HTML
--> --> -->Ambient VOC concentrations have been noted to vary with the emission intensities of dominant sources and with meteorological conditions; meteorological conditions, in particular, affect transport and chemical transformation (Filella and Pe?uelas, 2006a; Carter, 2010). Studies have reported that urban VOCs are derived from multiple sources—including vehicle exhaust, petrochemical industry emissions, fossil fuel volatilization, the use of chemical solvents, and solid fuel combustion (An et al., 2017; Tan et al., 2018; Sun et al., 2019a) However, VOC compositions and sources have varied significantly between seasons and locations. Specifically, a study observed that aromatic hydrocarbons in northern China originated mainly from the burning of biomass, biofuel, and coal, whereas those in southern China originated mainly from traffic-related emissions (Zhang et al., 2016). An et al. (2014) reported that industry was the primary contributor to summer VOCs in Nanjing, China, whereas the traffic source was the primary contributor in winter.
Such findings on variations in source contributions may also have been due to the different models used. Due to high uncertainty in source emission data and the lack of a localized VOC source inventory, source inventory-based methods are less popular than the receptor model is in existing source apportionment studies. For instance, positive matrix factorization (PMF) (Abeleira et al., 2017; Assan et al., 2018; Bari and Kindzierski, 2018; Chen et al., 2019) and chemical mass balance (CMB) (Watson et al., 2001; Srivastava, 2004; Feng et al., 2019) have both been widely used in VOC source apportionment. However, PMF requires large datasets, whereas CMB requires accurate localized VOC source profiles (Hopke, 2016). In cases with limited data samples or insufficiently localized source profile information, these two approaches cannot yield reliable source contributions, and a more advanced model is required. The Hybrid Environmental Receptor Model (HERM) is one such model, which was developed to take advantage of both CMB and PMF by allowing the use of incomplete source profiles as the model input (Chen and Cao, 2018).
Xi’an, a megacity in China with a population of over 10 million people, has frequently had PM2.5 and O3 pollution episodes over the past decade (Shen et al., 2008, 2009; Huang et al., 2014; Zhang et al., 2015). Xi’an’s surrounding basin-like terrain has compounded such pollution to make Xi’an the Chinese city with the worst air quality (Huang et al., 2014). Studies on VOCs in Xi’an have been considerably fewer relative to studies on VOCs in China’s more developed regions—such as the Beijing—Tianjin?Hebei region, Yangtze River Delta, and Pearl River Delta (Duan et al., 2008; Yuan et al., 2010; Wang et al., 2018). Thus, accurate and detailed findings on seasonal VOC concentrations and source apportionment in Xi’an have been lacking, where such findings are essential for pollution control and policymaking. Furthermore, Xi’an’s basin-like terrain minimizes the effect of long-range transport, which makes Xi’an a good candidate for testing the feasibility of HERM (Feng et al., 2016). In this study, nonmethane hydrocarbons (NMHCs) and oxygenated VOC (OVOC) samples, at an hourly temporal resolution, were collected both in summer and winter. This study aimed to (1) characterize the chemical profiles of total VOCs (TVOCs) in summer and winter in Xi’an and (2) conduct the source apportionment of seasonal TVOCs by using HERM.
2.1. NMHC and OVOC sampling
The sampling site was in the southeastern part of downtown Xi’an, and the sampling height was 15 m above the ground (Fig. 1). The site is a typical urban sampling site, having a superstation for atmospheric monitoring. A residential area, the Xi’an Jiaotong University campus, South Second Ring road, and Xingqing road (both of which are main roads) lie to the north, east, south, and west of the sampling site, respectively (Fig. 1). Four hourly (0800?0900, 1500?1600, 1900?2000, and 2300?2400 LST) ambient NMHC (n = 112) and OVOC (n = 112) samples were collected every day during a summer (16?28 July 2018) and winter (8?21 January 2019) campaign. The four hourly sampling periods were selected to represent the morning rush hour, the period when photochemical reactions are at high levels, the evening rush hour, and the period when human activity is at its lowest, respectively (Ho et al., 2004; Mazzuca et al., 2016). Figure1. Location of the sampling site and surrounding road distribution.
Figure1. Location of the sampling site and surrounding road distribution.The ambient NMHC sampling was conducted using a 3-L silonite-treated stainless steel canister with a flow rate of approximately 50 mL min?1, which was controlled by a sampling valve (Entech Instruments, Simi Valley, CA, USA) (Sun et al., 2019c). The OVOC samples were collected into acidified 2, 4-dintrophenylhydrazine (DNPH) cartridges (Waters Sep-Pak DNPH-silica, Milford, MA, USA) at a flow rate of 30 mL min?1. An O3 scrubber (Waters, Milford, MA) and particle filter were installed before the DNPH cartridges to remove O3 and particulate matter. After the sampling, the canisters were stored in a cool and dark place, and the wrapped DNPH-silica cartridges were stored in a 4°C refrigerator. Continual measurements of gaseous pollutants (CO, O3, SO2, and NOx) were also conducted using instruments listed in Table S1 in the Electronic Supplementary Material (ESM).
2
2.2. NMHC and OVOC chemical analysis
The NMHC samples were analyzed using a gas chromatography-mass selective detector/flame ionization detector (Agilent Technologies, Santa Clara, CA, USA). The procedures for analysis and temperature programming were identical to those used in the study conducted by Zhang et al. (2016). This study measured 57 NMHCs specified by the VOC monitoring network of photochemical assessment monitoring stations (PAMS), and the minimum detection limits (MDLs) are presented in Table S2 (Liu et al., 2012; Wei et al., 2014). The MDLs for the 57 NMHC species ranged from 4 to 81 pptv.The OVOC cartridge was first eluted with 2 mL of acetone-free acetonitrile and then analyzed through high-pressure liquid chromatography (Series 1200, Agilent Technologies). The analysis protocol used in this study was identical to that used by Dai et al. (2018). We quantified 20 target OVOCs (including monocarbonyls and dicarbonyls) in the cartridges. The estimated MDLs of the OVOCs ranged from 0.016 to 0.12 ppbv at the sampling volume of 1.8 m3 (Table S3).
2
2.3. Data analysis
32.3.1. Source apportionment model
This study discusses the source apportionment results obtained using HERM. For HERM, 4 fixed profiles with 57 hydrocarbons and 3 OVOCs were selected for data input (Table S6). HERM was constructed based on the multilinear engine (ME-2) solution to CMB problems, which differs from the current version of CMB software (EPA CMB v.8.2). Specifically, HERM analyzes either one or many samples in a single run, where it solves collinearity problems by inheriting non-negativity constraints (Chen and Cao, 2018):Here, Ei,k was the effective variance of pollutant i in source k, i denoted a certain pollutant, j and k denoted two different sources, the δi,j value was set to 0 or 1 when the source profile element Fi,j was specified or unknown/missing in the profiles, respectively, and β is an adjustable factor with a default value of 1; β is included to ensure that the model does not overwrite unspecified profiles due to zero uncertainty information. One crucial difference [Eq. (2)] between the CMB model and HERM is that χ2, χk2, and χi2 are defined in HERM when dealing with missing profiles (Fi,j):
where Ci,k is the measured concentration of a pollutant i in sample k, Fi,j is the source profile, and Si,j is the source contribution, I, J and K were the total number of pollutant i, source j and k, respectively.
When no profile information is input, the formulation of HERM becomes akin to that of PMF (

where


Therefore, HERM can solve profiles that are either not assigned or have some species missing. HERM algorithms are further detailed in Chen and Cao (2018).
