
Arbuscular mycorrhiza improves plant adaptation to phosphorus deficiency through regulating the expression of genes relevant to carbon and phosphorus metabolism
XULi-Jiao
通讯作者:
版权声明:2017植物生态学报编辑部本文是遵循CCAL协议的开放存取期刊,引用请务必标明出处。
基金资助:
展开
摘要
关键词:
Abstract
Keywords:
-->0
PDF (2693KB)元数据多维度评价相关文章收藏文章
本文引用格式导出EndNoteRisBibtex收藏本文-->
在热带和亚热带酸性土壤及温带钙化土壤中, 土壤有效磷含量很低, 而且施用的磷肥很快会被土壤固定, 难以被植物吸收利用(Fixen, 2002)。在作物生长发育的早期, 磷元素的获取至关重要(Plénet et al., 2000), 因此低磷胁迫成为制约作物生产的重要因素。在漫长的进化过程中, 植物也进化出一系列分子和生化机制来适应低磷胁迫。有研究表明, 玉米(Zea mays)根系中的多个功能基因, 如与糖酵解相关的甘油-3-磷酸转运蛋白基因(G3PT)、磷酸烯醇式丙酮酸羧化酶基因(PEPC)、三羧酸循环相关基因苹果酸合酶基因(MAS1)和无机焦磷酸化酶基因(TC289)等的表达水平在低磷胁迫下明显增高, 在改善植物磷营养方面具有重要作用(Carlos et al., 2008)。此外, 大多数植物可以与土壤中的丛枝菌根(AM)真菌形成共生体系。AM真菌帮助宿主植物吸收土壤中的磷, 而植物提供5%-20%的光合产物帮助AM真菌完成生活史(Wright et al., 1998)。研究表明, AM共生体系对植物适应低磷胁迫具有重要的积极作用。在植物中存在菌根特异性和菌根诱导性两种磷转运蛋白, 低磷环境下菌根上调磷转运蛋白的表达水平, 促进植物高效吸收利用土壤中的磷(Harrison et al., 2002; Casieri et al., 2013)。不同作物中的磷转运蛋白基因, 如玉米Pht1;6 (Nagy et al., 2009)、土豆(Solanum tuberosum) Pht1;3、番茄(Solanum lycopersicum) Pht1;4 (Nagy et al., 2005)和水稻(Oryza sativa) Pht1;13 (Guimil et al., 2005)在AM真菌侵染时会被强烈诱导表达上调。另一方面, 在AM真菌中已有3种磷转运蛋白(GvPT、GiPT和GmosPT)被鉴定出来。在缺磷的情况下, 这些磷转运蛋白会在根外菌丝中强烈表达, 促进真菌吸收磷并传输给宿主植物(Maldonado-Mendoza et al., 2001)。
宿主植物和AM真菌之间的碳磷交换是稳定菌根共生体系的基础(Bago et al., 2000)。目前, 关于菌根促进植物吸收磷的机理研究已有很多, 但是菌根真菌自身碳代谢研究还比较少。N-乙酰葡糖胺(GlcNAc)代谢是AM真菌中一种比较重要的碳代谢过程, 不仅参与几丁质的合成, 还为AM真菌的糖酵解过程提供前体物质, 真菌细胞壁的特征性组分几丁质即是GlcNAc的长链聚合物(Rich et al., 2014)。最近, Yoshihiro等(2015)构建了AM真菌中GlcNAc代谢通路: AM真菌菌丝中的GlcNAc转运蛋白NGT1能够将外源GlcNAc转运到菌丝内部, 继而由GlcNAc激酶a和激酶b (HXK1a和HXK1b)将GlcNAc磷酸化为GlcNAc-6-磷酸, 之后, GlcNAc-6-磷酸有两个下游通路, 一个是GlcNAc磷酸变位酶AGM1、UDP-GlcNAc焦磷酸化酶UAP1和几丁质合酶CHS1参与的几丁质合成代谢; 另一个是GlcNAc-6-磷酸去乙酰化酶DAC1和葡糖胺-6-磷酸异构酶NAG1作用形成果糖-6-磷酸进入糖酵解途径的分解代谢。考察磷胁迫下AM真菌中这些碳代谢相关基因表达的变化, 有助于更好地揭示菌根共生体系适应低磷胁迫的生理机制。
AM不仅能够直接参与和调节宿主植物碳磷代谢过程, 还可能通过菌根根际效应(mycorrhizosphere effects)调节相邻非菌根植物的生理代谢和生长发育(van der Heijden & Horton, 2009)。Barto等(2011)利用H形盆栽装置构建了可调控的菌根网络(common mycorrhizal networks, CMNs), 验证了CMNs在不同植株之间传输化感物质的可能性。然而, 在根外菌丝完全不接触未接种植物的情况下, AM是否能通过菌根分泌物作用调节植物的生理代谢还未见研究报道。基于此, 本试验采用分室培养系统, 在不同供磷条件下考察AM对宿主植物和相邻非菌根植物生长和生理的影响。在由供体室、缓冲室和受体室组成的三分室培养系统中, 采用微孔滤膜区隔不同分室, 可阻断接种处理供体植物根外菌丝与受体植物的直接联系, 从而考察来源于供体植物的AM分泌物对受体植物的可能作用。通过观测供体和受体植物生物量、磷浓度, 分析植物及AM真菌碳磷代谢相关基因的表达, 探讨AM直接和间接调控植物响应低磷胁迫的生理机制。
1 材料和方法
1.1 试验材料
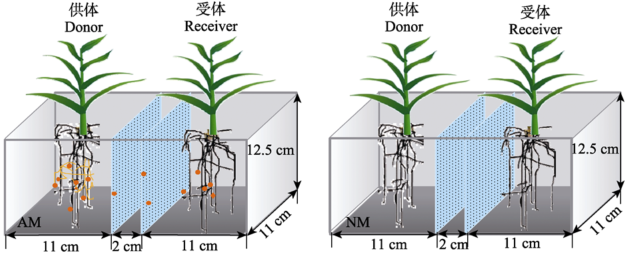
供试植物为已完成全基因组测序的模式作物材料玉米自交系‘B73’ (Schnable et al., 2009)。玉米种子由中国农业大学生物学院于静娟教授提供。供试AM真菌为根内球囊霉Rhizophagus irregularis ‘DAOM197198’, 接种剂为取自转移Ri T-DNA胡萝卜(Daucus carota var. sativa)根器官和AM真菌双重无菌培养体系的真菌孢子和被侵染的胡萝卜根段。供试土壤采自内蒙古鄂尔多斯市东胜区铜川镇枳机塔村(39.89° E, 110.02° N, 海拔1β367 m)。土壤基本理化性质如下: pH值8.69 (水浸提, 水土质量比2.5:1), 有机质含量22.01 g·kg-1; 有机碳含量12.77 g·kg-1; 有效磷含量4.46 mg·kg-1, 具体测定方法参见鲍士旦(2000)。土壤过2 mm筛后, 辐照灭菌(60Co, 25 kGy)。培养基质由灭菌土壤与河沙按质量比1.5:1混合组成。试验前土壤加入底肥NH4NO3-N 90 mg·kg-1, K2SO4-K 120 mg·kg-1。低磷处理加NaH2PO4-P 10 mg·kg-1, 高磷处理加NaH2PO4-P 100 mg·kg-1。分室培养系统采用2 mm厚的硬聚氯乙烯(PVC)板加工制成, 由2层0.45 μm微孔滤膜分成3个分室(AM分泌物供体室、缓冲室和受体室), 供体室和受体室体积相同并种植玉米, 中间为宽2 cm的缓冲室。培养系统尺寸(长×宽×高)为((11 + 2 + 11) × 11 × 12.5) cm3 (图1)。
 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图1分室培养系统示意图。以0.45 μm微孔滤膜区隔不同分室。AM和NM分别代表供体植物接种AM真菌和不接种对照处理。 处理分为高磷和低磷(10 mg?kg-1和100 mg?kg-1)两个水平, 每个处理三个重复(n = 3)。
-->Fig. 1Diagram of the compartment cultivation system. Different compartments were separated by microporous filter with pore size of 0.45 μm. AM and NM represent inoculation of donor plants with AM fungus and the non-mycorrhizal control respectively. There are two phosphorus levels (10 mg?kg-1 and 100 mg?kg-1), and three replications for each treatment (n = 3).
-->
1.2 试验方案
供体室玉米接种AM真菌, 作为AM分泌物的源, 同时设置不接种对照处理。受体室不进行接种处理。不同接种处理下均设低磷(10 mg·kg-1)和高磷(100 mg·kg-1)处理(所有分室磷浓度相同)。试验共有4个处理, 每个处理重复3次, 共12盆。玉米播种59天后进行试验收获。供体室接种AM真菌情况下, 供体室标记为AMD (AM Donor), 受体室标记为AMR (AM Receiver), 不接种情况下(non-mycorrhizal, NM), 供体室和受体室分别标记为NMD和NMR (图1)。1.3 植物培养与试验管理
‘B73’种子经清水浸泡3 h, 10%过氧化氢浸泡15 min后, 取出并用灭菌水冲洗数遍后放入装有3层湿润滤纸的培养皿中, 25 ℃催芽4天。供体室和受体室分别装入1 500 g施加过底肥的培养基质, 缓冲室装入250 g相同的培养基质。称质量浇水使土壤含水量达到14%质量含水量, 待水分渗透均匀后, 播入萌发的玉米种子。将2 000个AM真菌孢子附着在已萌发玉米种子的胚根部位, 并加入0.08 g风干的AM侵染的胡萝卜根段; 不接种处理加入0.08 g风干的无菌根侵染的胡萝卜根段。盆栽试验在人工气候室中完成, 温度为25 ℃/ 20 ℃, 每天光照16 h, 光强约700 μmol·m-2·s-1, 相对湿度为70%。在整个试验周期都维持14%的土壤含水量, 每天称质量补足损耗的水量, 使土壤含水量维持在设定水平。
1.4 测定指标和方法
1.4.1 生物量及菌根侵染率的测定试验收获时, 自培养基质表面将植物剪断。将玉米地上部和根系用去离子水冲洗两遍, 吸干表面水分, 分别称取鲜质量。取混匀的玉米根系 0.1 g, 经液氮冷冻后, 于-80 ℃保存, 用于RNA提取; 另取混匀根系样品3 g, 用于菌根侵染率测定。余下样品置于105 ℃烘箱中杀青10 min, 转为80 ℃烘干至恒质量, 称干质量。根系染色过程依据Phillips和Hayman (1970)的方法, 但有所简化: 将3 g根系放入10% KOH中, 90 ℃水浴锅中煮10 min, 用自来水冲洗干净后, 加入0.05%的台盼蓝90 ℃染色5 min。染色根段置于载玻片上, 然后加盖盖玻片观察, 每个样品观测30根段(李涛和陈保冬, 2012)。用根段频率法计算菌根侵染率(Biermann & Linderman, 1981)。
1.4.2 植物磷浓度的测定
取烘干的植物样品, 经粉碎机粉碎后, 称取样品约0.1 g, 以5 mL优级纯HNO3室温消化12 h后, 在微波消解仪(MARS5, CEM, Matthews, USA)中进行消解, 消解液定容至25 mL, 混匀后过滤, 用电感耦合等离子原子发射光谱仪(ICP-OES, Leeman Labs, Hudson, USA)测定样品中的磷浓度。
1.4.3 玉米和AM真菌碳磷代谢相关基因表达测定
取0.1 g玉米根部样品, 采用TRIZOL试剂(Invitrogen, Grand Island, New York, USA)提取玉米根系总RNA, 总RNA提取完成后经DNaseI消化, 用于合成cDNA。合成体系为20 μL, 所用试剂盒为Thermo反转录试剂盒(Thermo Scientific, 上海)。采用荧光定量PCR测定玉米碳磷代谢相关基因Pht1;2、Pht1;6、PEPC、G3PT、TC289、MAS1和AM真菌碳磷代谢相关基因GiPT、NGT1、HXK1b、AGM1、GlcNAc、UAP1、CHS1、DAC1、NAG1的表达量。定量PCR体系为25 μL, 其中包含12.5 μL SYBR? Premix ExTaqTM (TAKARA Biotechnology, 中国大连), 1.5 μL稀释5倍的cDNA模板和0.2 μmol·L-1特异性引物(附录I、附录II)。PCR程序为: (1) 95 ℃ 10 s; (2) 95 ℃ 15 s, 60 ℃ 60 s, 40个循环。60 ℃收集荧光数据。溶解曲线分析程序为: 70 ℃ 10 s, 然后以0.2 ℃·s-1的升温速率加热到100 ℃, 连续收集数据。玉米以Actin基因作为内参基因, AM真菌以EF1β作为内参基因, 每个样品做3个技术平行。在对照试验中, 分别在反应体系中加入每个RNA样品和水, 以取代cDNA模板, 以此排除基因组DNA污染和引物二聚体的形成。数据分析用2-ΔΔCt方法(Pfaffl, 2001)。试验所用仪器为Bio-Rad iQ5荧光定量PCR仪(Bio-Rad Laboratories, Hercules, USA), 数据分析软件为Bio-Rad iQ5Optical System Software。
