree to which rising atmospheric CO(2) will be offset by carbon (C) sequestration in forests depends in part on the capacity of trees and soil microbes to make physiological adjustments that can alleviate resource limitation. Here, we show for the first time that mature trees exposed to CO(2) enrichment increase the release of soluble C from roots to soil, and that such increases are coupled to the accelerated turnover of nitrogen (N) pools in the rhizosphere. Over the course of 3 years, we measured in situ rates of root exudation from 420 intact loblolly pine (Pinus taeda L.) roots. Trees fumigated with elevated CO(2) (200 p.p.m.v. over background) increased exudation rates (g C cm(-1) root h(-1) ) by 55% during the primary growing season, leading to a 50% annual increase in dissolved organic inputs to fumigated forest soils. These increases in root-derived C were positively correlated with microbial release of extracellular enzymes involved in breakdown of organic N (R(2) = 0.66; P = 0.006) in the rhizosphere, indicating that exudation stimulated microbial activity and accelerated the rate of soil organic matter (SOM) turnover. In support of this conclusion, trees exposed to both elevated CO(2) and N fertilization did not increase exudation rates and had reduced enzyme activities in the rhizosphere. Collectively, our results provide field-based empirical support suggesting that sustained growth responses of forests to elevated CO(2) in low fertility soils are maintained by enhanced rates of microbial activity and N cycling fuelled by inputs of root-derived C. To the extent that increases in exudation also stimulate SOM decomposition, such changes may prevent soil C accumulation in forest ecosystems. |
| [74] | A common finding in multiple CO2 enrichment experiments in forests is the lack of soil carbon (C) accumulation owing to microbial priming of ‘old’ soil organic matter (SOM). However, soil C losses may also result from the accelerated turnover of ‘young’ microbial tissues that are rich in nitrogen (N) relative to bulk SOM. We measured root-induced changes in soil C dynamics in a pine forest exposed to elevated CO2 and N enrichment by combining stable isotope analyses, molecular characterisations of SOM and microbial assays. We find strong evidence that the accelerated turnover of root-derived C under elevated CO2 is sufficient in magnitude to offset increased belowground inputs. In addition, the C losses were associated with accelerated N cycling, suggesting that trees exposed to elevated CO2 not only enhance N availability by stimulating microbial decomposition of SOM via priming but also increase the rate at which N cycles through microbial pools. |
| [75] | Key recent developments in litter decomposition research are reviewed. Long-term inter-site experiments indicate that temperature and moisture influence early rates of litter decomposition primarily by determining the plants present, suggesting that climate change effects will be small unless they alter the plant forms present. Thresholds may exist at which single factors control decay rate. Litter decomposes faster where the litter type naturally occurs. Elevated COconcentrations have little effect on litter decomposition rates. Plant tissues are not decay-resistant; it is microbial and biochemical transformations of materials into novel recalcitrant compounds rather than selective preservation of recalcitrant compounds that creates stable organic matter. Altering single characteristics of litter will not substantially alter decomposition rates. Nitrogen addition frequently leads to greater stabilization into humus through a combination of chemical reactions and enzyme inhibition. To sequester more C in soil, we need to consider not how to slow decomposition, but rather how to divert more litter into humus through microbial and chemical reactions rather than allowing it to decompose. The optimal strategy is to have litter transformed into humic substances and then chemically or physically protected in mineral soil. Adding N through fertilization and N-fixing plants is a feasible means of stimulating humification. |
| [76] | |
| [77] | Tree species can influence biogeochemistry through variation in the quantity and chemistry of their litter, and associated impacts on the soil heterotrophic community. However, the role that different plant traits play in these processes is not well understood, nor is it clear whether species effects on soils largely reflect a gymnosperm vs. angiosperm contrast. Using a replicated, long-term monoculture plot experiment, we examined variation in soils among 14 gymnosperm and angiosperm tree species 30 years after plot establishment, and assessed the role of litter chemistry vis-vis such variation. Differences in litter calcium concentrations among tree species resulted in profound changes in soil acidity and fertility that were similar within and among tree groups. Tree species rich in calcium were associated with increased native earthworm abundance and diversity, as well as increased soil pH, exchangeable calcium, per cent base saturation and forest floor turnover rate. |
| [78] | Arbuscular mycorrhizal fungi (AMF; phylum Glomeromycota) are ubiquitous in terrestrial ecosystems. Despite their acknowledged importance in ecology, most research on AMF has focused on effects on individual plant hosts, with more recent efforts aimed at the level of the plant community. Research at the ecosystem level is less prominent, but potentially very promising. Numerous human-induced disturbances (including global change and agro-ecosystem management) impinge on AMF functioning; hence study of this symbiosis from the ecosystem perspective seems timely and crucial. In this paper, I discuss four (interacting) routes via which AMF can influence ecosystem processes. These include indirect pathways (through changes in plant and soil microbial community composition), and direct pathways (effects on host physiology and resource capture, and direct mycelium effects). I use the case study of carbon cycling to illustrate the potentially pervasive influence of AMF on ecosystem processes. A limited amount of published research on AMF ecology is suited for direct integration into ecosystem studies (because of scale mismatch or ill-adaptation to the ools and flux paradigm of ecosystem ecology); I finish with an assessment of the tools (experimental designs, response variables) available for studying mycorrhizae at the ecosystem scale. |
| [79] | This paper explores the potential of using plant root and mycorrhizal fungal traits for understanding soil aggregation. It is established that using traits can increase fundamental understanding of the intricate relationship between plants, symbiotic fungi, and their immediate environment which is the soil aggregate. However, knowledge of root and mycorrhizal fungal traits could also have great applied significance. Such data could give rise to innovations such as tailored seed mixes (or fungal inoculum mixes) for grassland restoration, which maximize trait coverage in terms of soil aggregation within the available plant species pool, as well as setting priorities for conservation efforts through predicting which ecosystems are most prone to degradation in light of invasive species and imminent global change. Likewise, better information on plant traits could be used to foster crop breeding for sustainable agriculture, or for agroecosystem management to enhance soil stability (e.g. by selecting cover crops also for complementary trait values). |
| [80] | Abstract Contents Summary41 I. Introduction42 II. How mycorrhizal fungi can influence soil aggregation at various scales42 III. Effects of fungal mycelium: a mechanistic discussion44 IV. Role of fungal diversity48 V. Emerging foci, new directions and tools49 VI. Conclusions50 Acknowledgements50 References50 Summary In addition to their well-recognized roles in plant nutrition and communities, mycorrhizas can influence the key ecosystem process of soil aggregation. Here we review the contribution of mycorrhizas, mostly focused on arbuscular mycorrhizal fungi (AMF), to soil structure at various hierarchical levels: plant community; individual root; and the soil mycelium. There are a suite of mechanisms by which mycorrhizal fungi can influence soil aggregation at each of these various scales. By extension of these mechanisms to the question of fungal diversity, it is recognized that different species or communities of fungi can promote soil aggregation to different degrees. We argue that soil aggregation should be included in a more complete ultifunctional perspective of mycorrhizal ecology, and that in-depth understanding of mycorrhizas/soil process relationships will require analyses emphasizing feedbacks between soil structure and mycorrhizas, rather than a uni-directional approach simply addressing mycorrhizal effects on soils. We finish the discussion by highlighting new tools, developments and foci that will probably be crucial in further understanding mycorrhizal contributions to soil structure. |
| [81] | Glomalin is a soil proteinaceous substance produced by arbuscular mycorrhizal fungi. Most of the information available concerning this protein has been collected in relation to its role in soil aggregation. In this study, we explored the distribution of glomalin across soil horizons, decomposition of glomalin, and relationship with soil C and N in an agricultural field, a native forest, and an afforested system. Glomalin was present in A, B, and C horizons in decreasing concentrations. Land-use type significantly affected glomalin concentrations (mg cm-3), with native forest soils having the highest concentrations of the three land-use types in both A and B horizons. In terms of glomalin stocks (Mg ha-1), calculated based on corrected horizon weights, the agricultural area was significantly lower than both afforested and native forest areas. As measured after a 413 day laboratory soil incubation, glomalin was least persistent in the A horizon of the afforested area.. In agricultural soils and native soils, ca. 50% of glomalin was still remaining after this incubation, indicating that some glomalin may be in the slow or recalcitrant soil C fraction. Comparison of glomalin decomposition with CO2-C respired during incubation indicates that glomalin makes a large contribution to active soil organic C pools. Soil C and N were highly correlated with glomalin across all soils and within each land-use type, indicating that glomalin may be under similar controls as soil C. Our results show that glomalin may be useful as an indicator of land-use change effects on deciduous forest soils. |
| [82] | |
| [83] | Dissolved organic nitrogen (DON) is a potentially significant vector of N loss from forest ecosystems that has been characterized as an “N leak.” Although the term “leak” suggests a lack of regulation |
| [84] | . 菌根是土壤真菌与植物根系形成的共生体,存在于绝大多数植物(90%)的根系和生境中.菌根共有7种类型,在生态系统的过程和功能方面都扮演着十分重要的角色.为了增强对菌根在森林生态系统中重要功能的理解,文章基于全球森林数据库,在全球尺度上研究了不同菌根类型对森林树木净初级生产力(NPP)的影响.结果表明,森林树木NPP随菌根类型的不同而不同,AM类型菌根森林的NPP[679.49 g·m-2·a-1(以C计)]要显著高于含ECM类型菌根的森林[479.00 g·m-2·a-1(以C计)];菌根类型的不同对森林树木地上和地下及其各组分NPP的影响和贡献也存在着显著的不同,AM类型菌根对地下NPP的贡献要高于ECM菌根,而ECM菌根对地上NPP的贡献则较大.菌根类型对地上、地下NPP组分的影响分析则表明,AM类型的菌根对树叶和细根NPP的贡献较大,而ECM类型菌根则对树木主干和枝NPP的贡献较大.可见,森林树木总体NPP及其各组分NPP都随着菌根类型的不同而存在显著的差异. |
| [85] | . 菌根是由土壤中的菌根菌与植物根系形成的互惠共生体,在植物生产 力和生态系统碳循环过程中发挥着重要的作用.该文基于全球森林数据库,建立了包括全球森林菌根类型、净初级生产力(net primary productivity,NPP)和平均气温等指标的新数据库.在此基础上,分析了6种菌根类型(丛枝菌根(arbuscular mycorrhiza,AM)、AM+外生菌根(ectomycorrhiza,ECM)、AM+ ECM+内外生菌根(ectendomycorrhiza,EEM)、ECM、ECM+ EEM和ECM+ EEM+无菌根(nonmycorrhiza,NM))森林的总NPP、地上和地下NPP、树木主干NPP、树叶NPP,以及树木细根NPP对年平均气温 变化的响应.结果表明,不同菌根类型的森林总NPP、地上和地下NPP虽然都随气温的升高呈现上升的趋势,但其响应程度因菌根类型的不同而有所差异.除 AM和AM+ ECM+ EEM类型的森林外,其他4种菌根类型的森林总NPP都随年平均气温的增加而显著增加;随着菌根类型的不同,地上和地下NPP对年平均气温变化的响应程度 也存在差异,在AM+ECM类型的森林中,气温对地上NPP变异的解释率最高,达到57.27%、而地下NPP仅在ECM类型和ECM+ EEM类型的森林中呈现出与年平均气温显著的回归关系.树木主干、树叶和细根的NPP则随菌根类型的不同而变化,与气温变化呈现正、负相关关系.从AM与 ECM类型的森林的NPP来看,无论是总NPP还是各个组成部分的MPP,ECM类型的森林的MPP对气温的响应总是较AM类型更为敏感.可见,不同类型 的菌根通过影响森林不同部分的NPP对气温变化的响应程度而影响到森林NPP对气温变化的响应.这表明菌根类型是预测气温变化对森林NPP影响的重要指 标. |