3
2.3.2. Conditional function probability analysis
We used the conditional function probability (CPF) [Eq. (5)] to estimate the direction of different sources on the monitoring site, doing so by integrating source apportionment results and wind direction data (Chen et al., 2019):where mΔθ is the number of valid data points within the wind sector Δθ, and nΔθ is the total number of data points in the single wind sector Δθ. The settings for this function used in this study were identical to those used by Kim and Hopke (2004).
3
2.3.3. Formation potential analysis for O3 and SOA
The O3 formation potential (OFP) is often used to estimate the capacity of TVOCs in forming O3 through photochemical reactions. The maximum incremental reactivity (MIR) method was detailed by Carter (2010). The method is characterized by the following identity:where [VOCx] and MIRx are the concentration and MIR of an individual VOC specie x, respectively. A VOC-limited and high NOx condition in Carter’s study was selected for calculation to match conditions in the Guanzhong area of China.
Similarly, the SOA formation potential (SOAP) is typically calculated using the toluene-equivalent method, which is characterized as follows:
where Ix was increment in SOA mass concentration with species x, It was increment in SOA with toluene, SOAP is expressed as an index relative to toluene multiplied by 100. This method has been widely used and verified (Kleindienst et al., 2007; Hu et al., 2008; Derwent et al., 2010). In our study, the capability of toluene in SOA formation was then converted into mass-based values according to our toluene gas-to-particle chamber test results (Johnson et al., 2004). A high NOx condition and low NOx condition were chosen to represent winter and summer conditions in Xi’an, respectively, with the gas-to-particle ratios of 0.06 and 0.20, respectively.
3.1. Diurnal and daily variations of NMHCs and OVOCs
We noted that 54 of the 57 measured PAMS NMHCs and all of the 20 OVOCs had concentrations higher than their individual MDLs. The time series (four data points per day) of the concentrations of TVOCs by category in winter and summer are presented in Fig. 2. The campaign-average TVOC concentration was twice as large in winter (85.3 ± 60.6ppbv) than in summer (47.2 ± 31.6 ppbv). Previous studies have observed similar levels in other locations, which have been mostly attributed to the boundary layer height being lower in winter than in summer (Filella and Pe?uelas, 2006b; Liu et al., 2010; Wu et al., 2016). In addition, winter heating in rural areas around Xi’an resulted in greater coal and biomass burning, which resulted in the greater release of more VOCs into the atmosphere (Sun et al., 2018, 2019c). Similarly, Beijing was reported to have a much higher TVOC level in winter (216.05 ± 115.71 ppbv) than in summer (127.01 ± 39.67 ppbv); both of these levels were significantly higher than those in Xi’an (Gu et al., 2019). In winter, alkanes contributed almost 50% of the TVOCs, followed by OVOC (16.8%), acetylene (15.1%), alkenes (11.3%), and aromatics (8.1%). In summer, OVOC was the dominant contributor (47.9%), followed by alkanes (28.6%), alkenes (11.8%), aromatics (7.3%), and acetylene (4.5%). The higher proportion of OVOC in summer than in winter is attributable to secondary formation resulting from the higher photochemical reactivity under stronger UV radiation (Liu et al., 2019).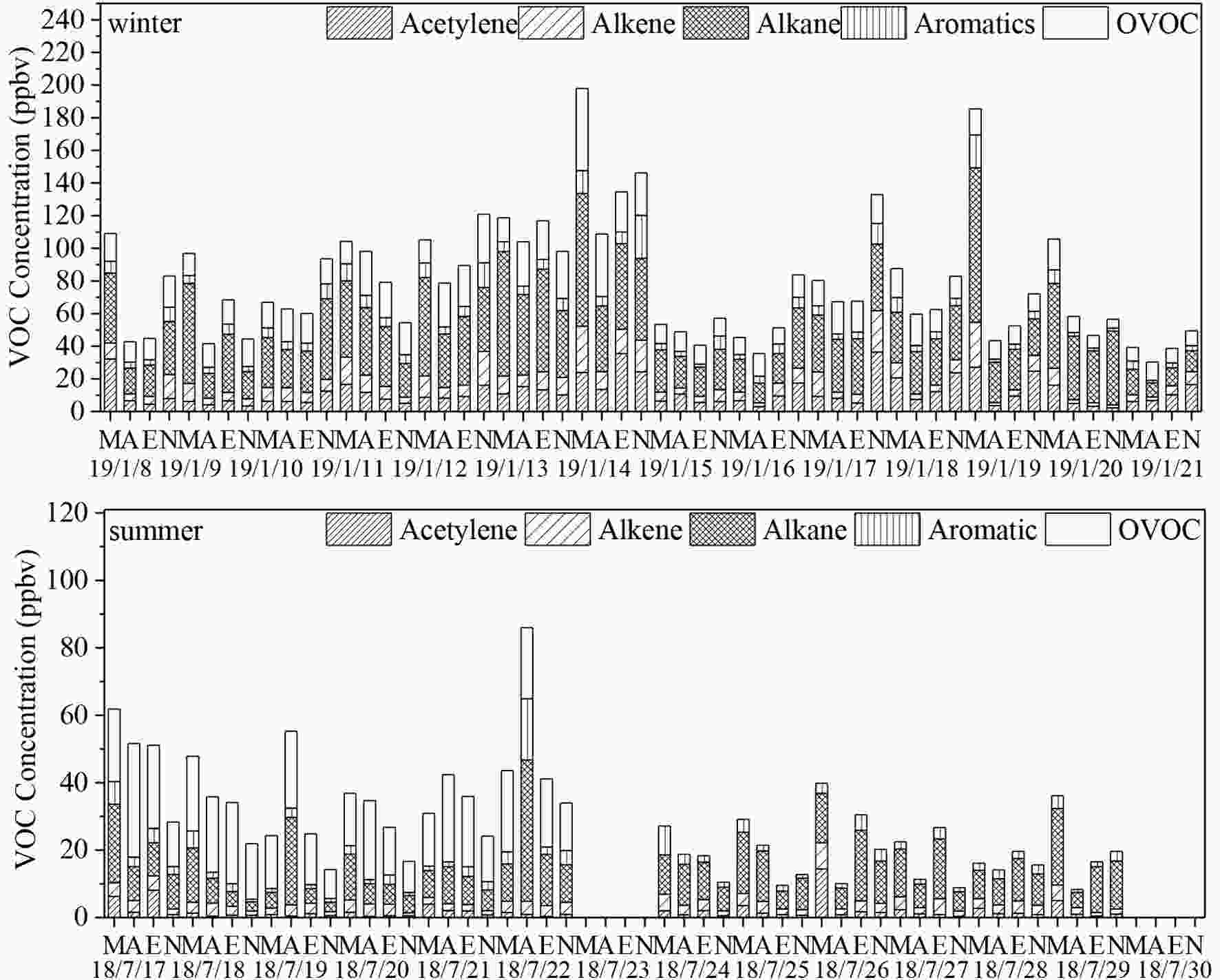 Figure2. Time series of the concentrations of PAMS NMHCs and OVOCs in the winter and summer campaign (M, A, E and N denote morning, afternoon, evening and night, respectively; the date format is as YY/MM/DD)(data absence on 18/7/23 and 18/7/30 were due to instrument failure).