1.4.4 数据分析
试验数据用平均值±标准偏差表示。用SPSS 13.0进行双因素方差分析, 分别检验接种处理和磷水平对供体植株或受体植株干质量和磷浓度、植物碳磷代谢相关基因表达的影响。若两因素交互作用显著, 则采用最小显著差异(LSD)法比较所有处理之间的差异显著性; 若二者交互作用不显著, 则按处理分组对数据进行t检验。对于菌根侵染率和AM真菌碳磷代谢相关基因表达, 采用t检验分析不同磷水平之间的差异显著性。采用Excel 2010生成柱形图。
2 结果
2.1 菌根侵染率
试验条件下, 未接种对照玉米根系未检测到菌根侵染迹象, 而接种处理供体玉米根内形成典型的菌根共生结构。低磷(10 mg·kg-1)条件下接种处理供体玉米菌根侵染率为68%, 丛枝丰度42%; 高磷(100 mg·kg-1)条件下供体玉米菌根侵染率降低至43%, 丛枝丰度23%。菌根侵染率在低磷和高磷处理之间存在显著差异。无论何种试验处理, 受体玉米根系均无菌根侵染。2.2 玉米生长情况
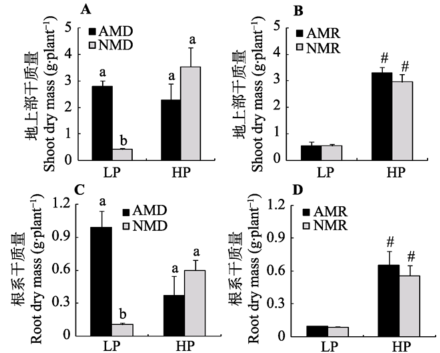
低磷条件下, 接种AM真菌显著提高了供体玉米地上部干质量, 接种处理玉米地上干质量约为不接种处理的5倍。高磷条件下, 接种处理对玉米地上部干重没有显著影响(图2A; 附录III)。无论高磷还是低磷条件下, 供体室接种处理对受体玉米地上部生物量都没有显著影响(图2B; 附录III)。接种处理对根系生物量的影响与地上部一致。低磷条件下, 供体室接种处理显著提高了供体玉米的根系生物量, 而高磷条件下, 接种对供体玉米的根系生物量没有显著影响(图2C; 附录III)。接种处理对受体室玉米根系生物量总体上没有明显影响(图2D; 附录III)。
 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图2不同磷浓度下接种AM真菌对玉米植株干质量的影响(平均值±标准偏差)。LP和HP分别代表低磷和高磷处理。AMD和NMD分别代表供体植物接种AM真菌和不接种对照处理; AMR和NMR分别代表受体植物受到AM分泌物处理和对照处理。柱形上方标示不同字母代表不同处理间在5%水平有显著性差异。“#”代表在相同接种处理下不同磷水平之间在5%水平差异显著。
-->Fig. 2Effects of mycorrhizal inoculation on maize dry mass under different P levels (mean ± SD). LP and HP refer to low P level (10 mg·kg-1) and high P level (100 mg·kg-1) respectively. AMD and NMD represent donor plants with and without AM fungus incubation, while AMR and NMR represent receiver plants with and without AM exudates respectively. Different letters above the columns indicate significant difference (p < 0.05) between corresponding treatments. # indicates significant difference (p < 0.05) between different P levels under the same inoculation treatment.
-->
接种处理和磷水平对供体玉米植株干质量表现出显著交互作用: 高磷显著提高了未接种对照植物地上部和根系干质量, 但对接种处理植物干质量没有显著影响(图2A、2C; 附录III)。对于受体植物而言, 无论供体室接种与否, 高磷水平均显著提高了植株地上部和根系干质量(图2B、2D; 附录III)。
2.3 接种AM真菌对玉米植株磷浓度的影响
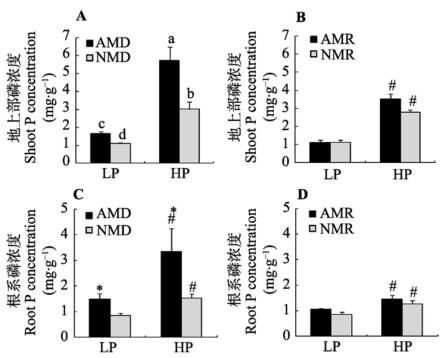
无论低磷还是高磷条件下, 接种AM真菌均显著提高了供体玉米地上部磷浓度(图3A; 附录III)。相同磷水平下, 供体室接种处理对受体玉米地上部磷浓度无显著性影响。总体上, 高磷处理显著提高了植株地上部磷浓度(图3B; 附录III)。接种AM真菌显著提高了供体玉米根系磷浓度, 低磷条件下接种处理供体玉米根系磷浓度比不接种对照提高了45.6% (图3C; 附录III)。相同磷水平下, AM真菌对受体玉米根系磷浓度无显著性影响(图3D; 附录III)。
 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图3不同磷浓度下接种AM真菌对玉米植株磷浓度的影响(平均值±标准偏差)。LP和HP分别代表低磷和高磷处理。AMD和NMD代表供体植物接种AM真菌和不接种对照处理; AMR和NMR代表供体和受体植物受到AM分泌物处理和对照处理。不同字母代表处理间在5%水平上有显著性差异。“#”代表在相同AM真菌分泌物受体不同磷浓度处理间在5%水平上差异显著。“*”代表在相同磷浓度处理下AM真菌处理间在5%水平上差异显著。
-->Fig. 3Effects of inoculation with AM fungus on maize P concentrations under different P levels (mean ± SD). LP and HP refer to low P level (10 mg·kg-1) and high P level (100 mg·kg-1) respectively. AMD and NMD represent donor plants with and without AM fungus incubation, while AMR and NMR represent receiver plants with and without AM exudates respectively. The different letters indicates significant difference (p < 0.05) between corresponding treatments. # indicates significant difference (p < 0.05) between different P levels under the same inoculation treatment; * indicates significant difference (p < 0.05) between inoculation treatments under the same P level.
-->
2.4 不同磷水平下AM真菌碳磷代谢基因表达情况
低磷条件下AM真菌中GlcNAc代谢和磷转运相关基因的表达水平总体显著高于高磷情形。与高磷情形相比, 低磷条件下GiPT、HXK1b、CHS1和NAG1表达水平提高了2-3倍(图4)。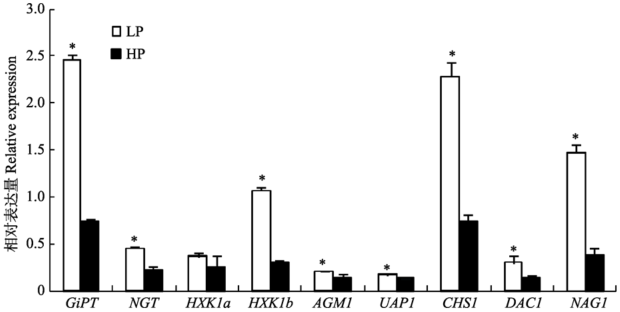 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图4不同磷水平下AM真菌碳磷代谢相关基因表达(平均值±标准偏差)。LP为低磷处理, HP为高磷处理; *表示不同磷水平之间差异显著(p < 0.05)。GiPT, AM真菌磷转运蛋白基因; NGT1, N-乙酰葡糖胺(GlcNAc)转运蛋白基因; HXK1b, GlcNAc激酶b基因; AGM1, GlcNAc磷酸变位酶基因; UAP1, UDP-GlcNAc焦磷酸化酶基因; CHS1, 几丁质合酶基因; DAC1, GlcNAc-6-磷酸去乙酰化酶基因; NAG1, 葡糖胺-6-磷酸异构酶基因。
-->Fig. 4Expression of AM fungal genes relevant to C and P metabolisms under different P levels (mean ± SD). LP refers to low P treatments, HP refers to high P treatments, * indicates significant difference (p < 0.05) between different P levels. GiPT, AM fungal P transporter gene; NGT1, GlcNAc transporter gene, HXK1b, GlcNAc kinase gene; AGM1, GlcNAc phosphomutase gene; UAP1, UDP GlcNAc pyrophosphorylase gene; CHS1, chitin synthase gene; DAC1, GlcNAc-6-phosphate deacetylase gene; NAG1, glucosamine- 6-phosphate isomerase gene.
-->
2.5 供体玉米碳磷代谢相关基因表达情况
低磷条件下, 接种AM真菌显著上调了供体玉米Pht1;2、Pht1;6、PEPC、TC289、G3PT、MAS1基因的表达水平。高磷条件下, 只有Pht1;2、Pht1;6和G3PT的表达量受接种处理上调(图5; 附录III)。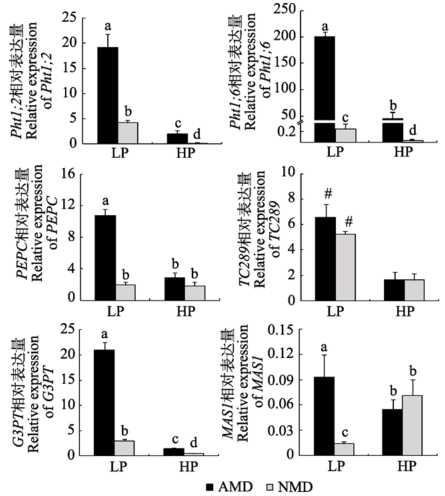 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图5不同磷水平下供体玉米碳磷代谢基因表达情况(平均值±标准偏差)。LP和HP分别代表低磷和高磷处理。AMD和NMD代表供体植物接种AM真菌和不接种对照处理。柱形上方标示不同字母代表相应处理之间在5%水平有显著性差异。“#”表示相同接种处理不同磷水平之间在5%水平差异显著。Pht1;2, Pht1;6, 磷转运蛋白基因; PEPC, 磷酸烯醇式丙酮酸羧化酶基因; G3PT, 甘油-3-磷酸转运蛋白基因; TC289, 无机焦磷酸化酶基因; MAS1, 苹果酸合酶基因。
-->Fig. 5Expression of genes relevant to C and P metabolism in maize roots from donor compartment under different P levels (mean ± SD). LP and HP refer to low P level (10 mg?kg-1) and high P level (100 mg?kg-1) respectively. AMD and NMD represent donor plants with and without AM fungus. Different letters above the columns indicate significant difference (p < 0.05) between corresponding treatments. # indicates significant difference (p < 0.05) between different P levels. Pht1;2, Pht1;6, P transporter genes; PEPC, phosphoenolpiruvate carboxylase gene; TC289, inorganic pyrophosphatase gene; G3PT, glycerol- 3-phosphate transporter gene; MAS1, malate synthase gene.
