HTML
--> --> -->In recent years, air pollution events have occurred more frequently in China as a result of rapid economic development. This phenomenon has attracted both public and research attention. Although particle pollution has been of the greatest concern, the control of ozone pollution is also urgent. Field studies in northern China have shown that summertime ozone concentrations increased by 2 ppbv yr–1, from 2003 to 2015, despite reduced NOx emissions (Sun et al., 2016). Extremely high ozone concentrations (> 200 ppbv) have also been frequently reported (Zhang et al., 2007, 2008; Xue et al., 2016), and regional ozone pollution has even occurred in the non-economically developed areas of western China (Tan et al., 2018). The Yangtze River Delta (YRD) is one of the three most developed regions in China and faces serious ozone pollution as a result of the rapid urbanization and industrialization that has taken place in recent years (Huang et al., 2011; Li et al., 2017; She et al., 2017). Due to the facts that VOCs are the key precursor of ozone formation and that previous studies have pointed out that ozone formation in the YRD is largely dependent on VOCs (Geng et al., 2009; Jin and Holloway, 2015), a full understanding of tropospheric VOCs in this area could assist in the development of more rational ozone control strategies.
Most field measurements of VOCs have focused upon the concentrations and composition of ground-level VOCs (Yuan et al., 2013; Cheng et al., 2016; An et al., 2017; Liu et al., 2018; Sheng et al., 2018; Wang et al., 2018). To truly understand the formation of ozone and secondary organic aerosol in the planetary boundary layer, especially the lowest few hundred meters, research on vertical profiles of VOCs becomes necessary. The study of vertical profiles of VOCs, in the troposphere, can help us better understand their concentration, distribution, and chemical changes which occur at different heights, thus deepening the understanding of their effects on ozone and secondary organic aerosol formation in the vertical direction. The most used techniques for studying vertical profiles are aircraft (Borbon et al., 2013), tethered balloons (Glaser et al., 2003; Sangiorgi et al., 2011; Tsai et al., 2012; Zhang et al., 2018), and towers (Lin et al., 2011; Mao et al., 2008; Zhang et al., 2020). Tethered balloons can sample up to a height of 1000 m (Sun et al., 2018; Wu et al., 2020), but require large space and only operate for short-flight distances. Tower-based sampling techniques are limited by the height and location of the tower. The maximum height of air samples successfully gathered by this technique was about 280 m (Mao et al., 2008). Aircraft techniques could overcome the limit of sampling heights and locations, and could be used to collect samples up to 12 km in height, but the interference from fuel-powered engines and the danger of low-altitude flight (lower than 500 m) limit the application and practicality of this technique in the context of near-surface, atmospheric research. Unmanned aerial vehicles (UAVs) have become a new option for vertical profile studies in recent years. Compared to the aforementioned techniques, UAVs are able to hover and require a small space for landing (Klemas, 2015). For the study of the vertical profiles of VOCs, UAVs can be equipped with stainless steel canisters to perform VOCs collection in ambient air (Vo et al., 2018), without the need for a tower, and can also flexibly hover at any height to obtain measurements.
In this work, vertical profiles of VOCs were explored by using a UAV equipped with stainless steel canisters on 8 and 9 September 2016 in the Shanghai’s Jinshan district, eastern China. This is the first attempt to use a UAV to collect air samples in the vertical direction over the Yangtze River Delta. We expect to achieve the following objectives: (1) describe the vertical distribution of VOCs and their evolutionary characteristics; (2) explore the consumed VOCs through transportation; (3) analyze the key species of VOCs in ozone and secondary organic aerosol formation during transportation, and; (4) discuss the source of VOCs in this area.