Figure2. Time series of the concentrations of PAMS NMHCs and OVOCs in the winter and summer campaign (M, A, E and N denote morning, afternoon, evening and night, respectively; the date format is as YY/MM/DD)(data absence on 18/7/23 and 18/7/30 were due to instrument failure).Large daily variations of TVOC concentrations (23.4?71.4 and 30.0?112.1 ppbv for summer and winter, respectively) were observed in this study. Table 1 presents a comparison of weekday with weekend data. The average concentrations of PAMS NMHCs were higher on weekends than in weekdays, both during summer and winter (p < 0.05). Alkanes contributed most to the increased PAMS NMHCs on weekends, likely because of higher traffic from weekend activities (e.g., shopping and social gatherings) (Zou et al., 2019). A previous study in Xi’an observed that the vehicle count at South Second Ring road (close to this study’s sampling site) was extremely high on weekends (Li et al., 2017a). These slightly higher NO2 and CO concentrations on weekends than on weekdays also indicated that vehicle count considerably affected PAMS NMHC concentrations (Table S4). Similar trends were also observed for OVOC concentrations, albeit with less significant weekend?weekday differences (p > 0.05) than those of PAMS NMHC. No significant weekend?weekday differences were noted for O3 (p > 0.05). This was because alkanes from vehicle exhausts generally have low photochemical reactivity and will not generate large amounts of OVOC and O3 through photochemical reactions (Hernández-Paniagua et al., 2018). The weekend?weekday difference for average OFP was also statistically nonsignificant (p > 0.05) during both winter and summer. This supported our hypothesis that high TVOC concentrations do not necessarily entail high O3 concentrations (Fig. S1 in the ESM). The weekend?weekday difference for temperature was also statistically nonsignificant (p > 0.05, Table S4). However, the considerably large variance in temperature between the seasons potentially led to large effects on biogenic emissions (i.e., isoprene) and combustion sources (i.e., ethylene), which had the winter-to-summer ratios of 6.5 and 0.02, respectively.
| Season | Category | Weekdays | Weekends | |||
| Avg | SD | Avg | SD | |||
| Summer | OVOCs | 18.9 | 6.2 | 19.9 | 4.2 | |
| NMHCs | 24.7a | 10.9 | 34.3b | 23.9 | ||
| Winter | OVOCs | 17.0 | 7.9 | 17.6 | 8.2 | |
| NMHCs | 56.8c | 29.6 | 73.2d | 33.8 | ||
| Notes: Na, N=8; Nb, N=4; Nc, N=10; Nd, N=4. | ||||||
Table1. Average concentrations (Avg) and standard deviations (SD) of OVOCs and NMHCs over weekdays and weekends (ppbv).
Diurnal variations in ambient TVOCs are controlled by their sources, transport, and chemical reactions (Lyu et al., 2016). The diurnal variations of TVOCs measured in this study are presented in Fig. 3. The TVOC patterns were similar between winter and summer; this pattern was of a single morning peak (M, 0800?0900 LST) before a gradual decline to the lowest value. The increasing planetary boundary layer during the day and the natural degradation of VOCs during the night were largely responsible for this diurnal pattern in summer (Menchaca-Torre et al., 2015; Wu et al., 2020). However, an obvious nighttime rebound (N, 2300?2400 LST) was only observed in winter. Space heating on cold nights potentially explains the slightly different diurnal pattern in winter (Zhang and Smith, 2007; Suryawanshi et al., 2016). In fact, the large increases of alkane, alkene, and aromatic concentrations in winter nights supported this hypothesis, and other studies have also noted this phenomenon (Wang et al., 2014; Cheng et al., 2018).
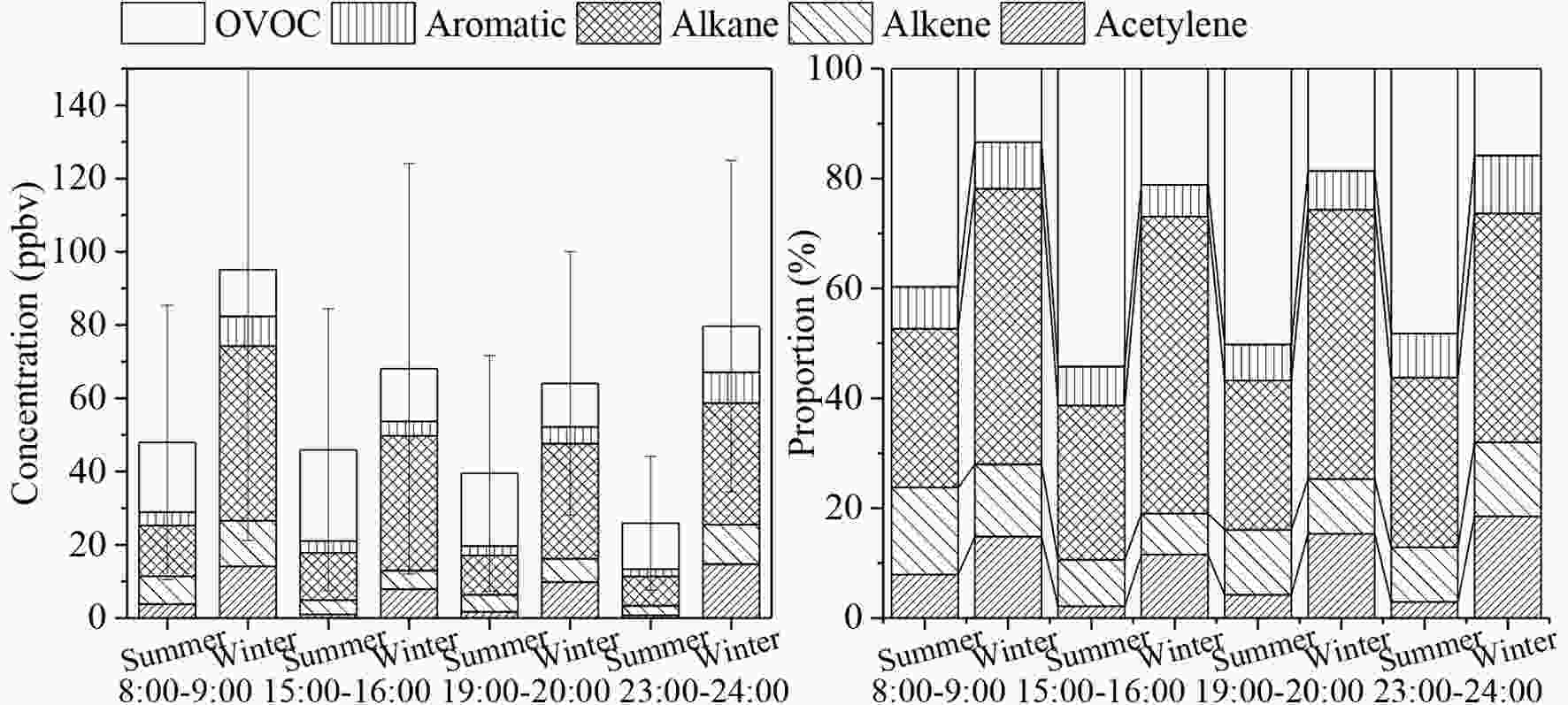 Figure3. Campaign-average TVOCs and proportions by category.