-->
2.6 受体玉米碳磷代谢相关基因表达情况
土壤磷水平和接种处理对受体玉米根系的Pht1;6、G3PT、PEPC、TC289、MAS1表达具有显著交互作用。高磷处理抑制这些基因的表达, 而供体室接种处理总体上提高了基因表达水平。Pht1;2、Pht1;6、G3PT、PEPC、TC289、MAS1等基因的表达受接种处理显著上调(图6; 附录III)。 显示原图|下载原图ZIP|生成PPT
显示原图|下载原图ZIP|生成PPT图6不同磷水平下受体玉米碳磷代谢相关基因表达情况(平均值±标准偏差)。LP和HP分别代表低磷和高磷处理。AMR和NMR代表供体和受体植物受到AM分泌物处理和对照处理。柱形上方标示不同字母代表相应处理之间在5%水平有显著性差异。“#”表示相同接种处理不同磷水平之间在5%水平差异显著, 而“$”代表在相同磷水平下不同接种处理之间在5%水平上差异显著。Pht1;2, Pht1;6, 磷转运蛋白基因; PEPC, 磷酸烯醇式丙酮酸羧化酶基因; G3PT, 甘油-3-磷酸转运蛋白基因; TC289, 无机焦磷酸化酶基因; MAS1, 苹果酸合酶基因。
-->Fig. 6Expression of genes relevant to C and P metabolism in maize roots from receiver compartment under different P levels (mean ± SD). LP and HP refer to low P level (10 mg?kg-1) and high P level (100 mg?kg-1) respectively. AMR and NMR represent receiver plants with and without AM exudates respectively. # indicates significant difference (p < 0.05) between different P levels, while $ indicates significant difference (p < 0.05) between inoculation treatments under the same P level. Pht1;2, Pht1;6, P transporter genes; PEPC, phosphoenolpiruvate carboxylase gene; TC289, inorganic pyrophosphatase gene; G3PT, glycerol-3-phosphate transporter gene; MAS1, malate synthase gene.
-->
3 讨论
在AM共生体系中, AM真菌促进植物对磷的吸收, 植物则为AM真菌提供碳水化合物。在低磷胁迫下, AM真菌不仅通过根外菌丝直接从土壤中获取磷, 还可以调控宿主植物磷转运蛋白基因的表达, 从而帮助植物抵御磷胁迫。本试验采用分室培养系统, 分析不同磷水平下AM共生体系对玉米生长和营养生理的影响。试验结果表明, 低磷条件下AM真菌不仅可以直接改善宿主植物磷营养状况, 还可能通过AM分泌物上调相邻非菌根植物碳磷代谢相关基因的表达, 进而调控植物对低磷胁迫的生理响应。本试验中, 低磷条件下玉米菌根侵染率显著高于高磷处理, 丛枝丰度也较高, 这与以往的研究结果一致(Gu et al., 2011)。在高磷水平下, 植物会限制碳水化合物向AM真菌的转运, 从而抑制AM共生体发育(Olsson et al., 2006; Nagy et al., 2009)。有研究表明, 磷酸盐能直接抑制R. intraradices根内菌丝的分化和丛枝的发育(Harrison et al., 2010)。Breuillin (2010)试验发现, 在10 mmol·L-1磷酸盐溶液处理
下, AM真菌丛枝发育不良, 仅有稀疏分枝并过早衰亡, 同时根内菌丝的生长和分化也受到抑制。此外, AM真菌中一些关键功能基因, 如磷酸盐转运蛋白基因的表达水平也会被高浓度磷酸盐抑制(Chen et al., 2007)。本实验中, 高磷条件下AM真菌碳磷代谢相关基因GiPT、NGT1、HXK1b、AGM1、UAP1、CHS1、DAC1和NAG1的表达水平较低, 也表明高磷抑制了AM共生体系的发育和正常功能。相应地, 低磷条件下GiPT、HXK1b、CHS1和NAG1基因表达水平显著升高, 则表明低磷胁迫会促进菌根共生体系的建成。
磷作为一种植物必需的营养元素, 在植物生长发育和代谢过程中均起着重要作用(Marschner, 1995)。在本试验中, 低磷条件下接种AM真菌显著促进了玉米生长, 达到和高磷条件下相近的生物量。实验土壤为碱性土(pH值8.69), 有效铁和有效磷较低而不利于植物生长(Tyler, 1999)。以往研究表明 R. irregularis可以在碱性土壤中与宿主植物形成良好的共生关系。AM不仅可以通过根外菌丝直接吸收土壤中的磷, 还可以分泌草酸并诱导植物根系分泌柠檬酸, 降低土壤的pH值以提高土壤磷的溶解度, 从而促进植物吸收更多的磷(Gardner et al., 1983; Cunningham & Kuiack, 1992)。分子水平的研究表明, 菌根磷转运蛋白基因位于菌根共生信号途径的下游, 在根内皮层细胞形成丛枝之后, 植物根内的菌根特异性磷转运蛋白被诱导表达并高效地向植物输送磷(Javot et al., 2007; Smith & Read, 2008)。本实验中, 低磷条件下接种AM真菌或AM分泌物都可以显著上调玉米根中Pht1;2和Pht1;6的表达水平, 尤其是Pht1;6基因在低磷条件下受到接种处理的强烈诱导, 表达量与不接种相比提高了近1β000倍。这表明低磷胁迫下AM共生体系特殊的磷吸收转运系统对于改善植物磷营养状况可能具有重要意义。
Carlos等(2008)利用基因芯片研究了磷饥饿对植物基因表达谱的影响, 表明碳代谢、氮代谢、脂代谢和磷代谢基因均有不同响应。本试验中, 低磷环境下AM不仅显著调节了供体植物碳磷代谢相关基因G3PT、PEPC、TC289和MAS1的表达, 还上调了受体植物中上述基因的表达。G3PT参与糖-磷酸盐/阴离子的逆向转运过程, 低磷胁迫下拟南芥(Arabidopsis thaliala)中G3PT家族的5个基因均呈现不同程度的上调, 进而调节根的生长或磷酸盐的平衡(Ramaiah et al., 2011)。PEPC不仅可以提高植物的光合速率和产量(Fukayama et al., 2003), 还参与促进C3植物的三羧酸循环, 为氨基酸合成提供碳骨架(Radchuk et al., 2007)。通过调控PEPC表达不仅提高豆类种子的蛋白含量, 也使蛋白质组分发生变化(Rolletschek et al., 2004)。TC289可以催化无机焦磷酸水解为正磷酸盐, 参与合成糖类、核酸和蛋白质等多种代谢途径中的焦磷酸水解过程(Rojas-Beltrán et al., 1999)。AM分泌物对于受体植物碳磷代谢基因的调控, 表明AM分泌物很可能通过扩散作用影响到非菌根植物, 上调植物碳代谢、磷转运相关基因的表达, 最终可能引起植物生理代谢过程的改变, 帮助植物抵御低磷胁迫。Kosuta等(2003)首次证明AM真菌可扩散性信号物质的存在, 这些信号物质可诱导植物基因MtEnod11的表达。Maillet等(2011)获得AM真菌信号物质菌根因子(Myc factor), 一种脂质几丁寡糖(Myc-LCOs)的化学结构。有研究表明AM真菌信号分子在诱导植物基因表达(Chabaud et al., 2011)、根内淀粉积累、糖代谢(Gutjahr et al., 2009)和侧根发生(Oláh et al., 2005)过程中作用活跃。限于研究条件, 本试验无法区分根系自身分泌物和AM特异分泌物, 也未能直接检测AM分泌物及相关信号物质, 至于是何种信号物质对植物响应低磷胁迫起到关键调节作用, 还需要进一步研究。
综上所述, 在植物和AM真菌相互作用的过程中, AM真菌能够通过对植物和自身碳磷代谢基因的调控促进共生体系的建成, 进而直接参与和调控宿主植物的磷营养。在AM真菌完全不接触邻体植物的情况下, AM分泌物也可能通过空间扩散作用调控植物基因表达, 帮助植物抵御低磷胁迫。在本试验中我们未能分析和测定AM分泌物的动态变化, 不能直接建立AM分泌物和植物对低磷胁迫生理响应的直接关联, 将来的研究可直接收集或纯化AM分泌物, 更为直接地考察AM分泌物的作用, 以期全面揭示AM共生体系调控植物磷营养的生理和分子机制。
附录I 玉米中碳磷代谢相关功能基因定量PCR引物序列
Appendix I附录I
附录I玉米中碳磷代谢相关功能基因定量PCR引物序列
Appendix IThe PCR primer sequences for functional genes in maize plants
| 基因 Gene | 正向引物 Forward primer | 反向引物 Reverse primer | 文献 Reference |
|---|---|---|---|
| Action | GTCCGTGCGTTTCCTTTTGT | AAACCGGCCTTGACCATTCC | Soderlund et al., 2009 |
| Pht1;2 | CCAACTTGCTTGGCTTTATCCT | AGCCTCCCCGGACATCTC | Schnable et al., 2009 |
| Pht1;6 | CTACAGCCAGAACCTGACCC | ACATGACGCCCATCAGTAGC | Schnable et al., 2009 |
| G3PT | TTCACCGCCTGCGTCCTT | TCGCTGGGCTCCTCTTGAG | Carlos et al., 2008 |
| PEPC | CACGCTGATCCTGACCATGA | TCGCAAACCGAGTATGTATCTT | Carlos et al., 2008 |
| TC289 | CCCTTGGCATGATCTGGAGAT | CCTTGCTGCCCCTTGGTAT | Carlos et al., 2008 |
| MAS1 | TGGACGCGTACAACCTCATC | CTGACTCCACTGCCGACAAA | Carlos et al., 2008 |
Pht1;2, Pht1;6, phosphorus transporter genes; PEPC, phosphoenolpiruvate carboxylase gene; G3PT, glycerol-3-phosphate transporter gene; TC289, inorganic pyrophosphatase gene; MAS1, malate synthase gene.
新窗口打开
附录II AM真菌中碳磷代谢相关功能基因定量PCR引物序列
Appendix II附录II
附录II AM真菌中碳磷代谢相关功能基因定量PCR引物序列
Appendix II The PCR primer sequences for AM fungal genes
| 基因 Gene | 正向引物 Forward primer | 反向引物 Reverse primer | 文献 Reference |
|---|---|---|---|
| EF1β | CCCATGCAGCTCGATGGTA | TGCCAGGAAGTGAAGAAAATGA | Yoshihiro et al., 2015 |
| NGT1 | TGGCGCAGCACTTTTGTG | CGTTCGGTAGGGTAAGATAACATGA | Yoshihiro et al., 2015 |
| HXK1a | CGATTGCCAACTGGTATGGA | GCGCAAATTAGTCCCACCTAAG | Yoshihiro et al., 2015 |
| HXK1b | GGAATCCCAACTGGCAAAGA | ACATTCGTAAATTTGTACCTCCAAGA | Yoshihiro et al., 2015 |
| AGM1 | AAAACAATTCGATCTGCTGAAGGT | ATGCTCGTAATTTTTCGATTGCT | Yoshihiro et al., 2015 |
| UAP1 | TGAACGCGTCAACCGAATC | CGGTACCGGGAGCAATTTC | Yoshihiro et al., 2015 |
| CHS1 | CGGCACAATTTAGGGATATAGTGA | GGTTCCCCATGAATCAAACTAGTAA | Yoshihiro et al., 2015 |
| DAC1 | TTTGGAAGAGTTGGTTAATTTTGGT | AATACGGTCGCGGACGAA | Yoshihiro et al., 2015 |
| NAG1 | GGCGTTAGCTCTTGCCAAGT | CGCCGAAACGGTAAACATG | Yoshihiro et al., 2015 |
| GiPT | CTGCTGTTGATTATTGTTGGC | GAACGGTTCCCATAATAGTG | Maldonado-Mendoza et al., 2001 |
GiPT, AM fungal P transporter gene; NGT1, GlcNAc transporter gene, HXK1b, GlcNAc kinase gene; AGM1, GlcNAc phosphomutase gene; UAP1, UDP GlcNAc pyrophosphorylase gene; CHS1, chitin synthase gene; DAC1, GlcNAc-6-phosphate deacetylase gene; NAG1, glucosamine-6-phosphate isomerase gene.