2.1. Sampling
The sampling site is located in Langxia (LX), which is a suburban site in Shanghai’s Jinshan district (30°79′N, 121°19′E, Fig. 1). There are two major industrial plants (SIZ—Shanghai Jinshan Second Industrial Zone and JPZ—Jinshan Petrochemical Zone) located 10 km to the southeast of the sampling site, which are the two major sources of VOC emission in Jinshan district. The SIZ contains a large number of industries, such as electronic information engineering, machinery manufacturing, biomedicine, iron and steel plants, synthetic/new materials, cement, new energy, and research and development technology (Qiu et al., 2019). The JPZ produces aviation kerosene, gasoline diesel, aromatic olefines, and emits VOCs via oil refineries, leakage of storage tanks, and water treatment processes (Kalabokas et al., 2001; Qiu et al., 2019). Five air sampling flights were conducted over this sampling site at 1000?1200 LST 8 and 9 September 2016, and 1400?1600 LST on 8 September 2016, and 0900?1000, 1300?1500, 1600?1800 LST on 9 September 2016. Five targeting heights of 50 m, 100 m, 200 m, 300 m and 400 m were obtained in all five sampling flights. All samples were transferred to the State Environmental Protection Key Laboratory of Urban Air Pollution Complex at Shanghai Academy of Environmental Sciences for the analysis of VOCs. An on-line monitoring station for VOCs was set up at Xinlian (XL), which is located on the northwest border of the SIZ.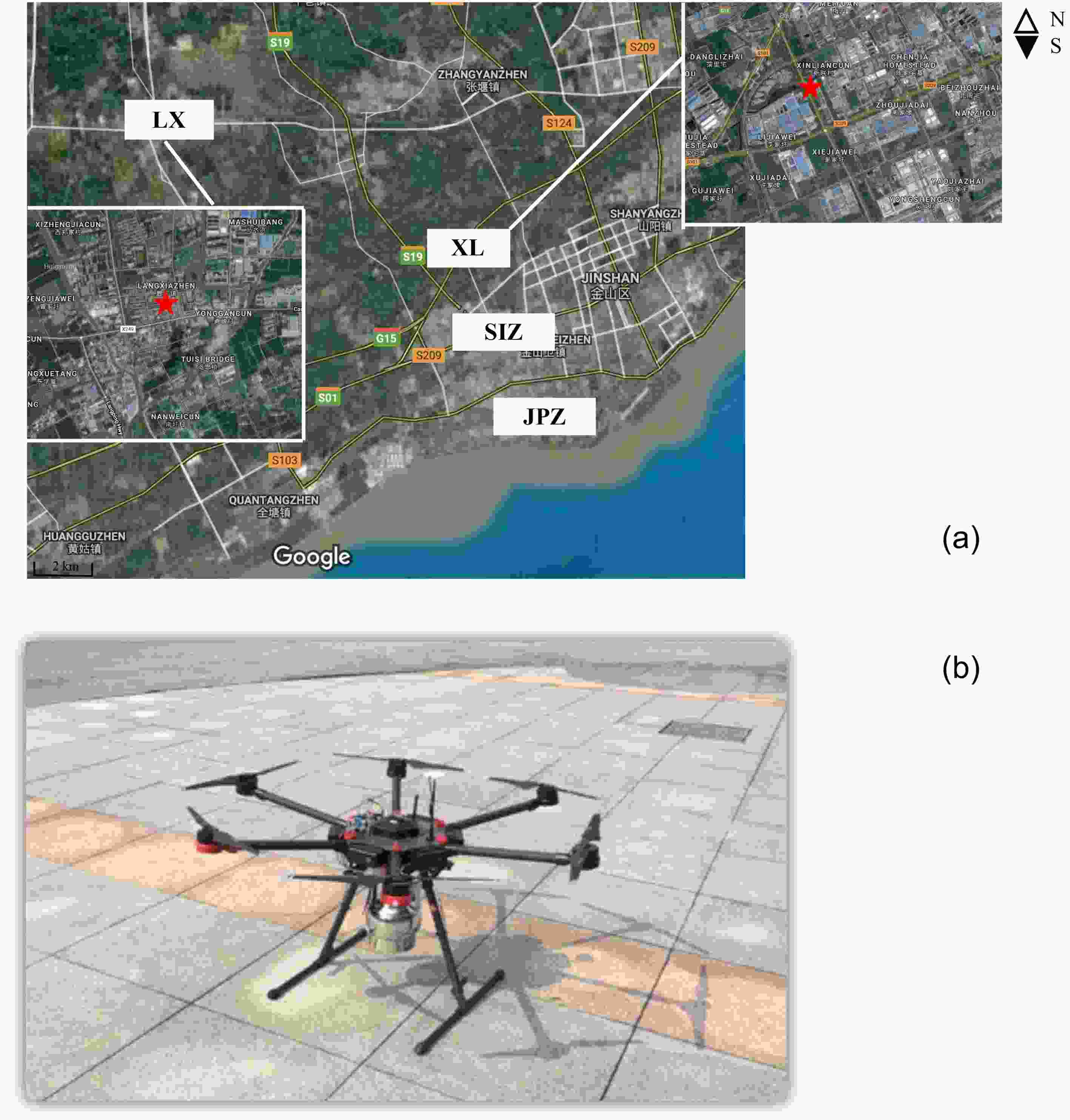 Figure1. (a) Locations of observation sites of Langxia (LX) and Xinlian (XL), and (b) unmanned aerial vehicle (UAV) used for volatile organic compounds (VOCs) sampling.