Figure3. Campaign-average TVOCs and proportions by category.2
3.2. Potential formation of O3 and SOA
Figure 4 presents the time series of OFPs in winter and summer calculated using the MIR method. The campaign-average OFPs were > 50% higher in summer (average: 304.3 ppbv) than in winter (average: 178.2 ppbv), despite summer having only half the TVOCs compared with winter. Alkenes were the dominant contributor of OFPs in both summer and winter, at 38.7% and 50.5%, respectively. Because the photochemical reactivity difference is not considered in our use of the MIR method, the seasonal differences in alkene-contributed OFPs were mainly caused by the different chemical compositions in this category. For example, biogenic alkenes (i.e., isoprene) had much higher levels in summer (94.8 ppbv) than in winter (0.25 ppbv). The very large seasonal difference in biogenic OFPs was primarily due to the deciduous vegetation in Xi’an; biogenic OFP concentrations were much higher in Guangzhou and exhibited a different pattern probably due to the dominance of evergreen vegetation in Guangzhou (Ou et al., 2015). The order of contributions of other VOC categories to the total OFPs, from high to low, was OVOC > aromatics > alkane > alkyne. OVOCs, especially formaldehyde and methylglyoxal, contributed greatly to OFPs due to their high photochemical reactivity and concentrations (Louie et al., 2013). The relatively low photochemical reactivity of alkanes and aromatics limited their potential in forming O3. The time series of OFPs indicated the ranges of 71.9?746 and 59.8?554 ppbv in summer and winter, respectively. These high calculated OFP values in this study was because the MIR method models an ideal capability of VOCs in forming O3, in which the complex reactions require high temperatures, high levels of solar radiation, and a proper NOx level (Carter, 2010). The higher temperature and stronger solar radiation in summer favor the transfer of OFPs to O3 (Table S4), and the higher OFPs in winter did not result in high O3 concentrations (Seco et al., 2011; Feng et al., 2016).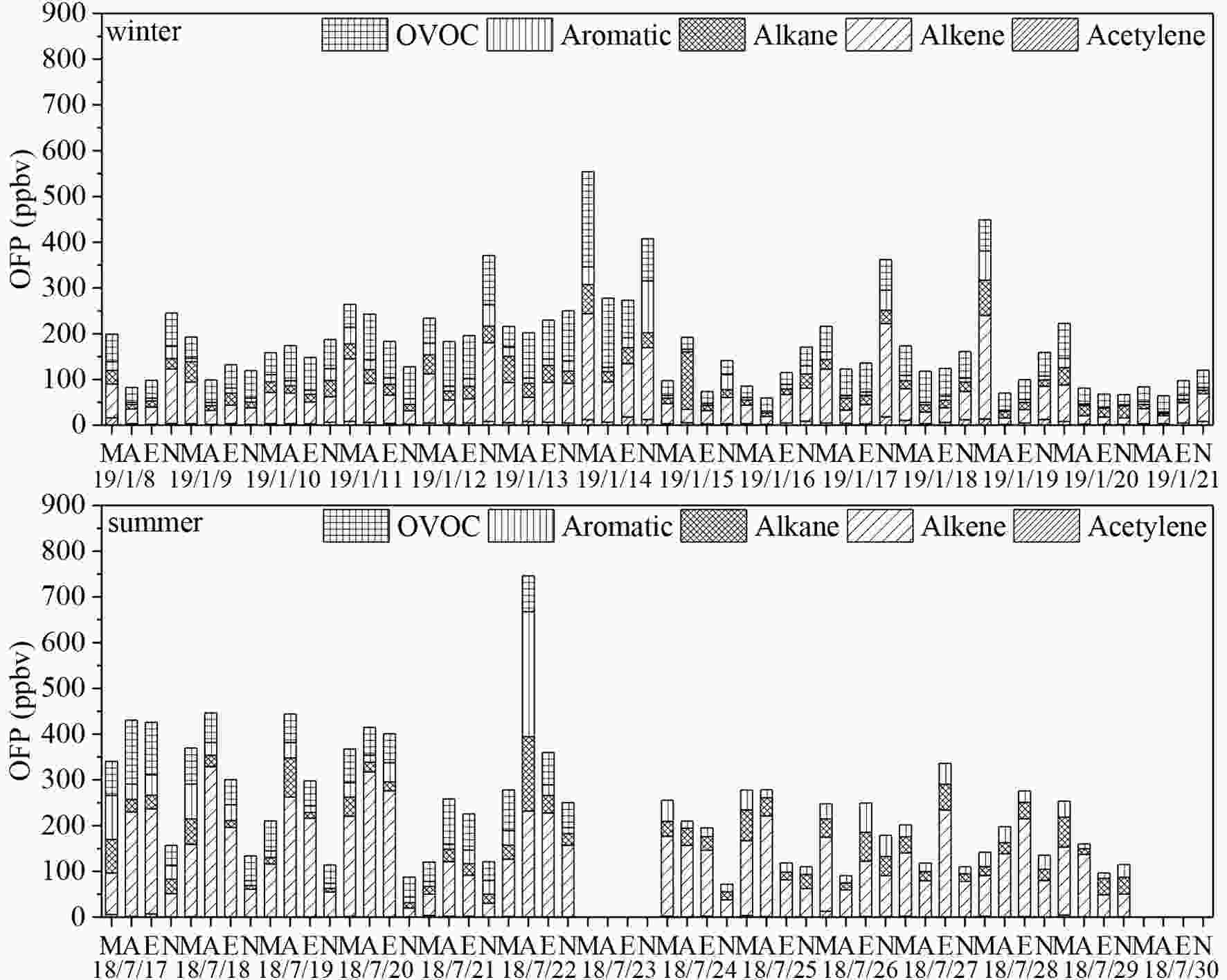 Figure4. Time series of OFPs by PAMS NMHCs and OVOCs in winter and summer (M, A, E and N denote morning, afternoon, evening and night, respectively; the date format is as YY/MM/DD) (data absence on 18/7/23 and 18/7/30 were due to instrument failure).
Figure4. Time series of OFPs by PAMS NMHCs and OVOCs in winter and summer (M, A, E and N denote morning, afternoon, evening and night, respectively; the date format is as YY/MM/DD) (data absence on 18/7/23 and 18/7/30 were due to instrument failure).SOAPs in winter and summer are presented in Fig. 5. In toluene-equivalent SOAPs, aromatic hydrocarbons constituted the dominant group because they demonstrated higher photo oxidation reactivity than alkane and alkenes did (Carter et al., 2012; Li et al., 2017a). OVOCs, the second largest contributor to SOAPs, contributed approximately 10% in winter and even less in summer, and benzaldehyde was the major species due to its extremely high toluene equivalence indexes. SOAPs from the group of alkanes and alkenes were negligible, (e.g., one to two orders of magnitude lower than those of the major groups at p < 0.05). According to the results of Huang et al. (2014), SOA occupied an average of 15.5% in Xi’an aerosols, and the campaign-average PM2.5 concentrations in winter and summer were 126.2 μg m?3 and 21.2 μg m?3, respectively (Shaanxi Environmental Monitoring Center, China). The calculated SOAPs had the proportions of 6.6% and 6.7% in SOA for summer and winter, respectively, which were lower than those reported in Beijing by Sun et al. (2016). This difference was possibly due to the SOA estimation methods used and the minimum OC/EC (organic carbon/elemental carbon) method potentially underestimating the actual SOA level (Huang et al., 2014).