新窗口打开
附录III 植物干质量、磷含量、碳磷代谢基因表达的双因素方差分析结果
Appendix III附录III
附录III 植物干质量、磷含量、碳磷代谢基因表达的双因素方差分析结果
Appendix III Two-way ANOVA of shoot and root dry mass, P concentrations and expression of genes related to C and P metabolisms as influenced by mycorrhizal inoculation and soil P levels
| 地上部干质量 Shoot dry mass | 根系干质量 Root dry mass | 地上部磷浓度 Shoot P concentration | 根系磷浓度 Root P concentration | Pht1;2 | Pht1;6 | G3PT | PEPC | TC289 | MAS1 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 供体植物 Donor | ||||||||||
| 接种处理 Inoculation treatment (I) | * | ** | * | ** | ** | ** | ** | ** | ns | ** |
| 磷水平 P levels (P) | ** | ns | ** | ** | ** | ** | ** | ** | ** | ns |
| 交互作用 I × P | ** | ** | ns | * | ** | ** | ** | ** | ns | ** |
| 受体植物 Receiver | ||||||||||
| 接种处理 Inoculation treatment (I) | ns | ns | ns | ns | ** | ** | ** | ** | ** | ** |
| 磷水平 P levels (P) | * | * | * | * | ** | ** | ** | ** | ** | ** |
| 交互作用 I × P | ns | ns | ns | ns | ns | ** | ** | ** | ** | ** |
Pht1;2, Pht1;6, phosphorus transporter genes; PEPC, phosphoenolpiruvate carboxylase gene; G3PT, glycerol-3-phosphate transporter gene; TC289, inor-ganic pyrophosphatase gene; MAS1, malate synthase gene. *, p < 0.05; **, p <0.01; ns, not significant.
新窗口打开
附录文献
Carlos CV, Enrique IL, Juan CP, Herrera-Estrella L (2008). Transcript profiling of Zea mays roots reveals gene responses to phosphate deficiency at the plant- and species-specific levels. Journal of Experimental Botany, 59, 2479-2497.Maldonado-Mendoza IE, Dewbre GR, Harrison MJ (2001). A phosphate transporter gene from the extra-radical mycelium of an arbuscular mycorrhizal fungus glomus intraradices is regulated in response to phosphate in the environment. Molecular Plant-Microbe Interactions, 14, 1140-1148.
Schnable PS, Ware D, Fulton RS, Stein JC, Wei F, Pasternak S, Liang C, Zhang J, Fulton L, Graves TA, Minx P, Reily AD, Courtney L, Kruchowski SS, Tomlinson C, Strong C, Delehaunty K, Fronick C, Courtney B, Rock SM, Belter E, Du F, Kim K, Abbott RM, Cotton M, Levy A, Marchetto P, Ochoa K, Jackson SM, Gillam B, Chen W, Yan L, Higginbotham J, Cardenas M, Waligorski J, Applebaum E, Phelps L, Falcone J, Kanchi K, Thane T, Scimone A, Thane N, Henke J, Wang T, Ruppert J, Shah N, Rotter K, Hodges J, Ingenthron E, Cordes M, Kohlberg S, Sgro J, Delgado B, Mead K, Chinwalla A, Leonard S, Crouse K, Collura K, Kudrna D, Currie J, He R, Angelova A, Rajasekar S, Mueller T, Lomeli R, Scara G, Ko A, Delaney K, Wissotski M, Lopez G, Campos D, Braidotti M, Ashley E, Golser W, Kim H, Lee S, Lin J, Dujmic Z, Kim W, Talag J, Zuccolo A, Fan C, Sebastian A, Kramer M, Spiegel L, Nascimento L, Zutavern T, Miller B, Ambroise C, Muller S, Spooner W, Narechania A, Ren L, Wei S, Kumari S, Faga B, Levy MJ, McMahan L, Van Buren P, Vaughn MW, Ying K, Yeh CT, Emrich SJ, Jia Y, Kalyanaraman A, Hsia AP, Barbazuk WB, Baucom RS, Brutnell TP, Carpita NC, Chaparro C, Chia JM, Deragon JM, Estill JC, Fu Y, Jeddeloh JA, Han Y, Lee H, Li P, Lisch DR, Liu S, Liu Z, Nagel DH, McCann MC, SanMiguel P, Myers AM, Nettleton D, Nguyen J, Penning BW, Ponnala L, Schneider KL, Schwartz DC, Sharma A, Soderlund C, Springer NM, Sun Q, Wang H, Waterman M, Westerman R, Wolfgruber TK, Yang L, Yu Y, Zhang L, Zhou S, Zhu Q, Bennetzen JL, Dawe RK, Jiang J, Jiang N, Presting GG, Wessler SR, Aluru S, Martienssen RA, Clifton SW, McCombie WR, Wing RA, Wilson RK (2009). The B73 maize genome: Complexity, diversity, and dynamics. Science, 326, 1112-1115.
Soderlund C, Descour A, Kudrna D, Bomhoff M, Boyd L, Currie J, Angelova A, Collura K, Wissotski M, Ashley E, Morrow D, Fernandes J, Walbot V, Yu Y (2009). Sequencing, mapping, and analysis of 27,455 maize full-length cDNAs. PLOS Genetics, 5, e1000740. doi: 10.1371/journal.pgen.1000740.
Yoshihiro K, Miki K, Katsuharu S, Kikuchi Y, Ezawa T, Maeshima M, Hata S, Fujiwara T (2015). Up-regulation of genes involved in N-acetylglucosamine uptake and metabolism suggests a recycling mode of chitin in intraradical mycelium of arbuscular mycorrhizal fungi. Mycorrhiza, 25, 411–417.
The authors have declared that no competing interests exist.
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
| [1] | Colonization of the land by plants some 400 million years ago was associated with the colonization of their primitive roots by soil-borne filamentous fungi (Nicolson, 1975;Simon et al., 1993; Taylor et al., 1995). Today, 90% to 95% of land plants still maintain some type of mycorrhizal association so that “mycorrhizas, not roots, are the chief organs of nutrient uptake by land plants” (Smith and Read, 1997). Of the several mycorrhizal symbioses, arbuscular mycorrhizas are much the most abundant. These are formed by a very wide variety of host plants (including angiosperms, gymnosperms, pteridophytes, and some mosses, lycopods, and psilotales) and a comparatively small group of aseptate filamentous fungi, the Glomales. |
| [2] | |
| [3] | Allelopathy, a phenomenon where compounds produced by one plant limit the growth of surrounding plants, is a controversially discussed factor in plant-plant interactions with great significance for plant community structure. Common mycorrhizal networks (CMNs) form belowground networks that interconnect multiple plant species; yet these networks are typically ignored in studies of allelopathy. We tested the hypothesis that CMNs facilitate transport of allelochemicals from supplier to target plants, thereby affecting allelopathic interactions. We analyzed accumulation of a model allelopathic substance, the herbicide imazamox, and two allelopathic thiophenes released from Tagetes tenuifolia roots, by diffusion through soil and CMNs. We also conducted bioassays to determine how the accumulated substances affected plant growth. All compounds accumulated to greater levels in target soils with CMNs as opposed to soils without CMNs. This increased accumulation was associated with reduced growth of target plants in soils with CMNs. Our results show that CMNs support transfer of allelochemicals from supplier to target plants and thus lead to allelochemical accumulation at levels that could not be reached by diffusion through soil alone. We conclude that CMNs expand the bioactive zones of allelochemicals in natural environments, with significant implications for interspecies chemical interactions in plant communities. |
| [4] | SUMMARY A standard method for the quantification of root colonization by vesicular-arbuscular (VA) mycorrhizal fungi is needed. From the examination of roots from three different host species, the estimation of the percentage of the length of root segments containing V A mycorrhizal fungal structures was found to be more accurate than the determination of the percentage of root segments with VA mycorrhizal fungal structures. It was no more time consuming, and was not influenced by segment size. Examination of a minimum of seven samples, each with 25 randomly selected 0-5 to 10 cm root segments, was needed for confidence limits to be within 10% of the mean It is proposed that, for the sake of comparability between studies, this procedure be adopted as a standard method. |
| [5] | Most terrestrial plants form arbuscular mycorrhiza (AM), mutualistic associations with soil fungi of the order Glomeromycota. The obligate biotrophic fungi trade mineral nutrients, mainly phosphate (P(i) ), for carbohydrates from the plants. Under conditions of high exogenous phosphate supply, when the plant can meet its own P requirements without the fungus, AM are suppressed, an effect which could be interpreted as an active strategy of the plant to limit carbohydrate consumption of the fungus by inhibiting its proliferation in the roots. However, the mechanisms involved in fungal inhibition are poorly understood. Here, we employ a transcriptomic approach to get insight into potential shifts in metabolic activity and symbiotic signalling, and in the defence status of plants exposed to high P(i) levels. We show that in mycorrhizal roots of petunia, a similar set of symbiosis-related genes is expressed as in mycorrhizal roots of Medicago, Lotus and rice. P(i) acts systemically to repress symbiotic gene expression and AM colonization in the root. In established mycorrhizal roots, P(i) repressed symbiotic gene expression rapidly, whereas the inhibition of colonization followed with a lag of more than a week. Taken together, these results suggest that P(i) acts by repressing essential symbiotic genes, in particular genes encoding enzymes of carotenoid and strigolactone biosynthesis, and symbiosis-associated phosphate transporters. The role of these effects in the suppression of symbiosis under high P(i) conditions is discussed. |
| [6] | Abstract Maize (Zea mays) is the most widely cultivated crop around the world; however, it is commonly affected by phosphate (Pi) deficiency in many regions, particularly in acid and alkaline soils of developing countries. To cope with Pi deficiency, plants have evolved a large number of developmental and biochemical adaptations; however, for maize, the underlying molecular basis of these responses is still unknown. In this work, the transcriptional response of maize roots to Pi starvation at 1, 3, 6, and 10 d after the onset of Pi deprivation was assessed. The investigation revealed a total of 1179 Pi-responsive genes, of which 820 and 363 genes were found to be either up- or down-regulated, respectively, by 2-fold or more. Pi-responsive genes were found to be involved in various metabolic, signal transduction, and developmental gene networks. A large set of transcription factors, which may be potential targets for crop breeding, was identified. In addition, gene expression profiles and changes in specific metabolites were also correlated. The results show that several dicotyledonous plant responses to Pi starvation are conserved in maize, but that some genetic responses appear to be more specific and that Pi deficiency leads to a shift in the recycling of internal Pi in maize roots. Ultimately, this work provides a more comprehensive view of Pi-responses in a model for economically important cereals and also sets a framework to produce Pi-specific maize microarrays to study the changes in global gene expression between Pi-efficient and Pi-inefficient maize genotypes. |
| [7] | Understanding the mechanisms that underlie nutrient use efficiency and carbon allocation along with mycorrhizal interactions is critical for managing croplands and forests soundly. Indeed, nutrient availability, uptake and exchange in biotrophic interactions drive plant growth and modulate biomass allocation. These parameters are crucial for plant yield, a major issue in the context of high biomass production. Transport processes across the polarized membrane interfaces are of major importance in the functioning of the established mycorrhizal association as the symbiotic relationship is based on a ‘fair trade’ between the fungus and the host plant. Nutrient and/or metabolite uptake and exchanges, at biotrophic interfaces, are controlled by membrane transporters whose regulation patterns are essential for determining the outcome of plant–fungus interactions and adapting to changes in soil nutrient quantity and/or quality. In the present review, we summarize the current state of the art regarding transport systems in the two major forms of mycorrhiza, namely ecto- and arbuscular mycorrhiza. |
| [8] | 61 The aim of this study was to investigate Ca(2+) responses to endosymbiotic arbuscular mycorrhizal (AM) fungi in the host root epidermis following pre-infection hyphopodium formation in both legumes and nonlegumes, and to determine to what extent these responses could be mimicked by germinated fungal spore exudate. 61 Root organ cultures of both Medicago truncatula and Daucus carota, expressing the nuclear-localized cameleon reporter NupYC2.1, were used to monitor AM-elicited Ca(2+) responses in host root tissues. 61 Ca(2+) spiking was observed in cells contacted by AM hyphopodia for both hosts, with highest frequencies correlating with the epidermal nucleus positioned facing the fungal contact site. Treatment with AM spore exudate also elicited Ca(2+) spiking within the AM-responsive zone of the root and, in both cases, spiking was dependent on the M. truncatula common SYM genes DMI1/2, but not on the rhizobial Nod factor perception gene NFP. 61 These findings support the conclusion that AM fungal root penetration is preceded by a SYM pathway-dependent oscillatory Ca(2+) response, whose evolutionary origin predates the divergence between asterid and rosid clades. Our results further show that fungal symbiotic signals are already generated during spore germination, and that cameleon-expressing root organ cultures represent a novel AM-specific bio-assay for such signals. |