Figure1. (a) Locations of observation sites of Langxia (LX) and Xinlian (XL), and (b) unmanned aerial vehicle (UAV) used for volatile organic compounds (VOCs) sampling.The collection of samples for analysis of VOCs was carried out by a UAV (M600, DJI, China, Fig. 1), which included a remote-controlled 3.2 L canister that was attached to the lower part of the UAV. The UAV had eight wings and was equipped with a power motor (DJI 6010) and six 4500 mAh LiPo 6s batteries (capable of sustaining flight and hovering for 30 minutes). The maximum loaded weight was 15 kg. To avoid interference with VOCs, the canister inlet was 20 cm above the UAV border. The UAV, equipped with the canister, travelled vertically at 2 m s–1, and hovered for one minute while sampling at each targeting height during each flight. Vertical profiles of temperature and relative humidity were obtained from a L-band sounding system which was acquired from the China Meteorological Administration (
2
2.2. VOCs analysis.
The concentrations and compositions of 52 VOCs [Table S1 in the electronic supplementary material (ESM)] captured in the sampling canisters were analyzed using a gas chromatography-mass spectrometry instrument (GC-MS, Agilent, GC model 7820A, MSD model 5977E). Briefly, a 500 mL full-air sample was taken from the sampling canister and concentrated by a three-stage, cryo-focusing, pre-concentration system (Entech Instruments, Inc., USA). The vaporized VOCs were then injected into the gas chromatography system by a helium carrier gas for analysis. A PLOT (Al2O3 KCl–1) column (15 m, 0.32 mm ID) connected to a flame ionization detector was used to quantify C2-C5 hydrocarbons, and a DB-624 column (30 m, 0.25 mm ID) connected to a mass spectrometer was used to analyze other VOCs species. The carrier for both columns was ultra-pure helium (> 99.999 %). Detailed information is described in Liu et al. (2008) and Wang et al. (2018).2
2.3. Quality assurance and quality control
The summa canisters (3.2 L each) were cleaned and evacuated (< 10 Pa) prior to sampling. One clean summa canister was selected for analysis of high-purity nitrogen to verify that the canisters were clean. Each canister was connected to a Teflon filter to remove particulate matter and moisture during sampling. The pressure of the canister was re-checked after each flight to ensure it was at 0 Torr. A commercial standard gas containing PAMs (29 alkanes, 11 alkenes, 16 aromatics) was used to identify compounds and confirm their retention times. The response of the instrument for the analysis of VOCs was calibrated after every eight samples using a 1 ppbv PAMs standard gas.2
2.4. Principal component analysis
Principal component analysis (PCA) has often been used to identify and quantify the major sources of VOCs (Bruno et al., 2001; Duan et al., 2008; Jia et al., 2016). In this study, PCA was used to determine the major source of VOCs in the study area. Statistical analysis was carried out using IBM SPSS Statistics 19 software. PCA was applied to the source profiles and source strengths in absolute VOCs concentrations measured at different heights. Twenty-four VOCs were used for the PCA calculation as they accounted for 65% of the total VOCs at LX. The varimax rotation of the matrix was used in this study to minimize the number of VOCs with high loadings on a factor. Only principal components whose eigenvalues were >1 were retained as a factor. More details regarding the method of PCA can be found in previous studies (Guo et al., 2004, 2007).2
2.5. Ozone formation potential
VOCs react with OH radicals, which efficiently convert NO to NO2 and result in the accumulation of ozone. However, individual VOCs can differ significantly in their effects on ozone formation. The ozone formation potential (OFP) is calculated following Eq. (1), which is used to represent the ozone formation probability of individual VOCs.The index i represents the concentration of a particular species of the VOCs (ppbv) and MIR represents the maximum incremental reactivity of that specie. (Carter et al., 1995).