| [86] | Since the 1900s, the link between soil biotic activity, soil organic matter (SOM) decomposition and stabilization, and soil aggregate dynamics has been recognized and intensively been studied. By 1950, many studies had, mostly qualitatively, investigated the influence of the five major factors (i.e. soil fauna, microorganisms, roots, inorganics and physical processes) on this link. After 1950, four theoretical mile-stones related to this subject were realized. The first one was when Emerson [Nature 183 (1959) 538] proposed a model of a soil crumb consisting of domains of oriented clay and quartz particles. Next, Edwards and Bremner [J. Soil Sci. 18 (1967) 64] formulated a theory in which the solid-phase reaction between clay minerals, polyvalent cations and SOM is the main process leading to microaggregate formation. Based on this concept, Tisdall and Oades [J. Soil Sci. 62 (1982) 141] coined the aggregate hierarchy concept describing a spatial scale dependence of mechanisms involved in micro- and macroaggregate formation. Oades [Plant Soil 76 (1984) 319] suggested a small, but very important, modification to the aggregate hierarchy concept by theorizing the formation of microaggregates within macroaggregates. Recent research on aggregate formation and SOM stabilization extensively corroborate this modification and use it as the base for furthering the understanding of SOM dynamics. The major outcomes of adopting this modification are: (1) microaggregates, rather than macroaggregates protect SOM in the long term; and (2) macroaggregate turnover is a crucial process influencing the stabilization of SOM. Reviewing the progress made over the last 50 years in this area of research reveals that still very few studies are quantitative and/or consider interactive effects between the five factors. The quantification of these relationships is clearly needed to improve our ability to predict changes in soil ecosystems due to management and global change. This quantification can greatly benefit from viewing aggregates as dynamic rather than static entities and relating aggregate measurements with 2D and 3D quantitative spatial information. |
| [87] | |
| [88] | Abstract A significant fraction of carbon stored in the Earth's soil moves through arbuscular mycorrhiza (AM) and ectomycorrhiza (EM). The impacts of AM and EM on the soil carbon budget are poorly understood. We propose a method to quantify the mycorrhizal contribution to carbon cycling, explicitly accounting for the abundance of plant-associated and extraradical mycorrhizal mycelium. We discuss the need to acquire additional data to use our method, and present our new global database holding information on plant species-by-site intensity of root colonization by mycorrhizas. We demonstrate that the degree of mycorrhizal fungal colonization has globally consistent patterns across plant species. This suggests that the level of plant species-specific root colonization can be used as a plant trait. To exemplify our method, we assessed the differential impacts of AM0002:0002EM ratio and EM shrub encroachment on carbon stocks in sub-arctic tundra. AM and EM affect tundra carbon stocks at different magnitudes, and via partly distinct dominant pathways: via extraradical mycelium (both EM and AM) and via mycorrhizal impacts on above- and belowground biomass carbon (mostly AM). Our method provides a powerful tool for the quantitative assessment of mycorrhizal impact on local and global carbon cycling processes, paving the way towards an improved understanding of the role of mycorrhizas in the Earth's carbon cycle. 0008 2015 The Authors. New Phytologist 0008 2015 New Phytologist Trust. |
| [89] | 1. Quantifying pathways and temporal dynamics of carbon (C) flux between plants and soil is critical to our understanding of the long-term fate of C stored in soils. The potential priming of old organic matter decomposition by fresh C input from plants means that the impact of environmental changes on the interactions between plant C allocation and soil C storage need to be better understood. We used forest girdling to investigate the partitioning of total soil CO2 efflux (RS) into autotrophic (RA) and heterotrophic (RH) flux components and their interaction with litter decomposition.2. The reduction in RS in girdled plots stabilized within two weeks at 65% of control plot values, indicating that RS is dominated by RH, and that only a small pool of available non-structural C remains in roots in late summer to sustain rhizosphere metabolic processes. RA contributions declined from 35% late in the growing season to about 25% in winter.3. Our results indicate that actual root respiration (RR) and respiration by ectyomycorrhizas and other rhizospheric organisms (RM) contribute c. 50% each to RA between September and early November. During winter, RA remained significantly greater than zero despite frequent sub-zero air temperatures, with RM being a dominant component of RA during this period.4. Forest girdling significantly increased the age of C in soil-respired CO2, consistent with the removal of contemporary C derived from RA. Partitioning of soil CO2 efflux on the basis of 14C results shows good agreement with the flux reduction observed between girdled and control plots.5. Litter bag incubations indicate a promoting influence of an intact C supply to the rhizosphere on decomposition, indicating a positive rhizosphere priming effect.6. Synthesis: Our results demonstrate significant contribution of mycorrhizas and other rhizosphere organisms to RS, and suggest a direct link between an intact rhizosphere and litter decomposition dynamics. These results highlight the tight coupling between autotroph activity and soil decomposition processes in forest soils, and add to the growing body of evidence that plant and soil processes cannot be treated separately. |
| [90] | Abstract Ecosystem carbon (C) balance is hypothesised to be sensitive to the mycorrhizal strategies that plants use to acquire nutrients. To test this idea, we coupled an optimality-based plant nitrogen (N) acquisition model with a microbe-focused soil organic matter (SOM) model. The model accurately predicted rhizosphere processes and C-N dynamics across a gradient of stands varying in their relative abundance of arbuscular mycorrhizal (AM) and ectomycorrhizal (ECM) trees. When mycorrhizal dominance was switched - ECM trees dominating plots previously occupied by AM trees, and vice versa - legacy effects were apparent, with consequences for both C and N stocks in soil. Under elevated productivity, ECM trees enhanced decomposition more than AM trees via microbial priming of unprotected SOM. Collectively, our results show that ecosystem responses to global change may hinge on the balance between rhizosphere priming and SOM protection, and highlight the importance of dynamically linking plants and microbes in terrestrial biosphere models. 2017 John Wiley & Sons Ltd/CNRS. |
| [91] | AbstractThree young northern temperate forest communities in the north-central United States were exposed to factorial combinations of elevated carbon dioxide (CO2) and tropospheric ozone (O3) for 1102years. Here, we report results from an extensive sampling of plant biomass and soil conducted at the conclusion of the experiment that enabled us to estimate ecosystem carbon (C) content and cumulative net primary productivity (NPP). Elevated CO2 enhanced ecosystem C content by 11%, whereas elevated O3 decreased ecosystem C content by 9%. There was little variation in treatment effects on C content across communities and no meaningful interactions between CO2 and O3. Treatment effects on ecosystem C content resulted primarily from changes in the near-surface mineral soil and tree C, particularly differences in woody tissues. Excluding the mineral soil, cumulative NPP was a strong predictor of ecosystem C content (r202=020.96). Elevated CO2 enhanced cumulative NPP by 39%, a consequence of a 28% increase in canopy nitrogen (N) content (g02N02m612) and a 28% increase in N productivity (NPP/canopy N). In contrast, elevated O3 lowered NPP by 10% because of a 21% decrease in canopy N, but did not impact N productivity. Consequently, as the marginal impact of canopy N on NPP (62NPP/62N) decreased through time with further canopy development, the O3 effect on NPP dissipated. Within the mineral soil, there was less C in the top 0.102m of soil under elevated O3 and less soil C from 0.1 to 0.202m in depth under elevated CO2. Overall, these results suggest that elevated CO2 may create a sustained increase in NPP, whereas the long-term effect of elevated O3 on NPP will be smaller than expected. However, changes in soil C are not well-understood and limit our ability to predict changes in ecosystem C content. |
| [92] | Summary Organic matter decomposition is the main process by which carbon (C) is lost from terrestrial ecosystems, and mycorrhizal associations of plants (i.e. arbuscular mycorrhizas (AM) and ectomycorrhizas (ECM)) may have different indirect effects on this loss pathway. AM and ECM plants differ in the soil decomposers they promote and the quality of litter they produce, which may result in different patterns of organic matter decomposition, and hence, soil C loss. To determine how mycorrhizal associations indirectly affect decomposer activity, we collected soils and litters from four AM and four ECM tree species from a mixed-deciduous temperate forest for a field and laboratory study. We first characterized in situ patterns in soil chemistry and soil microbial biomass among these eight tree species. We then conducted a microcosm experiment with mineral soils, leaf litter and fine roots originating from these tree species, where we reciprocally crossed litters and soils, and quantified the rate of heterotrophic respiration over a 140-day laboratory incubation. In natural forest conditions, AM tree soils contained lower total C and microbial biomass C:N relative to ECM tree soils. In our microcosm experiment, AM soils supported greater heterotrophic respiration than did ECM soils. The addition of AM litter stimulated respiration more than did ECM litter, owing to the lower C:N of AM litter. Matching the mycorrhizal identity of litter and soil resulted in a difference in total respiration, such that combinations of AM litters with AM soils lost more C than did combinations of ECM litters with ECM soils. Synthesis . Our findings demonstrate that AM and ECM trees have differing indirect effects on soil decomposer activity through the decomposers they cultivate and/or the quality of organic matter they produce. Mycorrhizal differences in litter quality accentuate these effects on soil C loss and may explain patterns in soil C dynamics in terrestrial ecosystems. |
| [93] | Abstract Plants buffer increasing atmospheric carbon dioxide (CO2) concentrations through enhanced growth, but the question whether nitrogen availability constrains the magnitude of this ecosystem service remains unresolved. Synthesizing experiments from around the world, we show that CO2 fertilization is best explained by a simple interaction between nitrogen availability and mycorrhizal association. Plant species that associate with ectomycorrhizal fungi show a strong biomass increase (30 00± 3%, P < 0.001) in response to elevated CO2 regardless of nitrogen availability, whereas low nitrogen availability limits CO2 fertilization (0 00± 5%, P = 0.946) in plants that associate with arbuscular mycorrhizal fungi. The incorporation of mycorrhizae in global carbon cycle models is feasible, and crucial if we are to accurately project ecosystem responses and feedbacks to climate change. Copyright 0008 2016, American Association for the Advancement of Science. |