| [9] | |
| [10] | An isolate of Penicillium bilaii previously reported to solubilize mineral phosphates and enhance plant uptake of phosphate was studied. Using agar media with calcium phosphate and the pH indicator alizarin red S, the influence of the medium composition on phosphate solubility and medium acidification was recorded. The major acidic metabolites produced by P. bilaii in a sucrose nitrate liquid medium were found to be oxalic acid and citric acid. Citric acid production was promoted under nitrogen-limited conditions, while oxalic acid production was promoted under carbon-limited conditions. Citric acid was produced in both growth and stationary phases, but oxalic acid production occurred only in stationary phase. When submerged cultures which normally produce acid were induced to sporulate, the culture medium shifted toward alkaline rather than acid reaction with growth. |
| [11] | |
| [12] | Phospho enol pyruvate carboxylase (PEPC) was overproduced in the leaves of rice plants by introducing the intact maize C 4 -specific PEPC gene. Maize PEPC in transgenic rice leaves underwent activity regulation through protein phosphorylation in a manner similar to endogenous rice PEPC but contrary to that occurring in maize leaves, being downregulated in the light and upregulated in the dark. Compared with untransformed rice, the level of the substrate for PEPC (phospho enol pyruvate) was slightly lower and the product (oxaloacetate) was slightly higher in transgenic rice, suggesting that maize PEPC was functioning even though it remained dephosphorylated and less active in the light. 14 CO 2 labeling experiments indicated that maize PEPC did not contribute significantly to the photosynthetic CO 2 fixation of transgenic rice plants. Rather, it slightly lowered the CO 2 assimilation rate. This effect was ascribable to the stimulation of respiration in the light, which was more marked at lower O 2 concentrations. It was concluded that overproduction of PEPC does not directly affect photosynthesis significantly but it suppresses photosynthesis indirectly by stimulating respiration in the light. We also found that while the steady-state stomatal aperture remained unaffected over a wide range of humidity, the stomatal opening under non-steady-state conditions was destabilized in transgenic rice. |
| [13] | Large quantities of citrate ions have been shown to be secreted by the roots ofLupinus albus. It is postulated that these react in the soil to form ferric hydroxy phosphate polymers which diffuse to the root surface where they are degraded by the action of reducing agents in the presence of an Fe II uptake mechanism balanced by hydrogen ion secretion. Some known chemical behaviour of Fe III and citrate which supports this postulate is reviewed. Evidence is also presented which suggests that much of the Fe absorbed circulates within the root system and is subsequently precipitated. |
| [14] | Most terrestrial plant roots form mutualistic symbiosis with soil-borne arbuscular mycorrhizal fungi (AMF), a characteristic feature of which is nutrient exchange between the two symbiotic partners. Phosphate (Pi) is the main benefit the host plants acquired from the AMF. It has long been a common realization that high Pi supply could suppress the AMF development. However, the direct molecular regulatory mechanisms underlying this plant directed suppression are lacking. Here, we reviewed the recent work providing the evidences that high Pi supply induces transcriptional alteration, leading to the inhibition of AMF development at different stages of AM symbiosis, and gave our view on potential cross-talk among Pi starvation, AM as well as phytohormone signaling. |
| [15] | Glomalean fungi induce and colonize symbiotic tissue called arbuscular mycorrhiza on the roots of most land plants. Other fungi also colonize plants but cause disease not symbiosis. Whole-transcriptome analysis using a custom-designed Affymetrix Gene-Chip and confirmation with real-time RT-PCR revealed 224 genes affected during arbuscular mycorrhizal symbiosis. We compared these transcription profiles with those from rice roots that were colonized by pathogens (Magnaporthe grisea and Fusarium moniliforme). Over 40% of genes showed differential regulation caused by both the symbiotic and at least one of the pathogenic interactions. A set of genes was similarly expressed in all three associations, revealing a conserved response to fungal colonization. The responses that were shared between pathogen and symbiont infection may play a role in compatibility. Likewise, the responses that are different may cause disease. Some of the genes that respond to mycorrhizal colonization may be involved in the uptake of phosphate. Indeed, phosphate addition mimicked the effect of mycorrhiza on 8% of the tested genes. We found that 34% of the mycorrhiza-associated rice genes were also associated with mycorrhiza in dicots, revealing a conserved pattern of response between the two angiosperm classes. |
| [16] | Arbuscular mycorrhizal fungi colonize the roots of most monocotyledons and dicotyledons despite their different root architecture and cell patterning. Among the cereal hosts of arbuscular mycorrhizal fungi, Oryza sativa (rice) possesses a peculiar root system composed of three different types of roots: crown roots; large lateral roots; and fine lateral roots. Characteristic is the constitutive formation of aerenchyma in crown roots and large lateral roots and the absence of cortex from fine lateral roots. Here, we assessed the distribution of colonization by Glomus intraradices within this root system and determined its effect on root system architecture. Large lateral roots are preferentially colonized, and fine lateral roots are immune to arbuscular mycorrhizal colonization. Fungal preference for large lateral roots also occurred in sym mutants that block colonization of the root beyond rhizodermal penetration. Initiation of large lateral roots is significantly induced by G. intraradices colonization and does not require a functional common symbiosis signaling pathway from which some components are known to be needed for symbiosis-mediated lateral root induction in Medicago truncatula. Our results suggest variation of symbiotic properties among the different rice root-types and induction of the preferred tissue by arbuscular mycorrhizal fungi. Furthermore, signaling for arbuscular mycorrhizal-elicited alterations of the root system differs between rice and M. truncatula. |
| [17] | Many plants have the capacity to obtain phosphate via a symbiotic association with arbuscular mycorrhizal (AM) fungi. In AM associations, the fungi release phosphate from differentiated hyphae called arbuscules, that develop within the cortical cells, and the plant transports the phosphate across a symbiotic membrane, called the periarbuscular membrane, into the cortical cell. In Medicago truncatula, a model legume used widely for studies of root symbioses, it is apparent that the phosphate transporters known to operate at the root-soil interface do not participate in symbiotic phosphate transport. EST database searches with short sequence motifs shared by known phosphate transporters enabled the identification of a novel phosphate transporter from M. truncatula, MtPT4. MtPT4 is significantly different from the plant root phosphate transporters cloned to date. Complementation of yeast phosphate transport mutants indicated that MtPT4 functions as a phosphate transporter, and estimates of the K(m) suggest a relatively low affinity for phosphate. MtPT4 is expressed only in mycorrhizal roots, and the MtPT4 promoter directs expression exclusively in cells containing arbuscules. MtPT4 is located in the membrane fraction of mycorrhizal roots, and immunolocalization revealed that MtPT4 colocalizes with the arbuscules, consistent with a location on the periarbuscular membrane. The transport properties and spatial expression patterns of MtPT4 are consistent with a role in the acquisition of phosphate released by the fungus in the AM symbiosis. |
| [18] | . In the arbuscular mycorrhizal (AM) symbiosis the reciprocal exchange of nutrients results in a nutritional benefit for both symbionts. The fungus acquires carbon from plant and the plant obtains mineral nutrients from the fungus. While there is evidence for the transfer of phosphorus (P), nitrogen, zinc and copper, current data suggest that P is transferred in the highest quantities and that symbiotic P transfer occurs in the vast majority of AM symbioses. Symbiotic phosphate Pi transfer requires transport proteins to move Pi across the membranes of the AM fungus and plant. In recent years, there has been tremendous progress in the identification of plant and fungal Pi transporter proteins involved in symbiotic Pi transport. Coupled with the physiological data a greater understanding of symbiotic Pi transfer has emerged. Here we summarize the current data about Pi transporters and their expression patterns and roles in AM symbiosis. |
| [19] | In response to the colonization by arbuscular mycorrhizal (AM) fungi, plants reprioritize their phosphate (Pi)-uptake strategies to take advantage of nutrient transfer via the fungus. The mechanisms underlying Pi transport are beginning to be understood, and recently, details of the regulation of plant and fungal Pi transporters in the AM symbiosis have been revealed. This review summarizes recent advances in this area and explores current data and hypotheses of how the plant Pi status affects the symbiosis. Finally, suggestions of an interrelationship of Pi and nitrogen (N) in the AM symbiosis are discussed. |
| [20] | Abstract Using dual cultures of arbuscular mycorrhizal (AM) fungi and Medicago truncatula separated by a physical barrier, we demonstrate that hyphae from germinating spores produce a diffusible factor that is perceived by roots in the absence of direct physical contact. This AM factor elicits expression of the Nod factor-inducible gene MtENOD11, visualized using a pMtENOD11-gusA reporter. Transgene induction occurs primarily in the root cortex, with expression stretching from the zone of root hair emergence to the region of mature root hairs. All AM fungi tested (Gigaspora rosea, Gigaspora gigantea, Gigaspora margarita, and Glomus intraradices) elicit a similar response, whereas pathogenic fungi such as Phythophthora medicaginis, Phoma medicaginis var pinodella and Fusarium solani f.sp. phaseoli do not, suggesting that the observed root response is specific to AM fungi. Finally, pMtENOD11-gusA induction in response to the diffusible AM fungal factor is also observed with all three M. truncatula Nod(-)/Myc(-) mutants (dmi1, dmi2, and dmi3), whereas the same mutants are blocked in their response to Nod factor. This positive response of the Nod(-)/Myc(-) mutants to the diffusible AM fungal factor and the different cellular localization of pMtENOD11-gusA expression in response to Nod factor versus AM factor suggest that signal transduction occurs via different pathways and that expression of MtENOD11 is differently regulated by the two diffusible factors. |
| [21] | , 在模拟干旱条件下,研究了接种丛枝菌根(AM)真菌Glomus intraradices对玉米(Zea mays)根部13种质膜水孔蛋白基因表达的影响,同时观测了AM真菌自身水孔蛋白基因的表达情况.结果表明,干旱条件下,除Zm PIP1;3、Zm PIP1;4、ZmPIP1;5和Zm PIP2;2之外的接种处理能显著提高根部其他8种质膜水孔蛋白基因的表达(Zm PIP2;7表达量未检测出),并且AM真菌菌丝中水孔蛋白基因GintAQP1表达也显著增强.与此同时,接种处理明显改善了植物水分状况,提高了叶片 水势.AM真菌增强宿主植物根部及自身的水孔蛋白基因的表达对于提高植物抗旱性具有潜在的重要贡献. |
| [22] | Arbuscular mycorrhiza (AM) is a root endosymbiosis between plants and glomeromycete fungi. It is the most widespread terrestrial plant symbiosis, improving plant uptake of water and mineral nutrients. Yet, despite its crucial role in land ecosystems, molecular mechanisms leading to its formation are just beginning to be unravelled. Recent evidence suggests that AM fungi produce diffusible symbiotic signals. Here we show that Glomus intraradices secretes symbiotic signals that are a mixture of sulphated and non-sulphated simple lipochitooligosaccharides (LCOs), which stimulate formation of AM in plant species of diverse families (Fabaceae, Asteraceae and Umbelliferae). In the legume Medicago truncatula these signals stimulate root growth and branching by the symbiotic DMI signalling pathway. These findings provide a better understanding of the evolution of signalling mechanisms involved in plant root endosymbioses and will greatly facilitate their molecular dissection. They also open the way to using these natural and very active molecules in agriculture. |