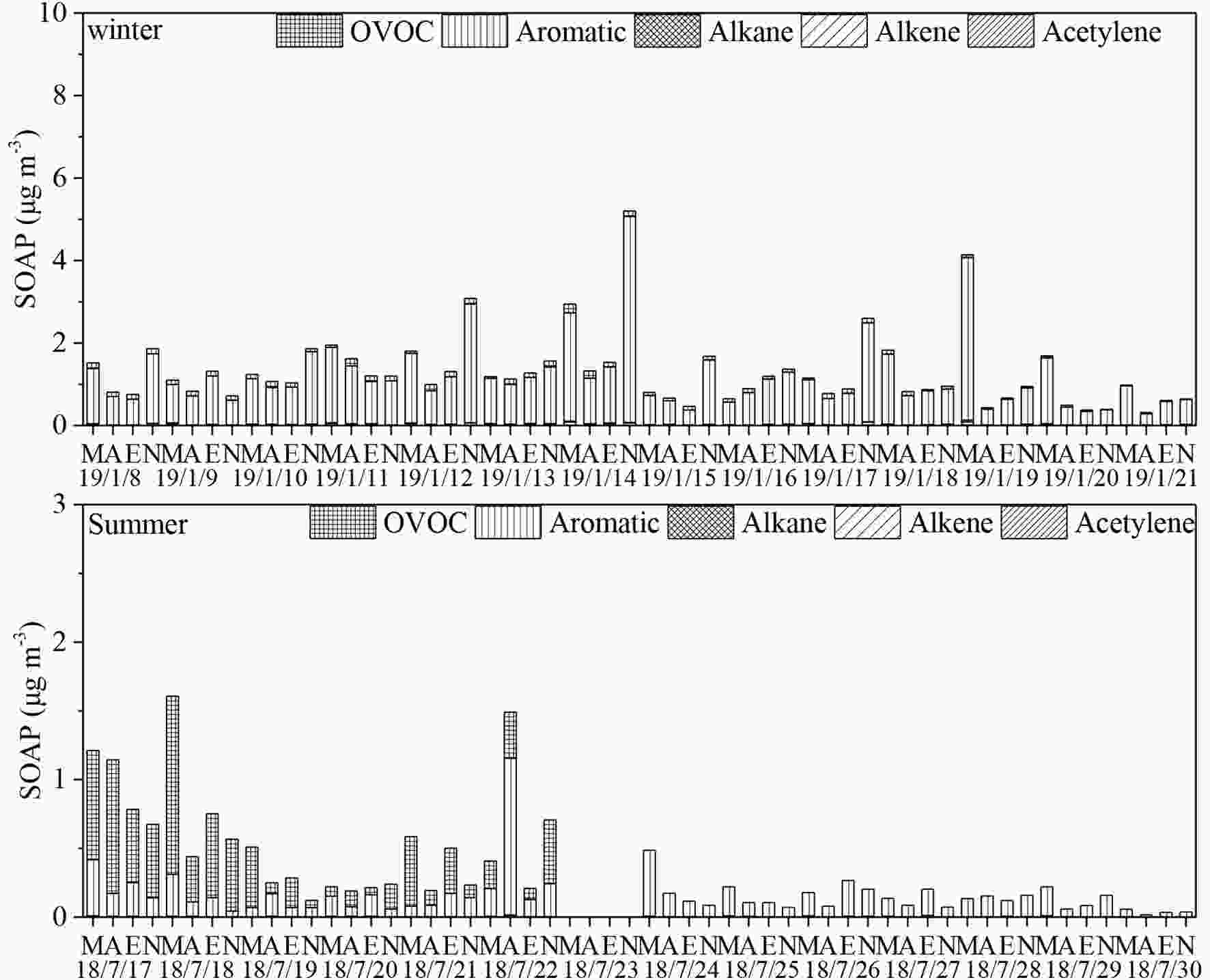 Figure5. Time series of SOAPs by PAMS NMHCs and OVOCs in winter and summer (M, A, E and N denote morning, afternoon, evening and night, respectively; the date format is as YY/MM/DD)(data absence on 18/7/23 were due to instrument failure).
Figure5. Time series of SOAPs by PAMS NMHCs and OVOCs in winter and summer (M, A, E and N denote morning, afternoon, evening and night, respectively; the date format is as YY/MM/DD)(data absence on 18/7/23 were due to instrument failure).2
3.3. Source identification by VOC composition and meteorological factors
The statistical results of the measured TVOCs are presented in Table S5a and Table S5b for the winter and summer campaign, respectively. The hourly average TVOCs had the ranges of 58.4?95.5 and 30.8?56.8 ppbv in winter and summer, respectively. The compositions in TVOCs significantly differed between summer and winter. For example, the proportions of acetylene and alkenes were twice as much in winter than in summer and as high as a factor of six during a nighttime period. Short-chain alkenes and alkynes are produced in large quantities from coal and biomass burning (Liu et al., 2017; Sun et al., 2019a), which explained their much higher fractions in winter in Xi’an. By contrast, isoprene exhibited much higher proportions in summer (0.94 ± 0.53 ppbv, 2.0%) than in winter (0.03 ± 0.07 ppbv, 0.04%) because isoprene primarily stems from biogenic emissions. High temperatures in summer favor lush vegetation growth, which resulted in high biogenic VOCs emissions (Li et al., 2018). By contrast, low temperatures in winter suppressed the emissions of isoprene from vegetation (Sharkey et al., 2007).Alkanes and aromatics also had higher concentrations and proportions in TVOCs during winter than during summer. The order of the prevailing alkane species, from high to low, was ethane > isopentane > isobutane in summer—which were all related with vehicle exhausts (Hwa et al., 2002)—and ethane > propane > n-butane in winter—which are similar to the chemical composition of combustion products, such as those from the burning of natural gas and biomass (Liu et al., 2008). For aromatics, the most abundant species were toluene and benzene in winter and summer, respectively. These results indicated the main VOC sources in Xi’an to be combustion in winter and vehicle exhausts in summer (Mo et al., 2016). OVOCs, the only category of TVOCs with higher concentrations in summer than winter, contributed approximately 50% of TVOC in summer but only 16.8% in winter, a phenomenon that was potentially due to stronger photochemical reactivity in summer, supported by summer’s high temperatures and atmospheric oxidability (expressed in terms of O3 concentration) (Wang et al., 2012; Shao et al., 2016).
Because the concentrations of selected VOC species are indicative of pollution sources, the 10 species with the highest campaign-average concentrations in NMHCs are listed in Fig. S2, and their hourly averages are listed in Fig. 6. The ranking of isopentane, a tracer of gasoline vehicle exhaust (Zhang et al., 2018b), decreased from the second in summer to the eighth in winter, indicating that contributions from vehicle exhausts in winter were not as significant as those in summer. Ethylene, which is primarily emitted from coal and biomass burning, ranked seventh in summer but fourth in winter, implying higher contributions from combustion sources in winter than in summer (Yuan et al., 2010). For the hourly average, species rankings and, by implication, relative source contributions, changed very little over time in winter (Fig. 6). By comparison, species rankings varied over time in summer, which indicated variations in the relative source contributions (i.e., traffic) for PAMS.