| [94] | |
| [95] | Human activities have greatly accelerated emissions of both carbon dioxide and biologically reactive nitrogen to the atmosphere(1,2). As nitrogen availability often limits forest productivity(3), it has long been expected that anthropogenic nitrogen deposition could stimulate carbon sequestration in forests(4). However, spatially extensive evidence for deposition-induced stimulation of forest growth has been lacking, and quantitative estimates from models and plot-level studies are controversial(5-10). Here, we use forest inventory data to examine the impact of nitrogen deposition on tree growth, survival and carbon storage across the northeastern and north-central USA during the 1980s and 1990s. We show a range of growth and mortality responses to nitrogen deposition among the region's 24 most common tree species. Nitrogen deposition (which ranged from 3 to 11 kg ha(-1) yr(-1)) enhanced the growth of 11 species and decreased the growth of 3 species. Nitrogen deposition enhanced growth of all tree species with arbuscular mycorrhizal fungi associations. In the absence of disturbances that reduced carbon stocks by more than 50%, above-ground biomass increment increased by 61 kg of carbon per kg of nitrogen deposited, amounting to a 40% enhancement over pre-industrial conditions. Extrapolating to the globe, we estimate that nitrogen deposition could increase tree carbon storage by 0.31 Pg carbon yr(-1). |
| [96] | Summary The water-stability of aggregates in many soils is shown to depend on organic materials. The organic binding agents have been classified into (a) transient , mainly polysaccharides, (b), temporary , roots and fungal hyphae, and (c) persistent , resistant aromatic components associated with polyvalent metal cations, and strongly sorbed polymers. The effectiveness of various binding agents at different stages in the structural organization of aggregates is described and forms the basis of a model which illustrates the architecture of an aggregate. Roots and hyphae stabilize macro-aggregates, defined as > 250 m diameter; consequently, macroaggregation is controlled by soil management (i.e. crop rotations), as management influences the growth of plant roots, and the oxidation of organic carbon. The water-stability of micro-aggregates depends on the persistent organic binding agents and appears to be a characteristic of the soil, independent of management. |
| [97] | Summary 6168 Numerous field studies have measured mycorrhizal dynamics under additions of nitrogen (N), phosphorus (P), or atmospheric CO 2 to test the hypothesis that plants should invest in mycorrhizal fungi when soil nutrients are limiting. 6168 Here meta-analyses were used to integrate nutrient responses across independent field-based studies. Responses were compared between ecto- and arbuscular mycorrhizal fungi, and among fertilizer types, methods of measurement, biomes, and lead investigators. Relationships between degree of response and study length, fertilization rates, total amounts of nutrients applied, and numbers of replicates were also tested. 6168 Across studies, mycorrhizal abundance decreased 15% under N fertilization and 32% under P fertilization. Elevated CO 2 elicited a 47% increase. Nitrogen effects varied significantly among studies, and P effects varied significantly among lead investigators. Most other factors did not affect mycorrhizal responses. 6168 These results support the plant investment hypothesis, and suggest that global standing stocks of mycorrhizal fungi may increase substantially under elevated CO 2 but decline moderately under P additions. Effects of N deposition may be difficult to predict for individual ecosystems, with a slightly negative influence overall. |
| [98] | Abstract In this review, we discuss the potential for mycorrhizal fungi to act as a source or sink for carbon (C) under elevated CO 2 and nitrogen deposition. Mycorrhizal tissue has been estimated to comprise a significant fraction of soil organic matter and below-ground biomass in a range of systems. The current body of literature indicates that in many systems exposed to elevated CO 2 , mycorrhizal fungi might sequester increased amounts of C in living, dead and residual hyphal biomass in the soil. Through this process, the fungi might serve as a negative feedback on the rise in atmospheric CO 2 levels caused by fossil fuel burning and deforestation. By contrast, a few preliminary studies suggest that N deposition might increase turnover rates of fungal tissue and negate CO 2 effects on hyphal biomass. If these latter responses are consistent among ecosystems, C storage in hyphae might decline in habitats surrounding agricultural and urban areas. When N additions occur without CO 2 enrichment, effects on mycorrhizal growth are inconsistent. We note that analyses of hyphal decomposition under elevated CO 2 and N additions are extremely sparse but are critical in our understanding of the impact of global change on the cycling of mycorrhizal C. Finally, shifts in the community composition of arbuscular and ectomycorrhizal fungi with increasing CO 2 or N availability are frequently documented. Since mycorrhizal groups vary in growth rate and tissue quality, these changes in species assemblages could produce unforeseeable impacts on the productivity, survivorship, or decomposition of mycorrhizal biomass. |
| [99] | Abstract Almost all land plants form symbiotic associations with mycorrhizal fungi. These below-ground fungi play a key role in terrestrial ecosystems as they regulate nutrient and carbon cycles, and influence soil structure and ecosystem multifunctionality. Up to 80% of plant N and P is provided by mycorrhizal fungi and many plant species depend on these symbionts for growth and survival. Estimates suggest that there are c. 50 000 fungal species that form mycorrhizal associations with c. 250 000 plant species. The development of high-throughput molecular tools has helped us to better understand the biology, evolution, and biodiversity of mycorrhizal associations. Nuclear genome assemblies and gene annotations of 33 mycorrhizal fungal species are now available providing fascinating opportunities to deepen our understanding of the mycorrhizal lifestyle, the metabolic capabilities of these plant symbionts, the molecular dialogue between symbionts, and evolutionary adaptations across a range of mycorrhizal associations. Large-scale molecular surveys have provided novel insights into the diversity, spatial and temporal dynamics of mycorrhizal fungal communities. At the ecological level, network theory makes it possible to analyze interactions between plant-fungal partners as complex underground multi-species networks. Our analysis suggests that nestedness, modularity and specificity of mycorrhizal networks vary and depend on mycorrhizal type. Mechanistic models explaining partner choice, resource exchange, and coevolution in mycorrhizal associations have been developed and are being tested. This review ends with major frontiers for further research. 2015 The Authors. New Phytologist 2015 New Phytologist Trust. |
| [100] | Summary Fine roots and mycorrhiza often represent the largest input of carbon (C) into soils and are therefore of primary relevance to the soil C balance. Arbuscular mycorrhizal (AM) fungi have previously been found to increase litter decomposition which may lead to reduced soil C stocks, but these studies have focused on immediate decomposition of relatively high amounts of high-quality litter and may therefore not hold in many ecological settings over longer terms. Here, we assessed the effect of mycorrhizal fungi on the fate of C and nitrogen (N) contained within a realistic amount of highly 13C-/15N-labelled root litter in soil. This litter was either added fresh or after a 3-month incubation period under field conditions to a hyphal in-growth core where mycorrhizal abundance was either reduced or not through rotation. After 3 months of incubation with a plant under glasshouse conditions, the effect of turning cores on residual 13C and 15N inside the cores was measured, as well as 13C incorporation in microbial signature fatty acids and 15N incorporation of plants. Turning of cores increased the abundance of fungal decomposers and 13C loss from cores, while 15N content of cores and plants was unaffected. Despite the difference in disturbance that turning the cores could have caused, the results suggest that mycorrhizal fungi and field incubation of litter acted to additively increase the proportion of 13C left in cores. Synthesis . Apart from stimulating litter decomposition as previously shown, mycorrhizas can also stabilize C during litter decomposition and this effect is persistent through time. |
| [101] | Nitrogen is a major nutrient that frequently limits primary productivity in terrestrial ecosystems. Therefore, the physiological responses of plants to soil nitrogen (N) availability have been extensively investigated, and the study of the soil N-cycle has become an important component of ecosystem ecology and biogeochemistry. The bulk of the literature in these areas has, however, overlooked the fact that most plants form mycorrhizal associations, and that nutrient uptake is therefore mediated by mycorrhizal fungi. It is well established that ecto- and ericoid mycorrhizas influence N nutrition of plants, but roles of arbuscular mycorrhizas in N nutrition are less well established; perhaps even more importantly, current conceptual models ignore possible influences of arbuscular mycorrhizal (AM) fungi on N-cycling processes. We review evidence for the interaction between the AM symbiosis with microbes and processes involved in soil N-cycling. We show that to date investigations have rather poorly addressed such interactions and discuss possible reasons for this. We outline mechanisms that could potentially operate with regards to AM fungal N-cycling interactions, discuss experimental designs aimed at studying these, and conclude by pointing out priorities for future research. |
| [102] | The knowledge of tree species effects on soil organic carbon (C) turnover based on rigorous experimental designs is limited for common European deciduous tree species. We assessed soil respiration, and rates of C turnover in six tree species in a more than 30-year-old common garden experiment replicated at six sites in Denmark. The studied tree species were the broadleaves beech ( Fagus sylvatica L.), pedunculate oak ( Quercus robur L.), lime ( Tilia cordata L.), sycamore maple ( Acer pseudoplatanus L.) and ash ( Fraxinus excelsior L.) and the conifer Norway spruce ( Picea abies (L.) Karst.). Rates of C turnover were estimated by (i) the ratio of estimated soil heterotrophic respiration ( R h) to C stock in forest floor and top mineral soil, (ii) the ratio of litterfall C to forest floor C, (iii) foliar mass loss in litterbags, and (iv) mineral soil C turnover assessed by laboratory incubation. Soil respiration differed significantly among several species and increased in the order beech < lime < spruce = oak = maple < ash. Soil respiration was temperature limited with no significant species difference in Q 10. Norway spruce soils were significantly driest, and soil respiration was also limited by soil moisture. Carbon turnover rates based on the ratio between R h and C stock were significantly higher in ash than in all other species except maple, and maple also had higher C turnover than spruce. A similar influence of tree species on C turnover was indicated by the litterfall C to forest floor C ratio and by foliar mass loss; rates of C turnover increased in the order spruce < beech < oak < ash = lime = maple with significant differences between several of the species. Mineral soil C turnover during laboratory incubation was highest for ash, maple and oak, and significantly lower for spruce. The indices of soil C turnover were largely consistent and some were significantly correlated. Differences in C turnover were for the most part attributable to variation in litter quality and microclimatic conditions. Litterfall foliar N, Ca and Mg concentrations and to some extent lignin concentration correlated best with C turnover indices that integrated the forest floor. The results suggests that specific traits of Norway spruce and these five common broadleaf forest species should be taken into account in the modelling of soil C stock dynamics over decades. |