3.1. VOCs vertical profile
Figure 2 presents the results of the average concentrations and compositions of VOCs at different heights in LX. The vertical profiles obtained in this study showed that concentrations increased at elevated heights, and reached a maximum at 100 m (36.1 ppbv) before decreasing continuously. Concentrations of the 52 VOCs measured in this study, at different heights, are listed in Table S1 in the ESM. Concentrations of VOCs (VOCs refers to the 52 species measured in this study) varied less below 200 m, and decreased by 21.2% from 100 m to 400 m. The concentrations of VOCs above 200 m were much lower compared to those below 200 m. Alkanes decreased by 17.3% from 100 m to 400 m. Alkenes and aromatics decreased by 43.0% and 20.5%, respectively, from 200 m to 400 m. Furthermore, we analyzed the average proportion of alkanes, alkenes, and aromatics in VOCs at different heights on 8 and 9 September 2016. The proportion of hydrocarbons did not appear to vary significantly between 50 m and 400 m. The major components of VOCs were alkanes (> 50 %) below 400 m, followed by aromatics (> 30 %). Figure2. (a) Overview of the vertical profile of 52 VOCs measured at Langxia (LX); (b) 24 hours backward trajectory in 3 heights (50 m, 100 m, 200 m) of Langxia (LX) on 9 September 2016 with 1° resolution GDAS data.
Figure2. (a) Overview of the vertical profile of 52 VOCs measured at Langxia (LX); (b) 24 hours backward trajectory in 3 heights (50 m, 100 m, 200 m) of Langxia (LX) on 9 September 2016 with 1° resolution GDAS data.By combining the conditions of the sampling site and surrounding areas, we inferred that air masses obtained at the sampling site were transported in from elsewhere. By applying a backward trajectory analysis [Hysplit4 (