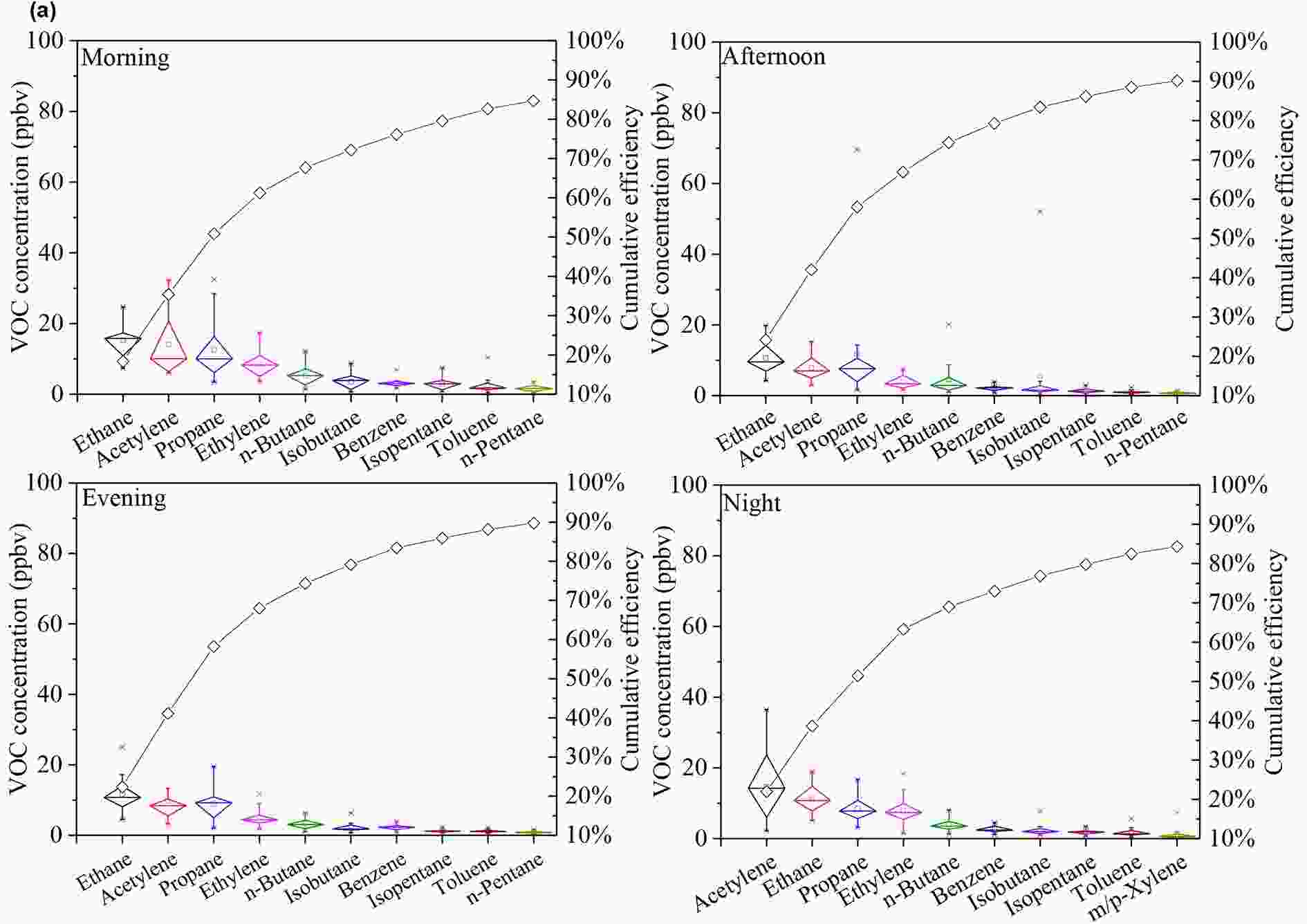 Figure6. Hourly averaged top 10 species in (a) winter and (b) summer. The box plots indicate the campaign-averaged VOC concentrations and the minimum, 25th, 50th, 75th, and maximum percentiles. A cumulative frequency curve is fitted to the top 10 species
Figure6. Hourly averaged top 10 species in (a) winter and (b) summer. The box plots indicate the campaign-averaged VOC concentrations and the minimum, 25th, 50th, 75th, and maximum percentiles. A cumulative frequency curve is fitted to the top 10 species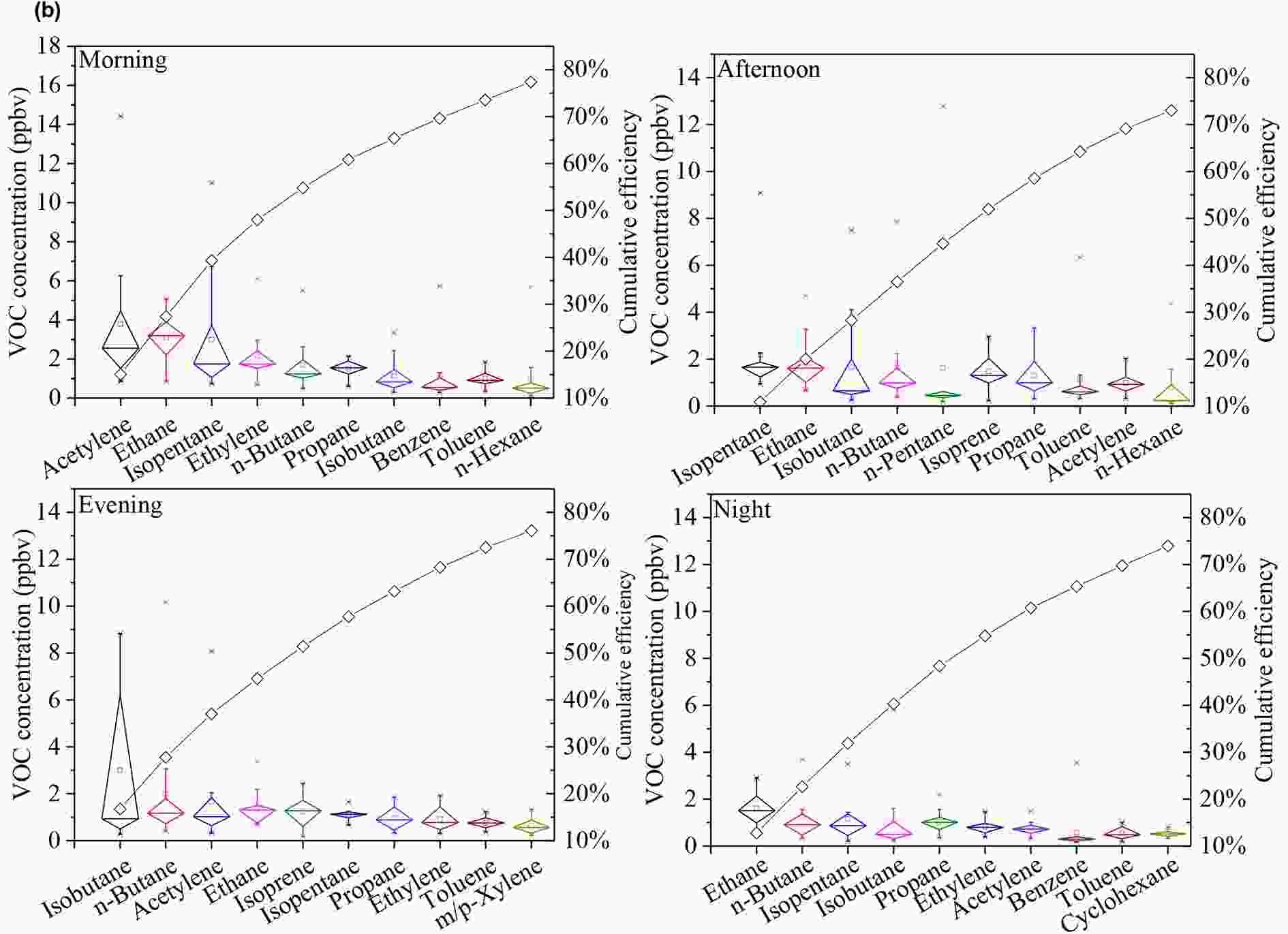 Figure6. (Continued)
Figure6. (Continued)The characteristics of the five selected VOC species (accounting for more than 50% of total PAMS NMHCs) were analyzed using CPF to determine their emission sources, as indicated in Fig. S3. In general, the main sources for the five VOC species were located northwest of the city, which is upwind of the city during winter (Fig. 1). The location map (Fig. 1) clearly indicates a main road (Xi’an Second Ring road) south of the sampling site. Vehicle exhaust with high concentrations of isopentane and short-chain alkanes (ethane and propane) can be brought to the site by southern winds (Li et al., 2017a). The results also indicated considerable sources in the northeast during winter, which potentially stemmed from human activity in the downtown area, that had its pollutants brought to the site by the prevailing wind direction (shown in Fig. S4). For isoprene, the CPF results resembled the wind rose, which indicated that isoprene was mainly affected by wind direction because large concentrations of vegetation (i.e., from the park) were located elsewhere in the city. The CPF results for toluene were unique in that sources differed neither between summer and winter nor between whether they were located in the southwest, northwest, or southeast. Because sources located in these three directions of the sampling site have large and constant toluene emissions (Huang and Hsieh, 2019), the heavy traffic and high population density in these three directions were highly related.