| [103] | For targeted use of tree species to sequester soil C we must identify the processes related to C input and output, particularly belowground, that control SOC stock differences. We should also study forms and stability of C along with bulk C stocks to assess whether certain broadleaves store C in more stable form. Joint cooperation is needed to support syntheses and process-oriented work on tree species and SOC, e.g. through an international network of common garden experiments. |
| [104] | The knowledge of tree species effects on soil C and N pools is scarce, particularly for European deciduous tree species. We studied forest floor and mineral soil carbon and nitrogen under six common European tree species in a common garden design replicated at six sites in Denmark. Three decades after planting the six tree species had different profiles in terms of litterfall, forest floor and mineral soil C and N attributes. Three groups were identified: (1) ash, maple and lime, (2) beech and oak, and (3) spruce. There were significant differences in forest floor and soil C and N contents and C/N ratios, also among the five deciduous tree species. The influence of tree species was most pronounced in the forest floor, where C and N contents increased in the order ash02=02lime02=02maple02<02oak02=02beech026102spruce. Tree species influenced mineral soil only in some of the sampled soil layers within 3002cm depth. Species with low forest floor C and N content had more C and N in the mineral soil. This opposite trend probably offset the differences in forest floor C and N with no significant difference between tree species in C and N contents of the whole soil profile. The effect of tree species on forest floor C and N content was primarily attributed to large differences in turnover rates as indicated by fractional annual loss of forest floor C and N. The C/N ratio of foliar litterfall was a good indicator of forest floor C and N contents, fractional annual loss of forest floor C and N, and mineral soil N status. Forest floor and litterfall C/N ratios were not related, whereas the C/N ratio of mineral soil (0–3002cm) better indicated N status under deciduous species on rich soil. The results suggest that European deciduous tree species differ in C and N sequestration rates within forest floor and mineral soil, respectively, but there is little evidence of major differences in the combined forest floor and mineral soil after three decades. |
| [105] | . |
| [106] | . |
| [107] | |
| [108] | The breakdown of organic nitrogen in soil is a potential rate-limiting step in nitrogen cycling. Arbuscular mycorrhizal (AM) fungi are root symbionts that might improve the ability of plants to compete for organic nitrogen products against other decomposer microbes. However, AM uptake of organic nitrogen, especially in natural systems, has traditionally been difficult to test. We developed a novel quantitative nanotechnological technique to determine in situ that organic nitrogen uptake by AM fungi can occur to a greater extent than has previously been assumed. Specifically, we found that AM fungi acquired recalcitrant and labile forms of organic nitrogen. Moreover, N enrichment of soil reduced plot-scale uptake of these compounds. Since most plants host AM fungi, AM use of organic nitrogen could widely influence plant productivity, especially where N availability is relatively low. |
| [109] | Global data sets provide strong evidence of convergence for leaf structure with leaf longevity such that species having thick leaves, low specific leaf area, low mass-based nitrogen concentrations, and low photosynthetic rates typically exhibit long leaf life span. Leaf longevity and corresponding leaf structure have also been widely linked to plant potential growth rate, plant competition, and nutrient cycling. We hypothesized that selection forces leading to variation in leaf longevity and leaf structure have acted simultaneously and in similar directions on the longevity and structure of the finest root orders. Our four-year study investigated the links between root and leaf life span and root and leaf structure among 11 north-temperate tree species in a common garden in central Poland. Study species included the hardwoods Acer pseudoplatanus L., Acer platanoides L., Fagus sylvatica L., Quercus robur L., and Tilia cordata Mill.; and the conifers Abies alba Mill., Larix decidua Mill., Picea abies (L.) Karst., Pinus nigra Arnold, Pinus sylvestris L., and Pseudotsuga menziesii (Mirbel) Franco. Leaf life span, estimated by phenological observations and needle cohort measurements, ranged from 0.5 to 8 yr among species. Median fine-root life span, estimated using minirhizotron images of individual roots, ranged from 0.5 to 2.5 yr among species. Root life span was not correlated with leaf life span, but specific root length was significantly correlated with specific leaf area. Root nitrogen: carbon ratio was negatively correlated with root longevity, which corroborates previous research that has suggested a trade-off between organ life span and higher organ N concentrations. Specific traits such as thickened outer tangential walls of the exodermis were better predictors of long-lived roots than tissue density or specific root length, which have been correlated with life span in previous studies. Although theories linking organ structure and function suggest that similar root and leaf traits should be linked to life span and that root and leaf life span should be positively correlated, our results suggest that tissue structure and longevity aboveground (leaves) can contrast markedly with that belowground (roots). |
| [110] | (4) Collectively, our results indicate that the effects of roots on nutrient cycling are consequential, particularly in forests where the C cost of mining nutrients from decomposing soil organic matter may be greatest (e.g., ECM-dominated stands). Further, our results suggest that small C fluxes from exudation may have disproportionate impacts on ecosystem N cycling in nutrient-limited forests. |
| [111] | Summary Despite the recognition that the capacity to acquire N is critical in plant response to CO 2 enrichment, there is little information on how elevated CO 2 affects root N uptake kinetics. The few available data indicate a highly variable pattern of response to elevated CO 2 , but it is presently unclear if the observed inconsistencies are caused by differences in experimental protocols or by true species differences. Furthermore, if there are interspecific variations in N uptake responses to elevated CO 2 , it is not clear whether these are associated with different functional groups. Accordingly, we examined intact root-system NH 4 + and NO 3 – uptake kinetic responses to elevated CO 2 in seedlings of six temperate forest tree species, representing (i) fast- vs. slow-growers and (ii) broad-leaves vs. conifers, that were cultured and assayed in otherwise similar conditions. In general, the species tested had a higher uptake capacity ( V max ) for NH 4 + than for NO 3 – . Species substantially differed in their NO 3 – and NH 4 + uptake capacities, but the interspecific differences were markedly greater for NO 3 – than NH 4 + uptake. Elevated CO 2 had a species-dependent effect on root uptake capacity for NH 4 + ranging from an increase of 215% in Acer negundo L. to a decrease of about 40% in Quercus macrocarpa Michx. In contrast, NO 3 – uptake capacity responded little to CO 2 in all the species except A. negundo in which it was significantly down-regulated at elevated CO 2 . Across species, the capacity for NH 4 + uptake was positively correlated with the relative growth rate (RGR) of species; however, the CO 2 effect on NH 4 + uptake capacity could not be explained by changes in RGR. The observed variation in NH 4 + uptake response to elevated CO 2 was also inconsistent with life-form differences. Other possible mechanisms that may explain why elevated CO 2 elicits a species-specific response in root N uptake kinetics are discussed. Despite the fact that the exact mechanism(s) for such interspecific variation remains unresolved, these differences may have a significant implication for competitive interactions and community responses to elevated CO 2 environment. We suggest that differential species responses in nutrient uptake capacity could be one potential mechanism for the CO 2 -induced shifts in net primary productivity and species composition that have been observed in experimental communities exposed to elevated levels of CO 2 . |
| [112] | |
| [113] | We studied the effects of mycorrhizal pitch pine (Pinus rigida) roots on litter decomposition, microbial biomass, nematode abundance and inorganic nutrients in the E horizon material of a spodosolic soil, using field microcosms created in a regenerating pitch pine stand in the New Jersey Pinelands. Pine roots stimulated litter decomposition by 18.7% by the end of the 29 month study. Both mass loss and N and P release from the litter were always higher in the presence of roots than in their absence. Nutrient concentrations in decomposing litter were similar, however, in the presence and absence of roots, which suggests that the roots present in the with-root treatment did not withdraw nutrients directly from the litter. The soil was slightly drier in the presence of roots, but there was no discernible effect on soil microbial biomass. The effects of roots on soil extractable inorganic nutrients were inconsistent. Roots, however, were consistently associated with higher numbers of soil nematodes. These results suggest that, in soils with low total C and N contents, roots stimulate greater activity of the soil biota, which contribute, in turn, to faster litter decomposition and nutrient release. |
1
2011
... 一般认为, AM真菌因不能通过分泌胞外酶获取复杂有机质中的N, 所以对植物获取N的过程没有影响, 其主要功能是促进植物对土壤磷的摄取能力.然而, 近年来越来越多的试验发现AM真菌能够促进植物对N的吸收, 且其吸收方式及能力随土壤N水平而变(
Whether in life or in death: Fresh perspectives on how plants affect biogeochemical cycling.
1
2015
... 与树种的叶习性和谱系分类(例如针叶裸子植物与阔叶被子植物)相比, 按菌根类型进行树种分类能更好地解释森林生态系统土壤C、N循环的变异性乃至森林生产力对全球变化的响应(
Slowed decomposition in ectomycorrhizal ecosystems is independent of plant chemistry.
2
2016
... 地上及地下凋落物输入是矿质土层C、N的主要输入过程; 不同菌根树种对这一过程影响的研究不多, 对其机理尚不清楚(
... 菌根对森林土壤C输出的减少效应主要是指菌根真菌与自由微生物的相互作用对分解过程产生了消极影响, 即加吉尔效应(
Ectomycorrhizal fungi slow soil carbon cycling.
1
2016
... 菌根对森林土壤C输出的减少效应主要是指菌根真菌与自由微生物的相互作用对分解过程产生了消极影响, 即加吉尔效应(
Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage.
2
2014
... 菌根是土壤真菌与植物根系形成的共生体, 存在于94%的维管植物根系中(
... 菌根对森林土壤C输出的减少效应主要是指菌根真菌与自由微生物的相互作用对分解过程产生了消极影响, 即加吉尔效应(
Long-term increase in nitrogen supply alters above- and below-ground ectomycorrhizal communities and increases the dominance of
1
2003
... 大气N沉降改变土壤养分状况, 影响菌根真菌及自由微生物的活性及群落结构, 进而调节土壤C、N循环过程(
Active and total microbial communities in forest soil are largely different and highly stratified during decomposition.
1
2012
... 菌根的存在也可能对森林土壤C输出无效应, 这就是生态位分化假说(
Going underground: Root traits as drivers of ecosystem processes.
1
2014
... 菌根是土壤真菌与植物根系形成的共生体, 存在于94%的维管植物根系中(
Ectomycorrhizal
1
2014
... 大气N沉降改变土壤养分状况, 影响菌根真菌及自由微生物的活性及群落结构, 进而调节土壤C、N循环过程(
Mycorrhizal associations and other means of nutrition of vascular plants: Understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis.
1
2009
... 菌根是土壤真菌与植物根系形成的共生体, 存在于94%的维管植物根系中(
Mycorrhizal type determines the magnitude and direction of root-induced changes in decomposition in a temperate forest.