| [23] | The majority of vascular flowering plants are able to form symbiotic associations with arbuscular mycorrhizal fungi. These symbioses, termed arbuscular mycorrhizas, are mutually beneficial, and the fungus delivers phosphate to the plant while receiving carbon. In these symbioses, phosphate uptake by the arbuscular mycorrhizal fungus is the first step in the process of phosphate transport to the plant. Previously, we cloned a phosphate transporter gene involved in this process. Here, we analyze the expression and regulation of a phosphate transporter gene (GiPT) in the extra-radical mycelium of the arbuscular mycorrhizal fungus Glomus intraradices during mycorrhizal association with carrot or Medicago truncatula roots. These analyses reveal that GiPT expression is regulated in response to phosphate concentrations in the environment surrounding the extra-radical hyphae and modulated by the overall phosphate status of the mycorrhiza. Phosphate concentrations, typical of those found in the soil solution, result in expression of GiPT These data imply that G. intraradices can perceive phosphate levels in the external environment but also suggest the presence of an internal phosphate sensing mechanism. |
| [24] | . |
| [25] | |
| [26] | Plants colonized by arbuscular mycorrhizal (AM) fungi take up phosphate (Pi)via the mycorrhizal and the direct Pi uptake pathway. Our understanding of the molecular mechanisms involved in the regulation of these pathways is just emerging.Here, we have analyzed the molecular physiology of mycorrhizal Pi uptake in the tomato (Solanum lycopersicum) variety Micro-Tom and integrated the data obtained with studies on chemical signaling in mycorrhiza-inducible Pi transporter gene regulation.At high plant phosphorus (P) status, the mycorrhizal Pi uptake pathway was almost completely repressed and the mycorrhiza-inducible Pi transporter genes were down-regulated. A high plant P status also suppressed the activation of the mycorrhiza-specific StPT3 promoter fragment by phospholipid extracts containing the mycorrhiza signal lysophosphatidylcholine.Our results suggest that the mycorrhizal Pi uptake pathway is controlled at least partially by the plant host. This control involves components in common |
| [27] | Summary Top of page Summary Introduction Results Discussion Experimental procedures Acknowledgements References Supporting Information Legumes form two different types of intracellular root symbioses, with fungi and bacteria, resulting in arbuscular mycorrhiza and nitrogen-fixing nodules, respectively. Rhizobial signalling molecules, called Nod factors, play a key role in establishing the rhizobium–legume association and genes have been identified in Medicago truncatula that control a Nod factor signalling pathway leading to nodulation. Three of these genes, the so-called DMI1 , DMI2 and DMI3 genes, are also required for formation of mycorrhiza, indicating that the symbiotic pathways activated by both the bacterial and the fungal symbionts share common steps. To analyse possible cross-talk between these pathways we have studied the effect of treatment with Nod factors on mycorrhization in M. truncatula. We show that Nod factors increase mycorrhizal colonization and stimulate lateral root formation. The stimulation of lateral root formation by Nod factors requires both the same structural features of Nod factors and the same plant genes ( NFP , DMI1 , DMI2 , DMI3 and NSP1 ) that are required for other Nod factor-induced symbiotic responses such as early nodulin gene induction and cortical cell division. A diffusible factor from arbuscular mycorrhizal fungi was also found to stimulate lateral root formation, while three root pathogens did not have the same effect. Lateral root formation induced by fungal signal(s) was found to require the DMI1 and DMI2 genes, but not DMI3 . The idea that this diffusible fungal factor might correspond to a previously hypothesized mycorrhizal signal, the ‘Myc factor’, is discussed. |
| [28] | Abstract Arbuscular mycorrhizal (AM) fungi depend on a C supply from the plant host and simultaneously provide phosphorus to the colonized plant. We therefore evaluated the influence of external P on C allocation in monoxenic Daucus carota-Glomus intraradices cultures in an AM symbiosis. Fungal hyphae proliferated from a solid minimal medium containing colonized roots into a C-free liquid minimal medium with high or low P availability. Roots and hyphae were harvested periodically, and the flow of C from roots to fungus was measured by isotope labeling. We also measured induction of a G. intraradices high-affinity P transporter to estimate fungal P demand. The prevailing hypothesis is that high P availability reduces mycorrhizal fungal growth, but we found that C flow to the fungus was initially highest at the high P level. Only at later harvests, after 100 days of in vitro culture, were C flow and fungal growth limited at high P availability. Thus, AM fungi can benefit initially from P-enriched environments in terms of plant C allocation. As expected, the P transporter induction was significantly greater at low P availability and greatest in very young mycelia. We found no direct link between C flow to the fungus and the P transporter transcription level, which indicates that a good C supply is not essential for induction of the high-affinity P transporter. We describe a mechanism by which P regulates symbiotic C allocation, and we discuss how this mechanism may have evolved in a competitive environment. |
| [29] | Use of the real-time polymerase chain reaction (PCR) to amplify cDNA products reverse transcribed from mRNA is on the way to becoming a routine tool in molecular biology to study low abundance gene expression. Real-time PCR is easy to perform, provides the necessary accuracy and produces reliable as well as rapid quantification results. But accurate quantification of nucleic acids requires a reproducible methodology and an adequate mathematical model for data analysis. This study enters into the particular topics of the relative quantification in real-time RT-PCR of a target gene transcript in comparison to a reference gene transcript. Therefore, a new mathematical model is presented. The relative expression ratio is calculated only from the real-time PCR efficiencies and the crossing point deviation of an unknown sample versus a control. This model needs no calibration curve. Control levels were included in the model to standardise each reaction run with respect to RNA integrity, sample loading and inter-PCR variations. High accuracy and reproducibility (<2.5% variation) were reached in LightCycler PCR using the established mathematical model. |
| [30] | Nonpigmented roots were cleared by heating (90$\circ$C for 1 hr) in 10% KOH, then rinsed and acidified with dilute HC1 and stained by simmering (5 min) in 0.05% trypan blue in lactophenol. Mycorrhizal infections showed clearly in onion, Liquid-ambar styraciflua, Coprosma robusta and gramineous roots so treated. Pigmented roots were cleared in 10% KOH for at least 2 hr, washed in fresh KOH and bleache... |
| [31] | Biomass accumulation by crops depends on both light interception by leaves and on the efficiency with which the intercepted light is used to produce dry matter. Our aim was to identify which of these processes were affected for maize ( Zea mays L., cv Volga) field crops grown under phosphorus (P) deficiency. In the preceding paper (Plénet et al., 2000), it was shown that P deficiency severely reduced leaf growth. In this paper, the effect of P deficiency on the radiation-use efficiency (RUE) was investigated. The experimental work was carried out in 1995, 1996 and 1997 on a long-term P fertilisation trial located on a sandy soil in the south-west of France. Three P fertilisation regimes have been applied since 1972: no- P (P0 treatment) and different rates of P fertiliser (P1.5: 1.5 times the grain P export and P3: 3 times the grain P export). These fertilisation regimes have led to contrasted levels of soil P supply. Only slight differences were observed between the P1.5 and P3 treatment for above-ground biomass accumulation and grain yield. Conversely the grain yield was significantly reduced in P0 (6111%). Above-ground biomass production was severely reduced, with the maximum difference between treatment (6160% in P0) occurring between 400 and 600 °C days after sowing. The lower biomass production in P0 was accounted for by the reduced amount of photosynthetically active radiation (PAR) absorbed by the canopy, which was itself the consequence of the reduced leaf area index (see Plénet et al., 2000). The calculated RUE were found to depend on the plant stage, especially during the pre-flowering period, and on the average air temperature. No effect of P deficiency was observed on the calculated RUE, even during the period when above-ground biomass accumulation was the most severely reduced. These results obtained in field crop conditions strengthen the idea that P deficiency affects plant growth, especially leaf growth, earlier and to a greater extent than photosynthesis per unit leaf area. |
| [32] | Seed maturation responds to endogenous and exogenous signals like nutrient status, energy and hormones. We recently showed that phosphoenolpyruvate carboxylase (PEPC) overexpression in Vicia narbonensis seeds alters seed metabolism and channels carbon into organic acids, resulting in greater seed storage capacity and increased protein content. Thus, these lines represent models with altered sink strength and improved nutrient status. Here we analyse seed developmental and metabolic parameters, and C/N partitioning in these seeds. Transgenic embryos take up more carbon and nitrogen. Changes in dry to FW ratio, seed fill duration and major seed components indicate altered seed development. Array-based gene expression analysis of embryos reveals upregulation of seed metabolism, especially during the transition phase and at late maturation, in terms of protein storage and processing, amino acid metabolism, primary metabolism and transport, energy and mitochondrial activity, transcriptional and translational activity, stress tolerance, photosynthesis, cell proliferation and elongation, signalling and hormone action and regulated protein degradation. Stimulated cell elongation is in accordance with upregulated signalling pathways related to gibberellic acid/brassinosteroids. We discuss that activated organic and amino acid production leads to a wide-range activation of nitrogen metabolism, including the machinery of storage protein synthesis, amino acid synthesis, protein processing and deposition, translational activity and the methylation cycle. We suggest that alpha-ketoglutarate (alpha-KG) and/or oxalacetate provide signals for coordinate upregulation of amino acid biosynthesis. Activation of stress tolerance genes indicates partial overlap between nutrient, stress and abscisic acid (ABA) signals, indicating a common interacting or regulatory mechanism between nutrients, stress and ABA. In conclusion, analysis of PEPC overexpressing seeds identified pathways responsive to metabolic and nutrient control on the transcriptional level and its underlying signalling mechanisms. |
| [33] | Phosphate (Pi) deficiency is one of the leading causes of loss in crop productivity. Plants respond to Pi deficiency by increasing Pi acquisition and remobilization involving organic and inorganic Pi transporters. Here, we report the functional characterization of a putative organic Pi transporter, Glycerol-3-phosphate permease (G3Pp) family, comprising five members (AtG3Pp1 to -5) in Arabidopsis (Arabidopsis thaliana). AtG3Pp1 and AtG3Pp2 showed 24-and 3-fold induction, respectively, in the roots of Pi-deprived seedlings, whereas Pi deficiency-mediated induction of AtG3Pp3 and -4 was evident in both roots and shoots. Furthermore, promoter-glucuronidase (GUS) fusion transgenics were generated for AtG3Pp2 to -5 for elucidation of their in planta role in Pi homeostasis. During Pi starvation, there was a strong expression of the reporter gene driven by AtG3Pp4 promoter in the roots, shoots, anthers, and siliques, whereas GUS expression was specific either to the roots (AtG3Pp3) or to stamens and siliques (AtG3Pp5) in other promoter-GUS fusion transgenics. Quantification of reporter gene activities further substantiated differential responses of AtG3Pp family members to Pi deprivation. A distinct pattern of reporter gene expression exhibited by AtG3Pp3 and AtG3Pp5 during early stages of germination also substantiated their potential roles during seedling ontogeny. Furthermore, an AtG3Pp4 knockdown mutant exhibited accentuated total lateral root lengths under +phosphorus and -phosphorus conditions compared with the wild type. Several Pi starvation-induced genes involved in root development and/or Pi homeostasis were up-regulated in the mutant. A 9-fold induction of AtG3Pp3 in the mutant provided some evidence for a lack of functional redundancy in the gene family. These results thus reflect differential roles of members of the G3Pp family in the maintenance of Pi homeostasis. |