By combining the vertical profiles of meteorological variables, the vertical profiles of VOCs concentrations showed two patterns. Figure 3 shows the results from 1000 to 1200 LST on 8 September and the results from 0900 to 1100 LST on 9 September. The concentrations of VOCs increased from the surface (50 m), reaching a maximum value at 200 m (40.4 ppbv) 8 on September and reaching a maximum value at 100 m (60.9 ppbv) on 9 September. Alkanes accounted for more than 50% of the total VOCs at each height. Analysis of the vertical profiles of temperature and relative humidity on 8 September, show that there was an inversion layer at 200 m, which traps the air masses, restricting their vertical movement, resulting in the accumulation of VOCs at 200 m. A similar change also appeared in the vertical profile on 9 September from 0900 to 1100 LST. Figure 4 shows the results from 1400 to 1600 LST on 8 September and the results from both 1300 to 1500 LST and from 1600 to 1800 LST on 9 September. These profiles were taken while the boundary layer was in a strong convective state. The concentration of VOCs decreased slowly with height, with the highest concentration at 50 m (41.8 ppbv on 8 September, 31.4 ppbv and 30.1 ppbv on 9 September) and the lowest concentration at 400 m (30.7 ppbv on 8 September and 19.9 ppbv on 9 September). In the profile from 1300 to 1500 LST on September 9, the minimum value of VOCs concentrations was located at 300 m (17.9 ppbv), and then increased slowly. It may be caused by long-distance transportation from the SIZ. Alkanes were the dominant component of VOCs at each height.