4
2015
... 与树种的叶习性和谱系分类(例如针叶裸子植物与阔叶被子植物)相比, 按菌根类型进行树种分类能更好地解释森林生态系统土壤C、N循环的变异性乃至森林生产力对全球变化的响应(
... AM、EM树种的根系特性也是矿质土层C、N输入量的一个重要影响因素(
... 不同菌根类型森林凋落物质量影响其凋落物层C、N输出过程, 即AM树种凋落物质量较高, 质量损失较快, 最终导致AM凋落物层C、N储量较低(
... 菌根及其与自由微生物之间的相互作用对土壤C输出过程的影响有增加、减少和无效3种.增加效应主要指根际激发效应, 即菌根通过分泌活性有机质提高自由微生物活性, 促进有机质分解, 从而增加C输出(
Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition.
1
2000
... 大气N沉降改变土壤养分状况, 影响菌根真菌及自由微生物的活性及群落结构, 进而调节土壤C、N循环过程(
Principles of Terrestrial Ecosystem Ecology. 2nd edn. Springer
1
2011
... 与C循环相比, 不同菌根类型森林土壤N循环的研究结果较一致(
Dominant mycorrhizal association of trees alters carbon and nutrient cycling by selecting for microbial groups with distinct enzyme function.
1
2017
... 土壤团聚体主要通过将微生物与有机质进行空间隔离的物理方式降低土壤有机质的周转速率, 提高土壤有机质的稳定性(
Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2.
3
2012
... 菌根及其与自由微生物之间的相互作用对土壤C输出过程的影响有增加、减少和无效3种.增加效应主要指根际激发效应, 即菌根通过分泌活性有机质提高自由微生物活性, 促进有机质分解, 从而增加C输出(
... 浓度升高条件下的AM农作物培养试验(
... CO2浓度升高引起AM和EM森林地上、地下生物量分配策略及其土壤有机质稳定性的差异会影响AM和EM森林土壤C、N循环(
Roots and associated fungi drive long-term carbon sequestration in boreal forest.
1
2013
... AM、EM树种的根系特性也是矿质土层C、N输入量的一个重要影响因素(
Carbon cycling traits of plant species are linked with mycorrhizal strategy.
2
2001
... 广义的森林土壤由凋落物层和矿质土层共同组成, 但多数研究并未同时报道不同菌根类型森林对这两个土层C储量的差异, 因此很难总结出整个森林土壤剖面C储量差异的普适性结论(
... 地上及地下凋落物输入是矿质土层C、N的主要输入过程; 不同菌根树种对这一过程影响的研究不多, 对其机理尚不清楚(
The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter?
5
2013
... 地上及地下凋落物输入是矿质土层C、N的主要输入过程; 不同菌根树种对这一过程影响的研究不多, 对其机理尚不清楚(
... AM、EM树种的根系特性也是矿质土层C、N输入量的一个重要影响因素(
... ).此外, 根据MEMS模型(
... 土壤有机质能够通过与铁铝矿物(铁铝氧化物、铁铝离子等)结合的物理化学方式或形成团聚体的物理方式降低其生物有效性, 从而提高其稳定性(
... 菌根及其与自由微生物之间的相互作用对土壤C输出过程的影响有增加、减少和无效3种.增加效应主要指根际激发效应, 即菌根通过分泌活性有机质提高自由微生物活性, 促进有机质分解, 从而增加C输出(
Bacterial growth efficiency in natural aquatic systems.
1998
Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long-term enhancement of forest productivity under elevated CO2.
3
2011
... CO2浓度升高引起AM和EM森林地上、地下生物量分配策略及其土壤有机质稳定性的差异会影响AM和EM森林土壤C、N循环(
... EM森林土壤C、N循环过程对CO2浓度升高的响应与AM不同.CO2浓度升高导致EM森林土壤有机质含量降低(
... 不同菌根类型森林土壤C、N循环对CO2浓度升高响应会影响植物养分限制程度, 最终造成森林生产力对气候变化的响应不同(
Revisiting the “Gadgil effect”: Do interguild fungal interactions control carbon cycling in forest soils?
3
2016
... 不同菌根类型森林凋落物质量影响其凋落物层C、N输出过程, 即AM树种凋落物质量较高, 质量损失较快, 最终导致AM凋落物层C、N储量较低(
... 菌根对森林土壤C输出的减少效应主要是指菌根真菌与自由微生物的相互作用对分解过程产生了消极影响, 即加吉尔效应(
... 关于菌根抑制分解过程的假说, 除了养分竞争假说之外, 还有化学抑制、菌寄生、水分限制假说.化学抑制假说是指与自由微生物相比, 菌根真菌从寄主根系获取C资源, 受C限制的程度较小, 因此可能会产生更多的次级代谢产物, 以限制自由微生物的活性(
Linking litter decomposition of above- and below-ground organs to plant-soil feedbacks worldwide.
2
2013
... AM、EM树种的根系特性也是矿质土层C、N输入量的一个重要影响因素(
... ), 但目前尚未有研究比较两者的相对大小.综上所述, 不同菌根类型树种根系特性对有机质输入过程的影响不一致, 这可能与测定过程中受很多不确定性因子干扰有关(
Suppression of litter decomposition by mycorrhizal roots of
1
1975
... 菌根对森林土壤C输出的减少效应主要是指菌根真菌与自由微生物的相互作用对分解过程产生了消极影响, 即加吉尔效应(
Mycorrhiza and litter decomposition.
1
1971
... 菌根对森林土壤C输出的减少效应主要是指菌根真菌与自由微生物的相互作用对分解过程产生了消极影响, 即加吉尔效应(
The impact of six European tree species on the chemistry of mineral topsoil in forest plantations on former agricultural land.
1
2004
... 关于矿质土层C循环相关过程的差异尚存分歧, 并且大多数研究并未分析两个菌根树种对矿质土层C输入过程的影响, 主要比较了二者C输出过程的差异(土壤呼吸、异养呼吸).在全球尺度上, AM森林矿质土层C含量比EM高8.0% (
Elevated carbon dioxide increases soil nitrogen and phosphorus availability in a phosphorus-limited
1
2016
... 不同菌根类型森林土壤C、N循环对CO2浓度升高响应会影响植物养分限制程度, 最终造成森林生产力对气候变化的响应不同(
Uptake and transport of organic and inorganic nitrogen by arbuscular mycorrhizal fungi.
1
2000
... 一般认为, AM真菌因不能通过分泌胞外酶获取复杂有机质中的N, 所以对植物获取N的过程没有影响, 其主要功能是促进植物对土壤磷的摄取能力.然而, 近年来越来越多的试验发现AM真菌能够促进植物对N的吸收, 且其吸收方式及能力随土壤N水平而变(
Tree species effects on soil organic matter dynamics: The role of soil cation composition.
2
2007
... 地上及地下凋落物输入是矿质土层C、N的主要输入过程; 不同菌根树种对这一过程影响的研究不多, 对其机理尚不清楚(
... AM、EM树种的根系特性也是矿质土层C、N输入量的一个重要影响因素(
Tree species effects on decomposition and forest floor dynamics in a common garden.
1
2006
... 土壤动物活动是凋落物及矿质土层有机质输入的又一影响因子(
An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material.
1
2001
... 菌根及其与自由微生物之间的相互作用对土壤C输出过程的影响有增加、减少和无效3种.增加效应主要指根际激发效应, 即菌根通过分泌活性有机质提高自由微生物活性, 促进有机质分解, 从而增加C输出(
Nutritional ecology of arbuscular mycorrhizal fungi.
1
2010
... 一般认为, AM真菌因不能通过分泌胞外酶获取复杂有机质中的N, 所以对植物获取N的过程没有影响, 其主要功能是促进植物对土壤磷的摄取能力.然而, 近年来越来越多的试验发现AM真菌能够促进植物对N的吸收, 且其吸收方式及能力随土壤N水平而变(
Arbuscular mycorrhiza and nitrogen: Implications for individual plants through to ecosystems.
2
2015
... 一般认为, AM真菌因不能通过分泌胞外酶获取复杂有机质中的N, 所以对植物获取N的过程没有影响, 其主要功能是促进植物对土壤磷的摄取能力.然而, 近年来越来越多的试验发现AM真菌能够促进植物对N的吸收, 且其吸收方式及能力随土壤N水平而变(
... ;
Decreases in soil moisture and organic matter quality suppress microbial decomposition following a boreal forest fire.
1
2015
... 关于菌根抑制分解过程的假说, 除了养分竞争假说之外, 还有化学抑制、菌寄生、水分限制假说.化学抑制假说是指与自由微生物相比, 菌根真菌从寄主根系获取C资源, 受C限制的程度较小, 因此可能会产生更多的次级代谢产物, 以限制自由微生物的活性(
球囊霉素相关土壤蛋白根际环境功能研究进展
2
2011
... 土壤团聚体主要通过将微生物与有机质进行空间隔离的物理方式降低土壤有机质的周转速率, 提高土壤有机质的稳定性(
... ;
Soil carbon and nitrogen cycling and storage throughout the soil profile in a sweetgum plantation after 11 years of CO2-enrichment.
5
2012
... CO2浓度升高引起AM和EM森林地上、地下生物量分配策略及其土壤有机质稳定性的差异会影响AM和EM森林土壤C、N循环(
... 浓度升高促进地下细根生长, 但没有缓解植物受养分限制的程度, 进而影响森林生产力(
... 浓度升高对土壤呼吸速率影响并不显著, 也未出现显著的激发效应(
... ;
... 不同菌根类型森林土壤C、N循环对CO2浓度升高响应会影响植物养分限制程度, 最终造成森林生产力对气候变化的响应不同(
Reduction of forest soil respiration in response to nitrogen deposition.
1
2010
... 大气N沉降改变土壤养分状况, 影响菌根真菌及自由微生物的活性及群落结构, 进而调节土壤C、N循环过程(
Elevated atmospheric carbon dioxide increases soil carbon.
2
2005
... CO2浓度升高引起AM和EM森林地上、地下生物量分配策略及其土壤有机质稳定性的差异会影响AM和EM森林土壤C、N循环(
... ).这可能是由于AM森林矿质土壤有机质以团聚体或有机质与矿物质结合体的形态存在, 稳定性较高, 微生物难以接触利用的缘故(
AM真菌在草原生态系统中的功能
1
2016
... 土壤团聚体主要通过将微生物与有机质进行空间隔离的物理方式降低土壤有机质的周转速率, 提高土壤有机质的稳定性(
Fungal secondary metabolism—From biochemistry to genomics.