| [34] | Abstract Plants engage in mutualistic interactions with microbes that improve their mineral nutrient supply. The most wide-spread symbiotic association is arbuscular mycorrhiza (AM), in which fungi of the order Glomeromycota invade roots and colonize the cellular lumen of cortical cells. The establishment of this interaction requires a dedicated molecular-genetic program and a cellular machinery of the plant host. This program is partially shared with the root nodule symbiosis (RNS), which involves prokaryotic partners collectively referred to as rhizobia. Both, AM and RNS are endosymbioses that involve intracellular accommodation of the microbial partner in the cells of the plant host. Since plant cells are surrounded by sturdy cell walls, root penetration and cell invasion requires mechanisms to overcome this barrier while maintaining the cytoplasm of the two partners separate during development of the symbiotic association. Here, we discuss the diverse functions of the cell wall compartment in establishment and functioning of plant symbioses with the emphasis on AM and RNS, and we describe the stages of the AM association between the model organisms Petunia hybrida and Rhizophagus irregularis. |
| [35] | |
| [36] | Summary An ambitious aim in plant breeding and biotechnology is to increase the protein content of crop seeds used for food and feed. Using an approach to manipulate assimilate partitioning, we succeeded in elevating the protein content in legume seeds up to 50%. Transgenic bean plants were generated which express a Corynebacterium glutamicum phosphoenolpyruvate carboxylase (PEPC) in a seed-specific manner. The bacterial enzyme was not feedback inhibited by malate. Transgenic seeds showed a higher [ 14 C]-CO 2 uptake and about a threefold increased incorporation of labelled carbon into proteins. Changed metabolite profiles of maturing cotyledons indicated a shift of metabolic fluxes from sugars/starch into organic acids and free amino acids. These changes were consistent with an increased carbon flow through the anaplerotic pathway catalysed by PEPC. Consequently, transgenic seeds accumulated up to 20% more protein per gram seed dry weight. Additionally, seed dry weight was higher by 20%~30%. We conclude that PEPC in seeds is a promising target for molecular plant breeding. |
| [37] | We report an improved draft nucleotide sequence of the 2.3-gigabase genome of maize, an important crop plant and model for biological research. Over 32,000 genes were predicted, of which 99.8% were placed on reference chromosomes. Nearly 85% of the genome is composed of hundreds of families of transposable elements, dispersed nonuniformly across the genome. These were responsible for the capture and amplification of numerous gene fragments and affect the composition, sizes, and positions of centromeres. We also report on the correlation of methylation-poor regions with Mu transposon insertions and recombination, and copy number variants with insertions and/or deletions, as well as how uneven gene losses between duplicated regions were involved in returning an ancient allotetraploid to a genetically diploid state. These analyses inform and set the stage for further investigations to improve our understanding of the domestication and agricultural improvements of maize. |
| [38] | |
| [39] | react-text: 292 Several studies pointed out soil properties as the prime determinant ofcerrado (the Brazilian savanna) physiognomies, and a gradient from campocerrado (a shrub savanna) to cerrado (a tallwoodland) has been correlated with a soil fertility gradient. Based on thishypothesis, we investigated soil-vegetation relationships in theP-de-Gigante Reserve (So Paulo State,SoutheasternBrazil). We randomly... /react-text react-text: 293 /react-text [Show full abstract] |
| [40] | Summary 1. Almost all plants are engaged in symbiotic relationships with mycorrhizal fungi. These soil fungi can promote plant growth by supplying limiting nutrients to plant roots in return for plant assimilates. 2. Many mycorrhizal fungi are not host specific and one fungal individual can colonize and interconnect a considerable number of plants. The existence of these so-called mycorrhizal networks implies that fungi have the potential to facilitate growth of other plants and distribute resources among plants irrespective of their size, status or identity. In this paper, we explore the significance of mycorrhizal fungal networks for individual plants and for plant communities. 3. We address the following questions: (i) are all plant species benefitting from mycorrhizal networks, (ii) is benefit dependent on the size or age of a plant, (iii) is fungal support related to the relative dominance of plants in a community, (iv) are there host dependent barriers and physiological constraints for support and (v) what is the impact of mycorrhizal networks on plant lant interactions and plant community dynamics? Moreover, using a review of published studies, we test whether mycorrhizal networks facilitate growth of small seedlings that establish between or near larger plants. 4. We found 60 cases where seedling species were grown together with larger plants with or without mycorrhizal fungal networks. Mycorrhizal networks promoted seedling growth in 48% of the cases (for 21 seedling species), while negative effects (25%) and no effects (27%) were also common. Seedlings associating with ectomycorrhizal fungi benefitted in the majority of the cases while effects on seedlings associating with arbuscular mycorrhizal fungi were more variable. Thus, the facilitative effects of mycorrhizal fungal networks depend on seedling species identity, mycorrhizal identity, plant species combinations and study system. We present a number of hypothetical scenarios that can explain the results based on cost enefit relationship of individual members in a network. 5. Synthesis. Overall, this review shows that mycorrhizal networks play a key role in plant communities by facilitating and influencing seedling establishment, by altering plant lant interactions and by supplying and recycling nutrients. |
| [41] | Abstract A comparative analysis of daily carbon (C) budgets and aspects of the C physiology of clover ( Trifolium repens L.) colonized by vesicular-arbuscular (VA) mycorrhizal fungi was carried out over a 70 d growth period under conditions designed to ensure that shoots of mycorrhizal (M) and non-mycorrhizal (NM) plants were of similar nutrient status. C budgets did not differ on day 24 but by day 42 M plants had a significantly higher rate of photosynthesis than their NM counterparts when expressed on a whole shoot basis or unit dry weight basis. As both sets of plants were of the same size it was concluded that this greater C gain was the result of increased sink strength provided by the mycorrhizal fungus. By day 53 M plants had become larger than their uncolonized counterparts and a sink-induced stimulation in the rate of photosynthesis was no longer apparent. M plants had higher root sucrose, glucose and fructose pools from day 24. Analyses suggested that these sugars were utilized for trehalose and lipid synthesis, for the production of the large extramatrical mycelium and for the support of the respiratory demands of the M root system. Increased C allocation to roots of M plants was associated with a stimulation of the activities of cell wall and cytoplasmic invertases and of sucrose synthase in roots colonized by VA fungi. Such increases in enzyme activity may provide the mechanism enabling increased partitioning of carbohydrate both to the M root system and the fungal symbiont. |
| [42] | Arbuscular mycorrhizal (AM) fungi colonize roots and form two kinds of mycelium, intraradical mycelium (IRM) and extraradical mycelium (ERM). Arbuscules are characteristic IRM structures that highly branch within host cells in order to mediate resource exchange between the symbionts. They are ephemeral structures and at the end of their life span, arbuscular branches collapse from the tip, fungal cytoplasm withdraws, and the whole arbuscule shrinks into fungal clumps. The exoskeleton of an arbuscule contains structured chitin, which is a polymer of N -acetylglucosamine (GlcNAc), whereas a collapsed arbuscule does not. The molecular mechanisms underlying the turnover of chitin in AM fungi remain unknown. Here, a GlcNAc transporter, RiNGT, was identified from the AM fungus Rhizophagus irregularis . Yeast mutants defective in endogenous GlcNAc uptake and expressing RiNGT took up 14 C-GlcNAc, and the optimum uptake was at acidic pH values (pH 4.0鈥4.5). The transcript levels of RiNGT in IRM in mycorrhizal Lotus japonicus roots were over 1000 times higher than those in ERM. GlcNAc-6-phosphate deacetylase ( DAC1 ) and glucosamine-6-phosphate isomerase ( NAG1 ) genes, which are related to the GlcNAc catabolism pathway, were also induced in IRM. Altogether, data suggest the existence of an enhanced recycling mode of GlcNAc in IRM of AM fungi. |
1
2000
... 宿主植物和AM真菌之间的碳磷交换是稳定菌根共生体系的基础(
1
... 供试土壤采自内蒙古鄂尔多斯市东胜区铜川镇枳机塔村(39.89° E, 110.02° N, 海拔1β367 m).土壤基本理化性质如下: pH值8.69 (水浸提, 水土质量比2.5:1), 有机质含量22.01 g·kg-1; 有机碳含量12.77 g·kg-1; 有效磷含量4.46 mg·kg-1, 具体测定方法参见
The fungal fast lane: Common mycorrhizal networks extend bioactive zones of allelochemicals in soils.
2011
Quantifying vesicular- arbuscular mycorrhizae: A proposed method towards standardization.
1
1981
... 试验收获时, 自培养基质表面将植物剪断.将玉米地上部和根系用去离子水冲洗两遍, 吸干表面水分, 分别称取鲜质量.取混匀的玉米根系 0.1 g, 经液氮冷冻后, 于-80 ℃保存, 用于RNA提取; 另取混匀根系样品3 g, 用于菌根侵染率测定.余下样品置于105 ℃烘箱中杀青10 min, 转为80 ℃烘干至恒质量, 称干质量.根系染色过程依据Phillips和Hayman (1970)的方法, 但有所简化: 将3 g根系放入10% KOH中, 90 ℃水浴锅中煮10 min, 用自来水冲洗干净后, 加入0.05%的台盼蓝90 ℃染色5 min.染色根段置于载玻片上, 然后加盖盖玻片观察, 每个样品观测30根段(
Phosphate systemically inhibits development of arbuscular mycorrhiza in Petunia hybrida and represses genes involved in mycorrhizal functioning.
2010
Transcript profiling of Zea mays roots reveals gene responses to phosphate deficiency at the plant-and species- specific levels.
1
2008
... 在热带和亚热带酸性土壤及温带钙化土壤中, 土壤有效磷含量很低, 而且施用的磷肥很快会被土壤固定, 难以被植物吸收利用(
Biotrophic transportome in mutualistic plant-fungal interactions.
1
2013
... 在热带和亚热带酸性土壤及温带钙化土壤中, 土壤有效磷含量很低, 而且施用的磷肥很快会被土壤固定, 难以被植物吸收利用(
Arbuscular mycorrhizal hyphopodia and germinated spore exudates trigger Ca2+ spiking in the legume and nonlegume root epidermis.
1
2011
... Carlos等(2008)利用基因芯片研究了磷饥饿对植物基因表达谱的影响, 表明碳代谢、氮代谢、脂代谢和磷代谢基因均有不同响应.本试验中, 低磷环境下AM不仅显著调节了供体植物碳磷代谢相关基因G3PT、PEPC、TC289和MAS1的表达, 还上调了受体植物中上述基因的表达.G3PT参与糖-磷酸盐/阴离子的逆向转运过程, 低磷胁迫下拟南芥(Arabidopsis thaliala)中G3PT家族的5个基因均呈现不同程度的上调, 进而调节根的生长或磷酸盐的平衡(
Conservation and divergence of both phosphate- and mycorrhiza-regulated physiological responses and expression patterns of phosphate transporters in solanaceous species.
1
2007
... 下, AM真菌丛枝发育不良, 仅有稀疏分枝并过早衰亡, 同时根内菌丝的生长和分化也受到抑制.此外, AM真菌中一些关键功能基因, 如磷酸盐转运蛋白基因的表达水平也会被高浓度磷酸盐抑制(
Production of citric and oxalic acids and solubilization of calcium phosphate by Penicillium bilaii.
1
1992
... 磷作为一种植物必需的营养元素, 在植物生长发育和代谢过程中均起着重要作用(
Soil test levels in North America.
1
2002
... 在热带和亚热带酸性土壤及温带钙化土壤中, 土壤有效磷含量很低, 而且施用的磷肥很快会被土壤固定, 难以被植物吸收利用(
Activity regulation and physiological impacts of maize C4-specific phosphoenolpyruvate carboxylase overproduced in transgenic rice plants.
1
2003
... Carlos等(2008)利用基因芯片研究了磷饥饿对植物基因表达谱的影响, 表明碳代谢、氮代谢、脂代谢和磷代谢基因均有不同响应.本试验中, 低磷环境下AM不仅显著调节了供体植物碳磷代谢相关基因G3PT、PEPC、TC289和MAS1的表达, 还上调了受体植物中上述基因的表达.G3PT参与糖-磷酸盐/阴离子的逆向转运过程, 低磷胁迫下拟南芥(Arabidopsis thaliala)中G3PT家族的5个基因均呈现不同程度的上调, 进而调节根的生长或磷酸盐的平衡(
The acquisition of phosphorus by Lupinus albus L.: 3. The probable mechanism by which phosphorus movement in the soil/ root interface is enhanced.
1
1983
... 磷作为一种植物必需的营养元素, 在植物生长发育和代谢过程中均起着重要作用(
How does phosphate status influence the development of the arbuscular mycorrhizal symbiosis?