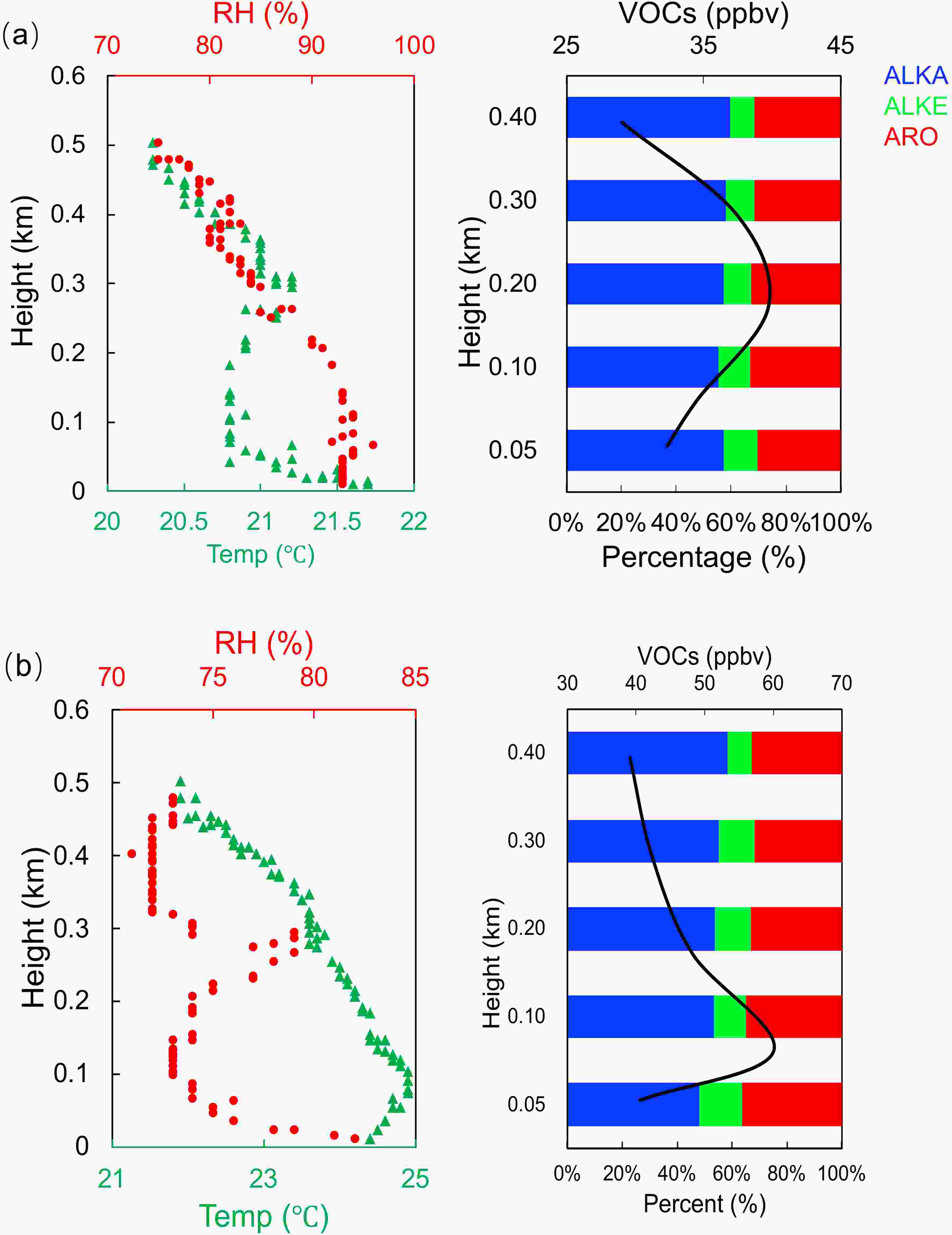 Figure3. Vertical profiles of the VOCs concentration and meteorological variables (temperature and relative humidity). (a) Vertical profile of VOCs concentrations during 1000?1200 LST on 8 September 2016, vertical profile of temperature and relative humidity at 0700 LST on 8 September; (b) Vertical profile of VOCs concentrations during 0900?1100 LST on 9 September 2016, vertical profile of temperature and relative humidity at 0700 LST on 9 September.
Figure3. Vertical profiles of the VOCs concentration and meteorological variables (temperature and relative humidity). (a) Vertical profile of VOCs concentrations during 1000?1200 LST on 8 September 2016, vertical profile of temperature and relative humidity at 0700 LST on 8 September; (b) Vertical profile of VOCs concentrations during 0900?1100 LST on 9 September 2016, vertical profile of temperature and relative humidity at 0700 LST on 9 September.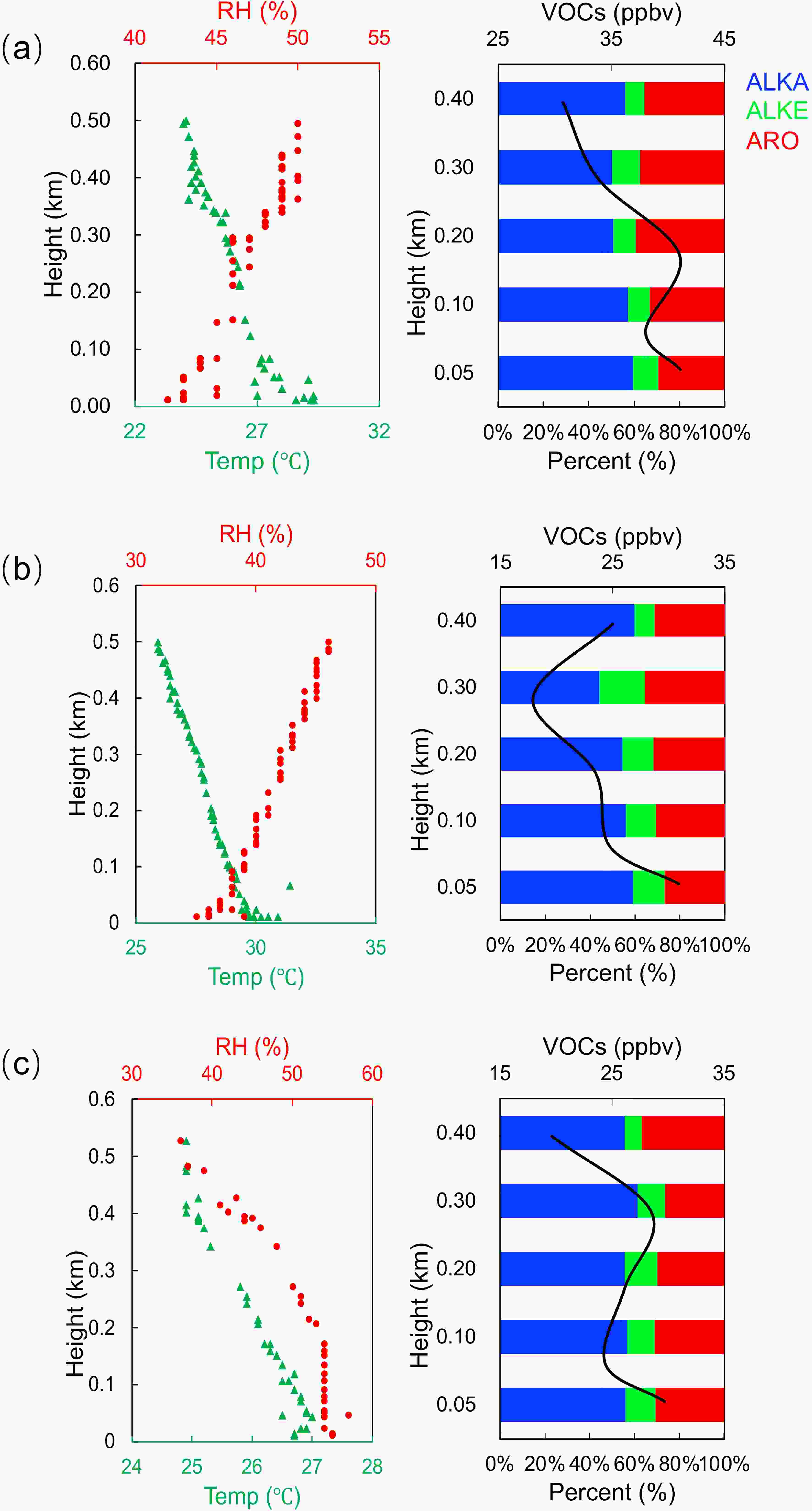 Figure4. Vertical profiles of the VOCs concentration and meteorological variables (temperature and relative humidity). (a) Vertical profile of VOCs concentrations during 1400?1600 LST on 8 September 2016, vertical profile of temperature and relative humidity at 1300 LST on 8 September; (b) Vertical profile of VOCs concentrations during 1300?1500 LST on 9 September 2016, vertical profile of temperature and relative humidity at 1300 LST on 9 September; (c) Vertical profile of VOCs concentrations during 1600?1800 LST on 9 September, vertical profile of temperature and relative humidity at 1900 LST on 9 September.