1
2005
... 关于菌根抑制分解过程的假说, 除了养分竞争假说之外, 还有化学抑制、菌寄生、水分限制假说.化学抑制假说是指与自由微生物相比, 菌根真菌从寄主根系获取C资源, 受C限制的程度较小, 因此可能会产生更多的次级代谢产物, 以限制自由微生物的活性(
Soil organic N—An under-rated player for C sequestration in soils?
1
2011
... 地上及地下凋落物输入是矿质土层C、N的主要输入过程; 不同菌根树种对这一过程影响的研究不多, 对其机理尚不清楚(
No globally consistent effect of ectomycorrhizal status on foliar traits.
2012
Ectomycorrhizas and retarded decomposition in a
1
2003
... 关于菌根抑制分解过程的假说, 除了养分竞争假说之外, 还有化学抑制、菌寄生、水分限制假说.化学抑制假说是指与自由微生物相比, 菌根真菌从寄主根系获取C资源, 受C限制的程度较小, 因此可能会产生更多的次级代谢产物, 以限制自由微生物的活性(
Tannins in nutrient dynamics of forest ecosystems—A review.
1
2003
... 菌根真菌功能及其共生植物凋落物差异是造成不同菌根类型森林土壤溶解性无机N (铵盐、硝酸盐)及DON淋溶损失不同的主要原因(
Carbon sequestration in soil.
1
2015
... 菌根是土壤真菌与植物根系形成的共生体, 存在于94%的维管植物根系中(
Below-ground frontiers in trait-based plant ecology.
1
2016
... 菌根是土壤真菌与植物根系形成的共生体, 存在于94%的维管植物根系中(
Arbuscular mycorrhizal fungi reduce decomposition of woody plant litter while increasing soil aggregation.
1
2015
... 菌根对森林土壤C输出的减少效应主要是指菌根真菌与自由微生物的相互作用对分解过程产生了消极影响, 即加吉尔效应(
Multiple factors influence the role of arbuscular mycorrhizal fungi in soil aggregation—A meta-analysis.
1
2014
... 土壤团聚体主要通过将微生物与有机质进行空间隔离的物理方式降低土壤有机质的周转速率, 提高土壤有机质的稳定性(
丛枝菌根共生体的氮代谢运输及其生态作用
1
2013
... 一般认为, AM真菌因不能通过分泌胞外酶获取复杂有机质中的N, 所以对植物获取N的过程没有影响, 其主要功能是促进植物对土壤磷的摄取能力.然而, 近年来越来越多的试验发现AM真菌能够促进植物对N的吸收, 且其吸收方式及能力随土壤N水平而变(
Similar below-ground carbon cycling dynamics but contrasting modes of nitrogen cycling between arbuscular mycorrhizal and ectomycorrhizal forests.
10
2016
... 广义的森林土壤由凋落物层和矿质土层共同组成, 但多数研究并未同时报道不同菌根类型森林对这两个土层C储量的差异, 因此很难总结出整个森林土壤剖面C储量差异的普适性结论(
... ;
... ;
... 关于矿质土层C循环相关过程的差异尚存分歧, 并且大多数研究并未分析两个菌根树种对矿质土层C输入过程的影响, 主要比较了二者C输出过程的差异(土壤呼吸、异养呼吸).在全球尺度上, AM森林矿质土层C含量比EM高8.0% (
... 与C循环相比, 不同菌根类型森林土壤N循环的研究结果较一致(
... ;
... 地上及地下凋落物输入是矿质土层C、N的主要输入过程; 不同菌根树种对这一过程影响的研究不多, 对其机理尚不清楚(
... ;
... 土壤团聚体主要通过将微生物与有机质进行空间隔离的物理方式降低土壤有机质的周转速率, 提高土壤有机质的稳定性(
... 不同菌根类型森林凋落物质量影响其凋落物层C、N输出过程, 即AM树种凋落物质量较高, 质量损失较快, 最终导致AM凋落物层C、N储量较低(
Spatial separation of litter decomposition and mycorrhizal nitrogen uptake in a boreal forest.
2
2007
... 菌根的存在也可能对森林土壤C输出无效应, 这就是生态位分化假说(
... ).该假说可从菌根真菌与自由微生物酶功能差异的角度进行阐释.在凋落物层, 来自于地上输入的活性底物浓度较高, 微生物需要产生降解细胞壁的水解酶对其进行利用; 而在较深土层活性底物浓度较低, 难分解的木质素和腐殖质底物较多, 因此微生物需要产生氧化酶才能加以利用(
Ectomycorrhizal fungi—Potential organic matter decomposers, yet not saprotrophs.
1
2015
... 菌根的存在也可能对森林土壤C输出无效应, 这就是生态位分化假说(
土壤有机碳稳定机制研究进展
2
2007
... 土壤有机质能够通过与铁铝矿物(铁铝氧化物、铁铝离子等)结合的物理化学方式或形成团聚体的物理方式降低其生物有效性, 从而提高其稳定性(
... 土壤团聚体主要通过将微生物与有机质进行空间隔离的物理方式降低土壤有机质的周转速率, 提高土壤有机质的稳定性(
Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions—A review.
2
2006
... 土壤有机质能够通过与铁铝矿物(铁铝氧化物、铁铝离子等)结合的物理化学方式或形成团聚体的物理方式降低其生物有效性, 从而提高其稳定性(
... 土壤团聚体主要通过将微生物与有机质进行空间隔离的物理方式降低土壤有机质的周转速率, 提高土壤有机质的稳定性(
Environmental and stoichiometric controls on microbial carbon-use efficiency in soils.
1
2012
... 大气N沉降改变土壤养分状况, 影响菌根真菌及自由微生物的活性及群落结构, 进而调节土壤C、N循环过程(
Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes.
1
2015
... 菌根是土壤真菌与植物根系形成的共生体, 存在于94%的维管植物根系中(
Slowed decomposition is biotically mediated in an ectomycorrhizal, tropical rain forest.
1
2010
... 菌根对森林土壤C输出的减少效应主要是指菌根真菌与自由微生物的相互作用对分解过程产生了消极影响, 即加吉尔效应(
Decay rates of leaf litters from arbuscular mycorrhizal trees are more sensitive to soil effects than litters from ectomycorrhizal trees.
7
2015
... 菌根是土壤真菌与植物根系形成的共生体, 存在于94%的维管植物根系中(
... 与树种的叶习性和谱系分类(例如针叶裸子植物与阔叶被子植物)相比, 按菌根类型进行树种分类能更好地解释森林生态系统土壤C、N循环的变异性乃至森林生产力对全球变化的响应(
... 植物对菌根真菌的碳分配策略及土壤养分对菌根真菌的限制与全球变化密切相关, 尤其是全球CO2浓度升高及N沉降格局的改变等全球性的C、N变化.CO2浓度升高可提高森林生产力, 从而提高植物对菌根真菌的C分配比例; 大气N沉降增加可提高土壤肥力, 减缓菌根真菌及自由微生物受养分限制的程度; 不同菌根真菌对它们响应不一(
... 大气N沉降改变土壤养分状况, 影响菌根真菌及自由微生物的活性及群落结构, 进而调节土壤C、N循环过程(
... ).不同菌根类型的森林土壤养分对植物及微生物的限制程度不同, 因而会造成土壤C、N循环对N沉降响应不同.N添加实验通常加大AM森林凋落物、土壤有机质的分解速率(
... )或者影响不显著(
... ); 也可能是由于EM森林凋落物及有机质C:N过高, 导致其分解过程对土壤养分的变化不敏感(
Mycorrhizal associations of dominant trees influence nitrate leaching responses to N deposition.
6
2014
... 与树种的叶习性和谱系分类(例如针叶裸子植物与阔叶被子植物)相比, 按菌根类型进行树种分类能更好地解释森林生态系统土壤C、N循环的变异性乃至森林生产力对全球变化的响应(
... 与C循环相比, 不同菌根类型森林土壤N循环的研究结果较一致(
... )、硝酸盐的淋溶损失(
... 菌根真菌功能及其共生植物凋落物差异是造成不同菌根类型森林土壤溶解性无机N (铵盐、硝酸盐)及DON淋溶损失不同的主要原因(
... 植物对菌根真菌的碳分配策略及土壤养分对菌根真菌的限制与全球变化密切相关, 尤其是全球CO2浓度升高及N沉降格局的改变等全球性的C、N变化.CO2浓度升高可提高森林生产力, 从而提高植物对菌根真菌的C分配比例; 大气N沉降增加可提高土壤肥力, 减缓菌根真菌及自由微生物受养分限制的程度; 不同菌根真菌对它们响应不一(
... 大气N沉降改变土壤养分状况, 影响菌根真菌及自由微生物的活性及群落结构, 进而调节土壤C、N循环过程(
Resource stoichiometry and the biogeochemical consequences of nitrogen deposition in a mixed deciduous forest.
1
2016
... 综上所述, 表现为无机养分经济(土壤C、N矿化速率较快)的AM森林可能更能适应N沉降增加的改变, 进而提高森林生产力; 而表现为有机养分经济(土壤C、N矿化速率较慢)的EM森林对N沉降增加的响应并不积极, 因此可能会造成树木死亡或者生产力降低(
Interactions among roots, mycorrhizas and free-living microbial communities differentially impact soil carbon processes.
3
2015
... 与树种的叶习性和谱系分类(例如针叶裸子植物与阔叶被子植物)相比, 按菌根类型进行树种分类能更好地解释森林生态系统土壤C、N循环的变异性乃至森林生产力对全球变化的响应(
... ).然而, 由于试验方法、研究尺度等限制, 不同菌根类型树种对森林土壤C、N循环过程的影响机制仍存在较大的不确定性(
... 不同菌根类型森林凋落物质量影响其凋落物层C、N输出过程, 即AM树种凋落物质量较高, 质量损失较快, 最终导致AM凋落物层C、N储量较低(
Synthesis of enzymes connected with mycoparasitism by ectomycorrhizal fungi.
1
2006
... 关于菌根抑制分解过程的假说, 除了养分竞争假说之外, 还有化学抑制、菌寄生、水分限制假说.化学抑制假说是指与自由微生物相比, 菌根真菌从寄主根系获取C资源, 受C限制的程度较小, 因此可能会产生更多的次级代谢产物, 以限制自由微生物的活性(
Tree species effects on coupled cycles of carbon, nitrogen, and acidity in mineral soils at a common garden experiment.
3
2012
... 关于矿质土层C循环相关过程的差异尚存分歧, 并且大多数研究并未分析两个菌根树种对矿质土层C输入过程的影响, 主要比较了二者C输出过程的差异(土壤呼吸、异养呼吸).在全球尺度上, AM森林矿质土层C含量比EM高8.0% (
... AM、EM树种的根系特性也是矿质土层C、N输入量的一个重要影响因素(
... 不同菌根类型森林土壤C、N循环对CO2浓度升高响应会影响植物养分限制程度, 最终造成森林生产力对气候变化的响应不同(
Effects of litter traits, soil biota, and soil chemistry on soil carbon stocks at a common garden with 14 tree species.