2
3.4. Source apportionment using HERM
HERM needs a certain sample number not less than the input species. In this study, some PAMS species were not detected or were in extremely low concentrations with rare variations, which were invalid inputs to HERM. In this situation, the actual species number input for HERM’s calculations was 46, which was smaller than the sample number (56) and made it possible for the separated source apportionment in summer and winter by HERM. In running HERM, four localized VOC source profiles—namely, biomass burning, coal combustion, gasoline vehicle exhaust, and diesel vehicle exhaust—were selected for model input (Li et al., 2017a; Sun et al., 2019b). Because VOC emissions from biomass burning and coal combustion varied significantly with different fuels and burning devices, two localized profiles from in-situ residential scenarios in Guanzhong plain were selected (Sun et al., 2019a). As biomass open burning has been legally banned by local government, residential use is the predominant source of biomass burning in Xi’an, which makes it representative in source apportionment (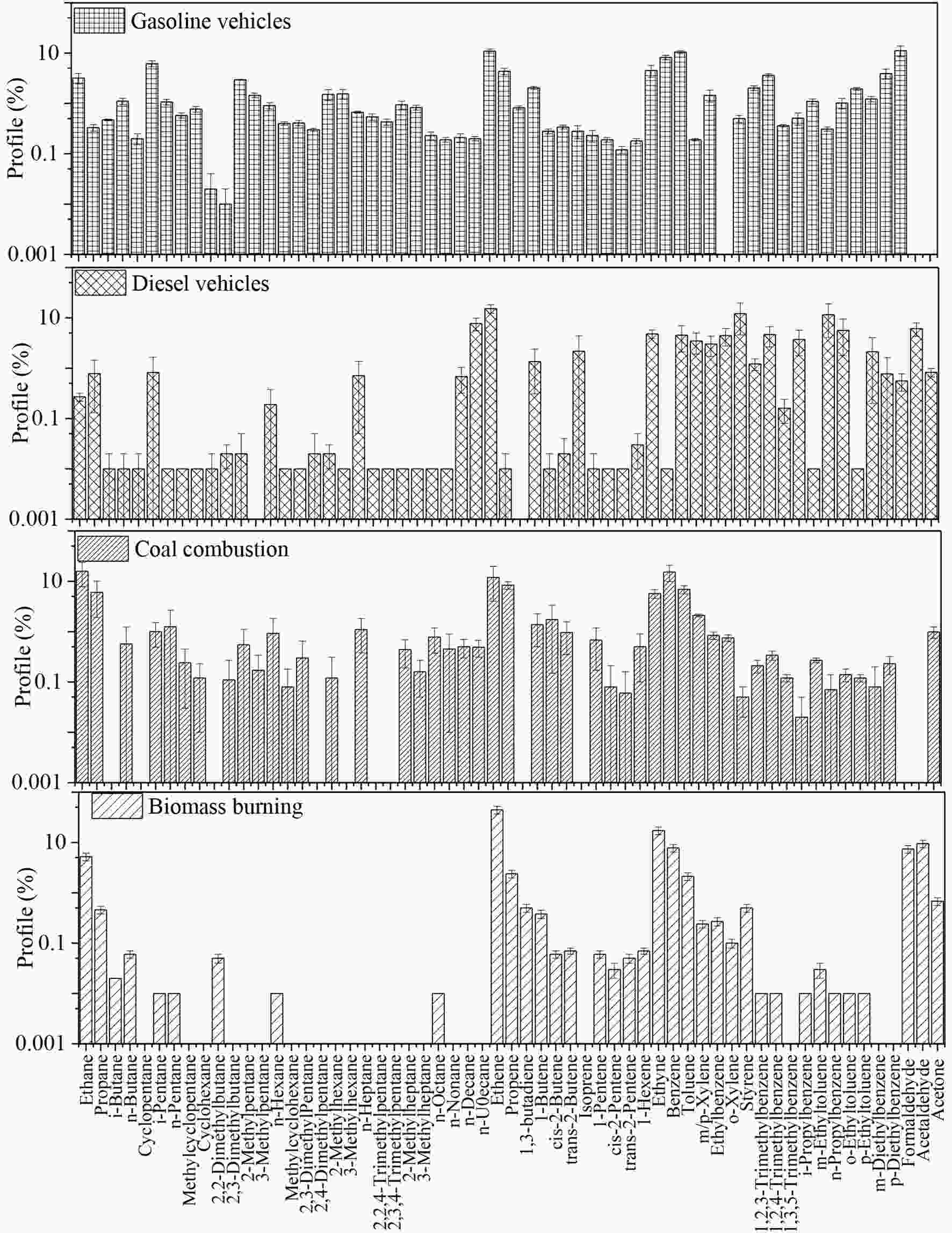 Figure7. Source profiles for HERM inputs and outputs
Figure7. Source profiles for HERM inputs and outputs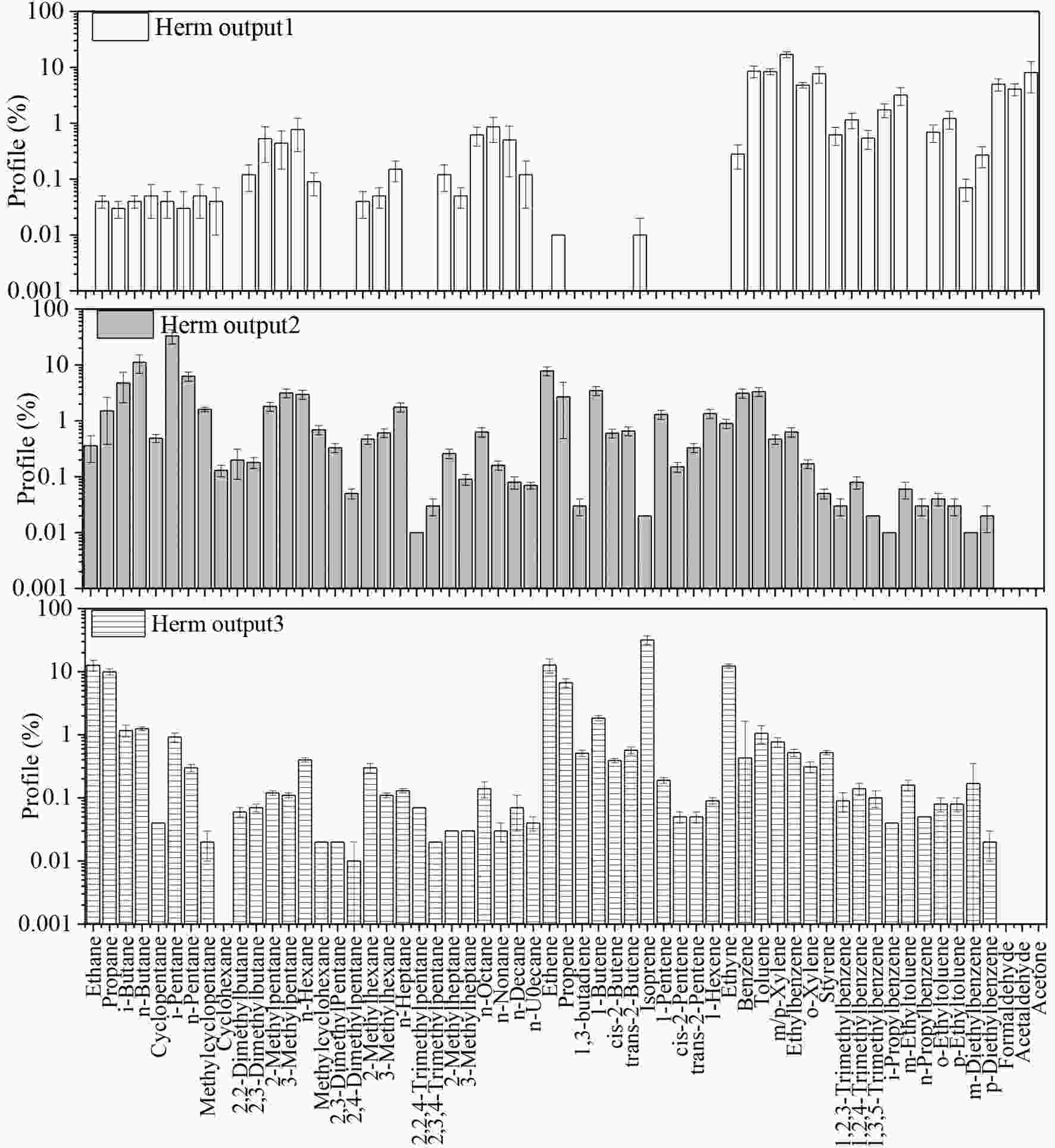 Figure7. (Continued)
Figure7. (Continued)The percentage contributions from the seven sources are presented in Fig. 8, and the relative contributions from these sources had significant seasonal variations. In winter, combustion-related sources (biomass burning and coal combustion) contributed most to the TVOC concentration (53.4%) because of urban and rural heating, as mentioned. Our previous study demonstrated that residential coal and biomass burning were both crucial sources of ambient TVOCs in Xi’an (Sun et al., 2019b). Traffic-related sources contributed more than one-third (34.3%) to the total TVOC concentration, in which diesel (18.6%) and gasoline (14.5%) vehicle exhausts were dominant and gasoline vapor (1.4%) was negligible. The VOC emission factors were higher for vehicles powered by diesel than by gasoline (Ho et al., 2009). Solvent use, usually in industrial processes (e.g., painting), only contributed 11.9% to the TVOC concentration in winter. By comparison, industrial emissions were the biggest contributor to ambient TVOCs in Xi’an in 2013 (Feng et al., 2016). This indicates that government-led efforts in industrial restructuring have improved air quality in Xi’an (Zhang et al., 2018a). The percentage contribution of BVOCs in winter was as low as 0.2%, which was because deciduous vegetation does not emit BVOCs and because emissions from evergreen vegetation are suppressed by low temperatures in winter (Kelly et al., 2018).