Figure4. Vertical profiles of the VOCs concentration and meteorological variables (temperature and relative humidity). (a) Vertical profile of VOCs concentrations during 1400?1600 LST on 8 September 2016, vertical profile of temperature and relative humidity at 1300 LST on 8 September; (b) Vertical profile of VOCs concentrations during 1300?1500 LST on 9 September 2016, vertical profile of temperature and relative humidity at 1300 LST on 9 September; (c) Vertical profile of VOCs concentrations during 1600?1800 LST on 9 September, vertical profile of temperature and relative humidity at 1900 LST on 9 September.2
3.2. Contribution of VOCs oxidants to SOA formation and ozone formation
The consumption of VOCs during transportation from XL to LX may have generated ozone and promoted the formation of SOA. In this section, we discuss the effects of the VOCs consumed during transportation by atmospheric oxidation. The loss of VOCs in the atmosphere depends on the photochemical age, which is defined as the time integrated exposure to OH radicals. A simple dilution chemistry model can be used to describe the changes of VOCs concentrations over the time. Twelve VOCs species with different OH reactivities were selected to calculate the OH exposure during the transportation process from XL to LX. Their concentrations at LX are given by Eq. (2):where




Species i and j were selected from 12 VOCs species that covered a wide range of OH reactivities from











Here, C0 is the initial concentration of the VOC in question (ppbv) that was measured at the XL site, D is a dilution factor, kOH,i is the reaction rate constant of the particular VOC with OH radicals, and

Here







| Height (m) | 8 Sep. | 9 Sep. | |||||||
| SOAFP (μg cm?3) | Top 3 species and its proportion of SOAFP | OFP (ppb) | Top 3 species and its proportion of OFP | SOAFP (μg cm?3) | Top 3 species and its proportion of SOAFP | OFP (ppb) | Top 3 species and its proportion of OFP | ||
| 50 | 21.2 | toluene ($ {\rm{52}}{\rm{.4\% \pm 0}}{\rm{.5\% }}$) m/p-xylene ($ 15.3\% \pm 0.4\% $) o-xylene ($ 11.2\% \pm 0.2\% $) | 469 | propene ($ 26.1\% \pm 0.4\% $) ethene ($ 20.6\% \pm 0.2\% $) m/p-xylene ($ 20.4\% \pm 0.2\% $) | 9.1 | toluene ($ 72.1\% \pm 0.1\% $) cyclohexane ($ 8.8\% \pm 0.0\% $) o-xylene ($ 5.2\% \pm 0.0\% $) | 112 | ethene ($ 21.3\% \pm 1.3\% $) Propene ($ 17.4\% \pm 1.0\% $) Toluene ($ 16.5\% \pm 1.0\% $) | |
| 100 | 22.5 | 471 | 9.1 | 112 | |||||
| 200 | 19.7 | 445 | 9.4 | 114 | |||||
| 300 | 21.0 | 466 | 9.2 | 113 | |||||
| 400 | 21.9 | 478 | 9.5 | 100 | |||||
Table1. Values of secondary organic aerosol formation potential (SOAFP) and ozone formation potential (OFP) of total VOCs during transportation at Langxia (LX) from heights of 50 m to 400 m and the top three volatile organic compounds (VOCs) species with the highest proportion of SOAFP and OFP on 8 and 9 September 2016
We also analyzed the impact of consumed VOCs to ozone formation during transportation. The analysis (Table 1) showed that the major VOCs species in the OFP calculation were propene (







2
3.3. Sources apportionment of VOCs using PCA
Twenty-four compounds out of the 52 VOCs measured at the LX site were selected for the PCA as they were the most abundant compounds. Kaiser’s criteria were adopted to determine the appropriate number of factors to be retained (only factors with eigenvalues > 1).Six factors were extracted from the application of the PCA to the vertical data (Table 2). The first factor explained 64.06 % of the total variance. High loadings were found on propene, 1-butene, hexane, benzene, and cyclohexane. C3?C4 alkenes were associated with combustion. Hexane and cyclohexane mainly came from an oil refinery or chemical plant that is related to the petrochemical industry. Benzene may have come from painting, combustion, or a chemical plant. When combined with other high loadings in factor 1, we assumed that benzene came from a chemical plant. Factor 1 featured the source of the VOCs from the petrochemical industry. The second factor explained 9.97 % of the total variance, whereby i-butane, butane, and propane showed the highest factor loading. Given that i-butane, butane, and propane are the characteristic products of LPG (liquefied petroleum gas) (Liu et al., 2008), we related the second factor to LPG. The third factor explained 6.26% of the total variance, and was highly loaded with m/p-xylene, o-xylene, and 2-methylhexane. The former two could have come from painting or vehicle emission, however, as 2-methylhexane is a tracer of gasoline evaporation (Liu et al., 2008), we determined that factor 3 was probably related to vehicle emissions. The fourth factor explained 4.74% of the total variance, and was highly loaded on 1,3,5-trimethylbenzene and 1,2,4-trimethylbenzene, which are directly emitted to the atmosphere from motor vehicle exhaust and the evaporation of solvents (Luo et al., 2019). The fifth factor explained 4.13% of the total variance, and was highly loaded on styrene, which we related to being sourced from the chemical plant. The sixth factor explained 3.56 % of the total variance, and was highly loaded on dodecane. As dodecane is mainly directly emitted from diesel vehicles, we determined that factor 6 was related to diesel vehicles emission.