1
2011
... 本试验中, 低磷条件下玉米菌根侵染率显著高于高磷处理, 丛枝丰度也较高, 这与以往的研究结果一致(
Comparative transcriptomics of rice reveals an ancient pattern of response to microbial colonization.
1
2005
... 在热带和亚热带酸性土壤及温带钙化土壤中, 土壤有效磷含量很低, 而且施用的磷肥很快会被土壤固定, 难以被植物吸收利用(
Glomus intraradices induces changes in root system architecture of rice independently of common symbiosis signaling.
1
2009
... Carlos等(2008)利用基因芯片研究了磷饥饿对植物基因表达谱的影响, 表明碳代谢、氮代谢、脂代谢和磷代谢基因均有不同响应.本试验中, 低磷环境下AM不仅显著调节了供体植物碳磷代谢相关基因G3PT、PEPC、TC289和MAS1的表达, 还上调了受体植物中上述基因的表达.G3PT参与糖-磷酸盐/阴离子的逆向转运过程, 低磷胁迫下拟南芥(Arabidopsis thaliala)中G3PT家族的5个基因均呈现不同程度的上调, 进而调节根的生长或磷酸盐的平衡(
A phosphate transporter from Medicago truncatula involved in the acquisition of phosphate released by arbuscular mycorrhizal fungi.
1
2002
... 在热带和亚热带酸性土壤及温带钙化土壤中, 土壤有效磷含量很低, 而且施用的磷肥很快会被土壤固定, 难以被植物吸收利用(
Phosphate transporters in arbuscular mycorrhizal symbiosis. In: Koltai H, Kapulnik Y eds. Arbuscular Mycorrhizas: Physiology and Function
1
2010
... 本试验中, 低磷条件下玉米菌根侵染率显著高于高磷处理, 丛枝丰度也较高, 这与以往的研究结果一致(
Phosphate in the arbuscular mycorrhizal symbiosis: Transport properties and regulatory roles.
1
2007
... 磷作为一种植物必需的营养元素, 在植物生长发育和代谢过程中均起着重要作用(
A diffusible factor from arbuscular mycorrhizal fungi induces symbiosis-specific MtENOD11 expression in roots of Medicago truncatula.
2003
丛枝菌根真菌通过上调根系及自身水孔蛋白基因表达提高玉米抗旱性
1
2012
... 试验收获时, 自培养基质表面将植物剪断.将玉米地上部和根系用去离子水冲洗两遍, 吸干表面水分, 分别称取鲜质量.取混匀的玉米根系 0.1 g, 经液氮冷冻后, 于-80 ℃保存, 用于RNA提取; 另取混匀根系样品3 g, 用于菌根侵染率测定.余下样品置于105 ℃烘箱中杀青10 min, 转为80 ℃烘干至恒质量, 称干质量.根系染色过程依据Phillips和Hayman (1970)的方法, 但有所简化: 将3 g根系放入10% KOH中, 90 ℃水浴锅中煮10 min, 用自来水冲洗干净后, 加入0.05%的台盼蓝90 ℃染色5 min.染色根段置于载玻片上, 然后加盖盖玻片观察, 每个样品观测30根段(
Fungal lipochitooligosaccharide symbiotic signals in arbuscular mycorrhiza.
2011
A phosphate transporter gene from the extra-radical mycelium of an arbuscular mycorrhizal fungus Glomus intraradices is regulated in response to phosphate in the environment.
1
2001
... 在热带和亚热带酸性土壤及温带钙化土壤中, 土壤有效磷含量很低, 而且施用的磷肥很快会被土壤固定, 难以被植物吸收利用(
Mineral Nutrition of Higher Plants. 2nd edn
1
1995
... 磷作为一种植物必需的营养元素, 在植物生长发育和代谢过程中均起着重要作用(
The characterization of novel mycorrhiza-specific phosphate transporters from Lycopersicon esculentum and Solanum tuberosum uncovers functional redundancy in symbiotic phosphate transport in solanaceous species.
1
2005
... 在热带和亚热带酸性土壤及温带钙化土壤中, 土壤有效磷含量很低, 而且施用的磷肥很快会被土壤固定, 难以被植物吸收利用(
Mycorrhizal phosphate uptake pathway in tomato is phosphorus-repressible and transcriptionally regulated.
2
2009
... 在热带和亚热带酸性土壤及温带钙化土壤中, 土壤有效磷含量很低, 而且施用的磷肥很快会被土壤固定, 难以被植物吸收利用(
... 本试验中, 低磷条件下玉米菌根侵染率显著高于高磷处理, 丛枝丰度也较高, 这与以往的研究结果一致(
Nod factors and a diffusible factor from arbuscular mycorrhizal fungi stimulate lateral root formation in Medicago truncatula via the DMI1/DMI2 signalling pathway.
1
2005
... Carlos等(2008)利用基因芯片研究了磷饥饿对植物基因表达谱的影响, 表明碳代谢、氮代谢、脂代谢和磷代谢基因均有不同响应.本试验中, 低磷环境下AM不仅显著调节了供体植物碳磷代谢相关基因G3PT、PEPC、TC289和MAS1的表达, 还上调了受体植物中上述基因的表达.G3PT参与糖-磷酸盐/阴离子的逆向转运过程, 低磷胁迫下拟南芥(Arabidopsis thaliala)中G3PT家族的5个基因均呈现不同程度的上调, 进而调节根的生长或磷酸盐的平衡(
Effect of P availability on temporal dynamics of carbon allocation and Glomus intraradices high-affinity P transporter gene induction in arbuscular mycorrhiza.
1
2006
... 本试验中, 低磷条件下玉米菌根侵染率显著高于高磷处理, 丛枝丰度也较高, 这与以往的研究结果一致(
A new mathematical model for relative quantification in real-time RT-PCR.
1
2001
... 取0.1 g玉米根部样品, 采用TRIZOL试剂(Invitrogen, Grand Island, New York, USA)提取玉米根系总RNA, 总RNA提取完成后经DNaseI消化, 用于合成cDNA.合成体系为20 μL, 所用试剂盒为Thermo反转录试剂盒(Thermo Scientific, 上海).采用荧光定量PCR测定玉米碳磷代谢相关基因Pht1;2、Pht1;6、PEPC、G3PT、TC289、MAS1和AM真菌碳磷代谢相关基因GiPT、NGT1、HXK1b、AGM1、GlcNAc、UAP1、CHS1、DAC1、NAG1的表达量.定量PCR体系为25 μL, 其中包含12.5 μL SYBR? Premix ExTaqTM (TAKARA Biotechnology, 中国大连), 1.5 μL稀释5倍的cDNA模板和0.2 μmol·L-1特异性引物(附录I、附录II).PCR程序为: (1) 95 ℃ 10 s; (2) 95 ℃ 15 s, 60 ℃ 60 s, 40个循环.60 ℃收集荧光数据.溶解曲线分析程序为: 70 ℃ 10 s, 然后以0.2 ℃·s-1的升温速率加热到100 ℃, 连续收集数据.玉米以Actin基因作为内参基因, AM真菌以EF1β作为内参基因, 每个样品做3个技术平行.在对照试验中, 分别在反应体系中加入每个RNA样品和水, 以取代cDNA模板, 以此排除基因组DNA污染和引物二聚体的形成.数据分析用2-ΔΔCt方法(
Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection.
1970
Growth analysis of maize field crops under phosphorus deficiency.
1
2000
... 在热带和亚热带酸性土壤及温带钙化土壤中, 土壤有效磷含量很低, 而且施用的磷肥很快会被土壤固定, 难以被植物吸收利用(
Ectopic expression of phosphoenolpyruvate carboxylase in Vicia narbonensis seeds: Effects of improved nutrient status on seed maturation and transcriptional regulatory networks.
1
2007
... Carlos等(2008)利用基因芯片研究了磷饥饿对植物基因表达谱的影响, 表明碳代谢、氮代谢、脂代谢和磷代谢基因均有不同响应.本试验中, 低磷环境下AM不仅显著调节了供体植物碳磷代谢相关基因G3PT、PEPC、TC289和MAS1的表达, 还上调了受体植物中上述基因的表达.G3PT参与糖-磷酸盐/阴离子的逆向转运过程, 低磷胁迫下拟南芥(Arabidopsis thaliala)中G3PT家族的5个基因均呈现不同程度的上调, 进而调节根的生长或磷酸盐的平衡(
Characterization of the phosphate starvation- induced glycerol-3-phosphate permease gene family in
1
2011
... Carlos等(2008)利用基因芯片研究了磷饥饿对植物基因表达谱的影响, 表明碳代谢、氮代谢、脂代谢和磷代谢基因均有不同响应.本试验中, 低磷环境下AM不仅显著调节了供体植物碳磷代谢相关基因G3PT、PEPC、TC289和MAS1的表达, 还上调了受体植物中上述基因的表达.G3PT参与糖-磷酸盐/阴离子的逆向转运过程, 低磷胁迫下拟南芥(Arabidopsis thaliala)中G3PT家族的5个基因均呈现不同程度的上调, 进而调节根的生长或磷酸盐的平衡(
The role of the cell wall compartment in mutualistic symbioses of plants.
1
2014
... 宿主植物和AM真菌之间的碳磷交换是稳定菌根共生体系的基础(
Identification of cytosolic Mg2+-dependent soluble inorganic pyrophosphatases in potato and phylogenetic analysis.
1
1999
... Carlos等(2008)利用基因芯片研究了磷饥饿对植物基因表达谱的影响, 表明碳代谢、氮代谢、脂代谢和磷代谢基因均有不同响应.本试验中, 低磷环境下AM不仅显著调节了供体植物碳磷代谢相关基因G3PT、PEPC、TC289和MAS1的表达, 还上调了受体植物中上述基因的表达.G3PT参与糖-磷酸盐/阴离子的逆向转运过程, 低磷胁迫下拟南芥(Arabidopsis thaliala)中G3PT家族的5个基因均呈现不同程度的上调, 进而调节根的生长或磷酸盐的平衡(
Seed-specific expression of a bacterial phosphoenolpyruvate carboxylase in Vicia narbonensis increases protein content and improves carbon economy.
1
2004
... Carlos等(2008)利用基因芯片研究了磷饥饿对植物基因表达谱的影响, 表明碳代谢、氮代谢、脂代谢和磷代谢基因均有不同响应.本试验中, 低磷环境下AM不仅显著调节了供体植物碳磷代谢相关基因G3PT、PEPC、TC289和MAS1的表达, 还上调了受体植物中上述基因的表达.G3PT参与糖-磷酸盐/阴离子的逆向转运过程, 低磷胁迫下拟南芥(Arabidopsis thaliala)中G3PT家族的5个基因均呈现不同程度的上调, 进而调节根的生长或磷酸盐的平衡(
The B73 maize genome: Complexity, diversity, and dynamics.
1
2009
... 供试植物为已完成全基因组测序的模式作物材料玉米自交系‘B73’ (
Arbuscular mycorrhizas.
1
2008
... 磷作为一种植物必需的营养元素, 在植物生长发育和代谢过程中均起着重要作用(
Plant distribution and soil-plant interactions on shallow soils.
1
1999
... 磷作为一种植物必需的营养元素, 在植物生长发育和代谢过程中均起着重要作用(
Socialism in soil? The importance of mycorrhizal fungal networks for facilitation in natural ecosystems.
1
2009
... AM不仅能够直接参与和调节宿主植物碳磷代谢过程, 还可能通过菌根根际效应(mycorrhizosphere effects)调节相邻非菌根植物的生理代谢和生长发育(
Mycorrhizal sink strength influences whole plant carbon balance of Trifolium repens L.
1
1998
... 在热带和亚热带酸性土壤及温带钙化土壤中, 土壤有效磷含量很低, 而且施用的磷肥很快会被土壤固定, 难以被植物吸收利用(
Up-regulation of genes involved in N-acetylglucosamine uptake and metabolism suggests a recycling mode of chitin in intraradical mycelium of arbuscular mycorrhizal fungi.
2015