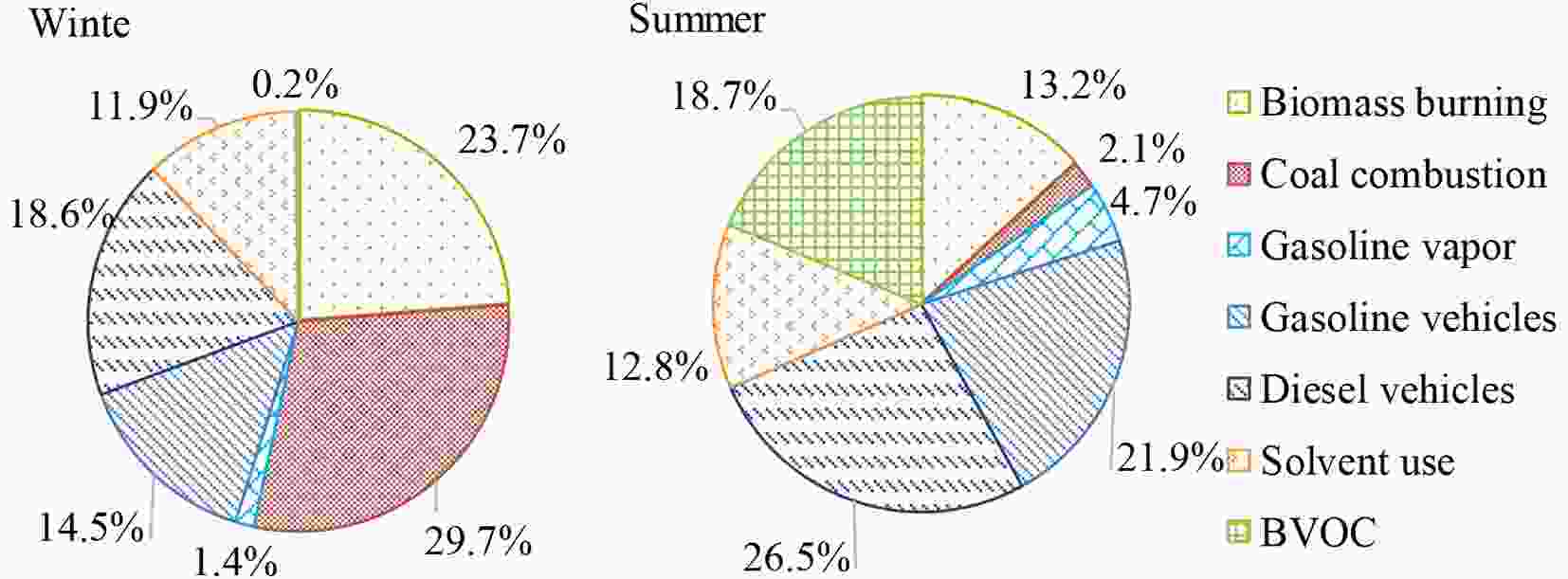 Figure8. Source apportionment results for TVOCs by HERM in winter and summer.
Figure8. Source apportionment results for TVOCs by HERM in winter and summer.In summer, traffic-related sources were the largest contributor to TVOCs (53.1%), in which diesel vehicle emissions, gasoline vehicle emissions, and gasoline vapor contributed 26.5%, 21.9% and 4.7%, respectively. However, traffic-related VOC emission intensities changed little between winter and summer (Tang et al., 2019). The contributions from solvent use were comparable between summer (12.8%) and winter (11.9%). Combustion sources contributed less in summer (15.3% in total) than in winter. Biomass burning still contributed 13.2% to the TVOC concentration, which was reflective of the common practices of residential and open biomass burning in Xi’an and surrounding areas. The percentage contribution from BVOCs to TVOCs was much higher in summer (18.7%) than in winter (0.2%) due to strong vegetation emissions in hot weather.
It should be noted that uncertainties in source apportionment widely exist in model execution, data measurement and source profile selection (Lee and Russell, 2007). The advanced HERM used in this study could decrease the uncertainty to some extent by adopting better algorithms and combining the advantages of CMB and PMF models (Chen and Cao, 2018). However, the limited sample number in this study, a mixture of source profiles from local studies and the literature, as well as the absence of certain profiles (i.e., industrial coal combustion), may increase the uncertainty of this study. A comprehensive future study is needed with larger sample sizes and longer sampling periods, along with far more local studies providing representative source profiles to reduce the uncertainty of VOC source apportionment.
Acknowledgements. This research was supported by the Natural Science Foundation of China (Grant No. 41907188), Natural Science Foundation of Shaanxi Province, China (Grant No. 2019JQ-386), and the China Postdoctoral Science Foundation (Grant No. 2019M653658).
Electronic supplementary material: Supplementary material is available in the online version of this article at