2
2015
... 关于矿质土层C循环相关过程的差异尚存分歧, 并且大多数研究并未分析两个菌根树种对矿质土层C输入过程的影响, 主要比较了二者C输出过程的差异(土壤呼吸、异养呼吸).在全球尺度上, AM森林矿质土层C含量比EM高8.0% (
... 土壤动物活动是凋落物及矿质土层有机质输入的又一影响因子(
Comments on “mycorrhizal association as a primary control of the CO2 fertilization effect”.
2017
Root and arbuscular mycorrhizal mycelial interactions with soil microorganisms in lowland tropical forest.
2
2013
... 菌根及其与自由微生物之间的相互作用对土壤C输出过程的影响有增加、减少和无效3种.增加效应主要指根际激发效应, 即菌根通过分泌活性有机质提高自由微生物活性, 促进有机质分解, 从而增加C输出(
... )、更有持续性(
Impact of tree species on soil carbon stocks and soil acidity in southern Sweden.
1
2006
... AM、EM树种的根系特性也是矿质土层C、N输入量的一个重要影响因素(
Organic nutrient uptake by mycorrhizal fungi enhances ecosystem carbon storage: A model-based assessment.
2
2011
... 菌根是土壤真菌与植物根系形成的共生体, 存在于94%的维管植物根系中(
... 菌根对森林土壤C输出的减少效应主要是指菌根真菌与自由微生物的相互作用对分解过程产生了消极影响, 即加吉尔效应(
Arbuscular mycorrhizal hyphae promote priming of native soil organic matter mineralisation.
2
2016
... 不同菌根类型森林凋落物质量影响其凋落物层C、N输出过程, 即AM树种凋落物质量较高, 质量损失较快, 最终导致AM凋落物层C、N储量较低(
... 菌根及其与自由微生物之间的相互作用对土壤C输出过程的影响有增加、减少和无效3种.增加效应主要指根际激发效应, 即菌根通过分泌活性有机质提高自由微生物活性, 促进有机质分解, 从而增加C输出(
The nature and dynamics of soil organic matter: Plant inputs, microbial transformations, and organic matter stabilization.
1
2016
... 土壤团聚体主要通过将微生物与有机质进行空间隔离的物理方式降低土壤有机质的周转速率, 提高土壤有机质的稳定性(
Ectomycorrhizal fungi and the enzymatic liberation of nitrogen from soil organic matter: Why evolutionary history matters.
1
2017
... 不同菌根类型森林土壤C、N循环对CO2浓度升高响应会影响植物养分限制程度, 最终造成森林生产力对气候变化的响应不同(
Ectomycorrhizal fungi contribute to soil organic matter cycling in sub-boreal forests.
2
2014
... 菌根对森林土壤C输出的减少效应主要是指菌根真菌与自由微生物的相互作用对分解过程产生了消极影响, 即加吉尔效应(
... 菌根的存在也可能对森林土壤C输出无效应, 这就是生态位分化假说(
The mycorrhizal- associated nutrient economy: A new framework for predicting carbon-nutrient couplings in temperate forests.
5
2013
... 菌根是土壤真菌与植物根系形成的共生体, 存在于94%的维管植物根系中(
... 与树种的叶习性和谱系分类(例如针叶裸子植物与阔叶被子植物)相比, 按菌根类型进行树种分类能更好地解释森林生态系统土壤C、N循环的变异性乃至森林生产力对全球变化的响应(
... 与C循环相比, 不同菌根类型森林土壤N循环的研究结果较一致(
... 地上及地下凋落物输入是矿质土层C、N的主要输入过程; 不同菌根树种对这一过程影响的研究不多, 对其机理尚不清楚(
... 大气N沉降改变土壤养分状况, 影响菌根真菌及自由微生物的活性及群落结构, 进而调节土壤C、N循环过程(
Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation.
2
2011
... EM森林土壤C、N循环过程对CO2浓度升高的响应与AM不同.CO2浓度升高导致EM森林土壤有机质含量降低(
... 不同菌根类型森林土壤C、N循环对CO2浓度升高响应会影响植物养分限制程度, 最终造成森林生产力对气候变化的响应不同(
Roots and fungi accelerate carbon and nitrogen cycling in forests exposed to elevated CO2.
3
2012
... 菌根是土壤真菌与植物根系形成的共生体, 存在于94%的维管植物根系中(
... EM森林土壤C、N循环过程对CO2浓度升高的响应与AM不同.CO2浓度升高导致EM森林土壤有机质含量降低(
... ,
Litter decomposition: What controls it and how can we alter it to sequester more carbon in forest soils?
2
2010
... 地上及地下凋落物输入是矿质土层C、N的主要输入过程; 不同菌根树种对这一过程影响的研究不多, 对其机理尚不清楚(
... 土壤动物活动是凋落物及矿质土层有机质输入的又一影响因子(
Mycorrhizas and nutrient cycling in ecosystems—A journey towards relevance?
1
2003
... EM真菌能通过产生胞外酶促进复杂有机质分解, 从而获取土壤中的DON, 缓解自身及植物受到的养分限制(
Linking litter calcium, earthworms and soil properties: A common garden test with 14 tree species.
1
2005
... 土壤动物活动是凋落物及矿质土层有机质输入的又一影响因子(
Arbuscular mycorrhizae and terrestrial ecosystem processes.
1
2004
... 土壤团聚体主要通过将微生物与有机质进行空间隔离的物理方式降低土壤有机质的周转速率, 提高土壤有机质的稳定性(
Plant root and mycorrhizal fungal traits for understanding soil aggregation.
1
2015
... 土壤团聚体主要通过将微生物与有机质进行空间隔离的物理方式降低土壤有机质的周转速率, 提高土壤有机质的稳定性(
Mycorrhizas and soil structure.
4
2006
... 土壤团聚体主要通过将微生物与有机质进行空间隔离的物理方式降低土壤有机质的周转速率, 提高土壤有机质的稳定性(
... ).若AM根系生物量显著高于EM, 则推测前者根系通过物理作用促进土壤团聚过程的程度可能更大; 而由于EM菌丝的根状菌索(rhizomorphs)及其生命周期(平均11个月)较长于AM菌丝(5-32天), EM菌丝通过缠绕、网捕等物理过程改变土壤团聚过程的效应可能更明显.二是根系及菌丝分泌物对土壤颗粒的胶结作用也能促使土壤团聚体的形成(
... ;
... ).EM真菌则可通过生产疏水蛋白影响土壤团聚过程(
Glomalin, an arbuscular-mycorrhizal fungal soil protein, responds to land-use change.
1
2003
... 土壤团聚体主要通过将微生物与有机质进行空间隔离的物理方式降低土壤有机质的周转速率, 提高土壤有机质的稳定性(
The role of arbuscular mycorrhizal fungi and glomalin in soil aggregation: Comparing effects of five plant species.
2002
Patterns of DON and DOC leaching losses across a natural N availability gradient in temperate hardwood forests.
4
2017
... 与C循环相比, 不同菌根类型森林土壤N循环的研究结果较一致(
... 地上及地下凋落物输入是矿质土层C、N的主要输入过程; 不同菌根树种对这一过程影响的研究不多, 对其机理尚不清楚(
... 菌根真菌功能及其共生植物凋落物差异是造成不同菌根类型森林土壤溶解性无机N (铵盐、硝酸盐)及DON淋溶损失不同的主要原因(
... EM森林土壤C:N高于AM森林, 可能会进一步抑制无机N的淋溶损失(
a). 菌根类型对森林树木净初级生产力的影响
1
2012
... AM、EM树种的根系特性也是矿质土层C、N输入量的一个重要影响因素(
b). 不同菌根类型的森林净初级生产力对气温变化的响应
1
2012
... 菌根是土壤真菌与植物根系形成的共生体, 存在于94%的维管植物根系中(
A history of research on the link between (micro) aggregates, soil biota, and soil organic matter dynamics.
1
2004
... 土壤团聚体主要通过将微生物与有机质进行空间隔离的物理方式降低土壤有机质的周转速率, 提高土壤有机质的稳定性(
Stabilization of newly formed amino acid metabolites in soil by clay minerals.
1
1972
... 土壤有机质能够通过与铁铝矿物(铁铝氧化物、铁铝离子等)结合的物理化学方式或形成团聚体的物理方式降低其生物有效性, 从而提高其稳定性(
Quantitative assessment of the differential impacts of arbuscular and ectomycorrhiza on soil carbon cycling.
4
2015
... 菌根是土壤真菌与植物根系形成的共生体, 存在于94%的维管植物根系中(
... ;
... 与树种的叶习性和谱系分类(例如针叶裸子植物与阔叶被子植物)相比, 按菌根类型进行树种分类能更好地解释森林生态系统土壤C、N循环的变异性乃至森林生产力对全球变化的响应(
... 不同菌根类型森林凋落物质量影响其凋落物层C、N输出过程, 即AM树种凋落物质量较高, 质量损失较快, 最终导致AM凋落物层C、N储量较低(
Dynamics and pathways of autotrophic and heterotrophic soil CO2 efflux revealed by forest girdling.
1
2010
... 菌根及其与自由微生物之间的相互作用对土壤C输出过程的影响有增加、减少和无效3种.增加效应主要指根际激发效应, 即菌根通过分泌活性有机质提高自由微生物活性, 促进有机质分解, 从而增加C输出(
Feedbacks between plant N demand and rhizosphere priming depend on type of mycorrhizal association.
1
2017
... 菌根及其与自由微生物之间的相互作用对土壤C输出过程的影响有增加、减少和无效3种.增加效应主要指根际激发效应, 即菌根通过分泌活性有机质提高自由微生物活性, 促进有机质分解, 从而增加C输出(
Elevated carbon dioxide and ozone alter productivity and ecosystem carbon content in northern temperate forests.
2
2014
... EM森林土壤C、N循环过程对CO2浓度升高的响应与AM不同.CO2浓度升高导致EM森林土壤有机质含量降低(
... ;
Mycorrhizal associations of trees have different indirect effects on organic matter decomposition.
3
2016
... 广义的森林土壤由凋落物层和矿质土层共同组成, 但多数研究并未同时报道不同菌根类型森林对这两个土层C储量的差异, 因此很难总结出整个森林土壤剖面C储量差异的普适性结论(
... AM、EM树种的根系特性也是矿质土层C、N输入量的一个重要影响因素(
... 不同菌根类型森林凋落物质量影响其凋落物层C、N输出过程, 即AM树种凋落物质量较高, 质量损失较快, 最终导致AM凋落物层C、N储量较低(
Mycorrhizal association as a primary control of the CO2 fertilization effect.