| VOCs species | 1 | 2 | 3 | 4 | 5 | 6 |
| ethane | 0.64 | 0.17 | ?0.07 | ?0.34 | 0.07 | 0.04 |
| propene | 0.93 | 0.20 | 0.22 | 0.02 | 0.02 | 0.00 |
| propane | 0.32 | 0.82 | 0.34 | 0.13 | 0.02 | 0.04 |
| i-butane | 0.34 | 0.86 | 0.24 | 0.09 | ?0.03 | 0.07 |
| 1-butene | 0.88 | 0.30 | 0.29 | 0.13 | 0.08 | ?0.02 |
| butane | 0.52 | 0.78 | 0.26 | 0.16 | 0.08 | 0.07 |
| i-pentane | 0.36 | 0.65 | 0.13 | 0.32 | 0.34 | ?0.01 |
| n-pentane | 0.67 | 0.58 | 0.31 | 0.14 | 0.18 | 0.09 |
| cyclopentane | 0.59 | 0.59 | 0.27 | 0.20 | 0.31 | ?0.06 |
| 2-methylpentane | 0.77 | 0.44 | 0.23 | 0.14 | 0.25 | ?0.08 |
| 3-methylpentane | 0.67 | 0.64 | 0.26 | 0.12 | 0.16 | ?0.03 |
| hexane | 0.83 | 0.38 | 0.29 | 0.10 | 0.09 | ?0.01 |
| methylcyclopentane | 0.71 | 0.43 | 0.48 | 0.14 | 0.13 | ?0.02 |
| Benzene | 0.90 | 0.29 | 0.25 | 0.04 | 0.02 | ?0.02 |
| cyclohexane | 0.83 | 0.25 | 0.46 | 0.05 | 0.00 | 0.01 |
| 2-methyl-hexane | 0.10 | 0.42 | 0.73 | 0.12 | 0.36 | 0.13 |
| toluene | 0.37 | 0.72 | 0.52 | 0.09 | 0.12 | 0.11 |
| ethylbenzene | 0.61 | 0.42 | 0.65 | 0.08 | 0.00 | 0.02 |
| m/p-xylene | 0.45 | 0.24 | 0.84 | 0.06 | 0.02 | 0.02 |
| styrene | 0.12 | 0.11 | 0.10 | 0.11 | 0.94 | 0.03 |
| o-xylene | 0.37 | 0.29 | 0.85 | 0.08 | 0.03 | 0.05 |
| dodecane | ?0.04 | 0.08 | 0.09 | 0.01 | 0.03 | 0.99 |
| 1,3,5-trimethylbenzene | 0.03 | 0.18 | 0.03 | 0.96 | 0.07 | 0.01 |
| 1,2,4-trimethylbenzene | 0.06 | 0.17 | 0.12 | 0.95 | 0.09 | 0.01 |
Table2. Rotated component matrix of 24 volatile organic compounds (VOCs) from 50 m to 400 m
Overall these six factors explained 92.71% of the total variance and could be classified according to the following source categories: 1) petrochemical industry, 2) LPG, 3) vehicle emission. As factor 4 was related to motor vehicle emission and factor 6 was related to diesel vehicle emission, we combined these with factor 3 as vehicle emission sources. As factor 5 was highly related to a chemical plant, this was combined with factor 1 as a petrochemical industry source.
In this study, we did a preliminary study on the distribution and impact of VOCs upon atmospheric oxidation below 400 m. However, the distribution of VOCs and their effect on oxidative properties of the troposphere are still worthy of further research.
Acknowledgements. This work was supported by the National Natural Science Foundation of China (Grant Nos. 41830106, 21607104), the National Key Research and Development Plan (Grant Nos. 2017YFC0210004, 2018YFC0213801), the Shanghai Science and Technology Commission of Shanghai Municipality (18QA 403600), and the Shanghai Environmental Protection Bureau (2017-2). The author gratefully thanks the science team of Peking University, as well as the team from Jinshan Environmental Monitor Station for their general support. The authors gratefully acknowledge the NOAA Air Resources Laboratory (ARL) for the provision of the HYSPLIT transport and dispersion model and/or READY website (
Electronic supplementary material: Supplementary material is available in the online version of this article at