6
2016
... 菌根是土壤真菌与植物根系形成的共生体, 存在于94%的维管植物根系中(
... 与树种的叶习性和谱系分类(例如针叶裸子植物与阔叶被子植物)相比, 按菌根类型进行树种分类能更好地解释森林生态系统土壤C、N循环的变异性乃至森林生产力对全球变化的响应(
... 植物对菌根真菌的碳分配策略及土壤养分对菌根真菌的限制与全球变化密切相关, 尤其是全球CO2浓度升高及N沉降格局的改变等全球性的C、N变化.CO2浓度升高可提高森林生产力, 从而提高植物对菌根真菌的C分配比例; 大气N沉降增加可提高土壤肥力, 减缓菌根真菌及自由微生物受养分限制的程度; 不同菌根真菌对它们响应不一(
... CO2浓度升高引起AM和EM森林地上、地下生物量分配策略及其土壤有机质稳定性的差异会影响AM和EM森林土壤C、N循环(
... 不同菌根类型森林土壤C、N循环对CO2浓度升高响应会影响植物养分限制程度, 最终造成森林生产力对气候变化的响应不同(
... ), 因此AM生态系统在土壤可利用N含量较低时可能不足以维持其生物量的增长(
Response to comment on “Mycorrhizal association as a primary control of the CO2 fertilization effect”.
4
2017
... 菌根是土壤真菌与植物根系形成的共生体, 存在于94%的维管植物根系中(
... 与树种的叶习性和谱系分类(例如针叶裸子植物与阔叶被子植物)相比, 按菌根类型进行树种分类能更好地解释森林生态系统土壤C、N循环的变异性乃至森林生产力对全球变化的响应(
... 不同菌根类型森林土壤C、N循环对CO2浓度升高响应会影响植物养分限制程度, 最终造成森林生产力对气候变化的响应不同(
... 浓度升高条件下能够维持生物量的增长(
Increased tree carbon storage in response to nitrogen deposition in the US.
2
2010
... 植物对菌根真菌的碳分配策略及土壤养分对菌根真菌的限制与全球变化密切相关, 尤其是全球CO2浓度升高及N沉降格局的改变等全球性的C、N变化.CO2浓度升高可提高森林生产力, 从而提高植物对菌根真菌的C分配比例; 大气N沉降增加可提高土壤肥力, 减缓菌根真菌及自由微生物受养分限制的程度; 不同菌根真菌对它们响应不一(
... 综上所述, 表现为无机养分经济(土壤C、N矿化速率较快)的AM森林可能更能适应N沉降增加的改变, 进而提高森林生产力; 而表现为有机养分经济(土壤C、N矿化速率较慢)的EM森林对N沉降增加的响应并不积极, 因此可能会造成树木死亡或者生产力降低(
Organic matter and water-stable aggregates in soils.
2
1982
... 土壤团聚体主要通过将微生物与有机质进行空间隔离的物理方式降低土壤有机质的周转速率, 提高土壤有机质的稳定性(
... ).AM与EM树种根系分泌物数量及分泌时间的差异可能会造成土壤团聚过程的不同.AM生产的球囊霉素(glomalin)相关土壤蛋白(GRSP)能够作为胶结剂直接影响土壤团聚体的形成及稳定(
A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies.
4
2004
... 植物对菌根真菌的碳分配策略及土壤养分对菌根真菌的限制与全球变化密切相关, 尤其是全球CO2浓度升高及N沉降格局的改变等全球性的C、N变化.CO2浓度升高可提高森林生产力, 从而提高植物对菌根真菌的C分配比例; 大气N沉降增加可提高土壤肥力, 减缓菌根真菌及自由微生物受养分限制的程度; 不同菌根真菌对它们响应不一(
... CO2浓度升高引起AM和EM森林地上、地下生物量分配策略及其土壤有机质稳定性的差异会影响AM和EM森林土壤C、N循环(
... 大气N沉降改变土壤养分状况, 影响菌根真菌及自由微生物的活性及群落结构, 进而调节土壤C、N循环过程(
... ;
Mycorrhizal fungi have a potential role in soil carbon storage under elevated CO2 and nitrogen deposition.
1
2000
... 植物对菌根真菌的碳分配策略及土壤养分对菌根真菌的限制与全球变化密切相关, 尤其是全球CO2浓度升高及N沉降格局的改变等全球性的C、N变化.CO2浓度升高可提高森林生产力, 从而提高植物对菌根真菌的C分配比例; 大气N沉降增加可提高土壤肥力, 减缓菌根真菌及自由微生物受养分限制的程度; 不同菌根真菌对它们响应不一(
Mycorrhizal ecology and evolution: The past, the present, and the future.
3
2015
... 菌根是土壤真菌与植物根系形成的共生体, 存在于94%的维管植物根系中(
... 一般认为, AM真菌因不能通过分泌胞外酶获取复杂有机质中的N, 所以对植物获取N的过程没有影响, 其主要功能是促进植物对土壤磷的摄取能力.然而, 近年来越来越多的试验发现AM真菌能够促进植物对N的吸收, 且其吸收方式及能力随土壤N水平而变(
... EM真菌能通过产生胞外酶促进复杂有机质分解, 从而获取土壤中的DON, 缓解自身及植物受到的养分限制(
Do arbuscular mycorrhizal fungi stabilize litter-derived carbon in soil?
2
2016
... 菌根及其与自由微生物之间的相互作用对土壤C输出过程的影响有增加、减少和无效3种.增加效应主要指根际激发效应, 即菌根通过分泌活性有机质提高自由微生物活性, 促进有机质分解, 从而增加C输出(
... 菌根对森林土壤C输出的减少效应主要是指菌根真菌与自由微生物的相互作用对分解过程产生了消极影响, 即加吉尔效应(
Arbuscular mycorrhiza and soil nitrogen cycling.
3
2012
... 菌根是土壤真菌与植物根系形成的共生体, 存在于94%的维管植物根系中(
... 一般认为, AM真菌因不能通过分泌胞外酶获取复杂有机质中的N, 所以对植物获取N的过程没有影响, 其主要功能是促进植物对土壤磷的摄取能力.然而, 近年来越来越多的试验发现AM真菌能够促进植物对N的吸收, 且其吸收方式及能力随土壤N水平而变(
... ).此外, Veresoglou等(2012)认为, AM真菌对铵态N的吸收比细根更有效, 因此在土壤铵态N发生短暂脉冲效应时, AM真菌的生态重要性更为突出.然而, 关于AM真菌促进吸收、运输N素的研究仅基于室内培养研究, 且大多数以球囊霉科为研究对象(
Soil respiration and rates of soil carbon turnover differ among six common European tree species.
1
2012
... 不同菌根类型森林凋落物质量影响其凋落物层C、N输出过程, 即AM树种凋落物质量较高, 质量损失较快, 最终导致AM凋落物层C、N储量较低(
Do tree species influence soil carbon stocks in temperate and boreal forests?
3
2013
... 广义的森林土壤由凋落物层和矿质土层共同组成, 但多数研究并未同时报道不同菌根类型森林对这两个土层C储量的差异, 因此很难总结出整个森林土壤剖面C储量差异的普适性结论(
... ,
... 地上及地下凋落物输入是矿质土层C、N的主要输入过程; 不同菌根树种对这一过程影响的研究不多, 对其机理尚不清楚(
Carbon and nitrogen in forest floor and mineral soil under six common European tree species.
1
2008
... 土壤动物活动是凋落物及矿质土层有机质输入的又一影响因子(
丛枝菌根网络的生态学功能研究进展
1
2015
... 菌根是土壤真菌与植物根系形成的共生体, 存在于94%的维管植物根系中(
树种对土壤有机碳密度的影响: 5种温带树种同质园试验
1
2015
... 地上及地下凋落物输入是矿质土层C、N的主要输入过程; 不同菌根树种对这一过程影响的研究不多, 对其机理尚不清楚(
Interaction between Laccaria laccata and Trichoderma virens in co-culture and in the rhizosphere of Pinus sylvestris grown in vitro.
1
2002
... 关于菌根抑制分解过程的假说, 除了养分竞争假说之外, 还有化学抑制、菌寄生、水分限制假说.化学抑制假说是指与自由微生物相比, 菌根真菌从寄主根系获取C资源, 受C限制的程度较小, 因此可能会产生更多的次级代谢产物, 以限制自由微生物的活性(
Organic nitrogen uptake by arbuscular mycorrhizal fungi in a boreal forest.
1
2012
... 一般认为, AM真菌因不能通过分泌胞外酶获取复杂有机质中的N, 所以对植物获取N的过程没有影响, 其主要功能是促进植物对土壤磷的摄取能力.然而, 近年来越来越多的试验发现AM真菌能够促进植物对N的吸收, 且其吸收方式及能力随土壤N水平而变(
Comparisons of structure and life span in roots and leaves among temperate trees.
2
2006
... AM、EM树种的根系特性也是矿质土层C、N输入量的一个重要影响因素(
... 土壤有机质能够通过与铁铝矿物(铁铝氧化物、铁铝离子等)结合的物理化学方式或形成团聚体的物理方式降低其生物有效性, 从而提高其稳定性(
Root-induced changes in nutrient cycling in forests depend on exudation rates.
1
2014
... 菌根及其与自由微生物之间的相互作用对土壤C输出过程的影响有增加、减少和无效3种.增加效应主要指根际激发效应, 即菌根通过分泌活性有机质提高自由微生物活性, 促进有机质分解, 从而增加C输出(
Interspecies variation in nitrogen uptake kinetic responses of temperate forest species to elevated CO2: Potential causes and consequences.
1
2001
... 不同菌根类型森林土壤C、N循环对CO2浓度升高响应会影响植物养分限制程度, 最终造成森林生产力对气候变化的响应不同(
Ectomycorrhizal fungi in association with
1
2014
... 总之, 有关AM真菌通过影响土壤团聚过程改变土壤C、N稳定性的报道较多, 而对EM真菌的研究报道很有限(
The effects of mycorrhizal roots on litter decomposition, soil biota, and nutrients in a spodosolic soil.
3
1996
... 不同菌根类型森林凋落物质量影响其凋落物层C、N输出过程, 即AM树种凋落物质量较高, 质量损失较快, 最终导致AM凋落物层C、N储量较低(
... 菌根及其与自由微生物之间的相互作用对土壤C输出过程的影响有增加、减少和无效3种.增加效应主要指根际激发效应, 即菌根通过分泌活性有机质提高自由微生物活性, 促进有机质分解, 从而增加C输出(
... ).采用挖壕、树干环割等试验方法的很多研究在EM森林发现根际激发效应(

