 ,, 郭庆港, 苏振贺, 王培培, 董丽红, 胡卿, 鹿秀云, 张晓云, 李社增, 马平
,, 郭庆港, 苏振贺, 王培培, 董丽红, 胡卿, 鹿秀云, 张晓云, 李社增, 马平 ,河北省农林科学院植物保护研究所/河北省农业有害生物综合防治工程技术研究中心/农业农村部华北北部作物有害生物综合治理重点实验室,河北保定 071000
,河北省农林科学院植物保护研究所/河北省农业有害生物综合防治工程技术研究中心/农业农村部华北北部作物有害生物综合治理重点实验室,河北保定 071000Characterization of Fungal Community Structure in the Rhizosphere Soil of Healthy and Diseased-Verticillium Wilt Potato Plants and Carbon Source Utilization
ZHAO WeiSong ,, GUO QingGang, SU ZhenHe, WANG PeiPei, DONG LiHong, HU Qing, LU XiuYun, ZHANG XiaoYun, LI SheZeng, MA Ping
,, GUO QingGang, SU ZhenHe, WANG PeiPei, DONG LiHong, HU Qing, LU XiuYun, ZHANG XiaoYun, LI SheZeng, MA Ping ,Plant Protection Institute of Hebei Academy of Agricultural and Forestry Sciences/IPM Centre of Hebei Province/Key Laboratory of IPM on Crops in Northern Region of North China, Ministry of Agriculture and Rural Affairs, Baoding 071000, Hebei
,Plant Protection Institute of Hebei Academy of Agricultural and Forestry Sciences/IPM Centre of Hebei Province/Key Laboratory of IPM on Crops in Northern Region of North China, Ministry of Agriculture and Rural Affairs, Baoding 071000, Hebei通讯作者:
责任编辑: 岳梅
收稿日期:2020-04-16接受日期:2020-05-19网络出版日期:2021-01-16
| 基金资助: |
Received:2020-04-16Accepted:2020-05-19Online:2021-01-16
作者简介 About authors
赵卫松,Tel:0312-5927076;E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (1226KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
赵卫松, 郭庆港, 苏振贺, 王培培, 董丽红, 胡卿, 鹿秀云, 张晓云, 李社增, 马平. 马铃薯健株与黄萎病株根际土壤真菌群落结构及其对碳源利用特征[J]. 中国农业科学, 2021, 54(2): 296-309 doi:10.3864/j.issn.0578-1752.2021.02.006
ZHAO WeiSong, GUO QingGang, SU ZhenHe, WANG PeiPei, DONG LiHong, HU Qing, LU XiuYun, ZHANG XiaoYun, LI SheZeng, MA Ping.
开放科学(资源服务)标识码(OSID):

0 引言
【研究意义】马铃薯(Solanum tuberosum)是我国第4大粮食作物[1]。马铃薯产业是农民增收的主要来源之一,对保障我国粮食安全具有重要意义。目前,我国是马铃薯种植面积和产量最大的国家,分别占世界马铃薯总面积和总产量的25%和22%[2]。近年来,由于马铃薯连作导致土传病害有逐年加重的趋势,给马铃薯产业带来了较大的经济损失。马铃薯黄萎病又称早死病或早熟病,是典型的土传兼种传维管束病害,轻者减产20%—30%,重者损失达50%以上,严重影响马铃薯的产量和品质[3,4]。在我国新疆、河北、甘肃、陕西、内蒙古、宁夏、黑龙江、吉林、贵州等地已有关于马铃薯黄萎病发生的报道[5,6,7,8,9]。因此,针对马铃薯黄萎病的防治工作至关重要。研究马铃薯健康植株和黄萎病株根际土壤的真菌群落结构,有助于揭示黄萎病发生与真菌群落两者之间的互作关系,可为进一步筛选对马铃薯黄萎病有拮抗作用的有益微生物和病害防治提供新思路。【前人研究进展】目前,有关马铃薯黄萎病的研究主要集中在抗性品种筛选、病原菌种类鉴定、致病力测定、病害防治技术等方面[5,6,7,8,9,10]。微生物群落结构与植物健康状况之间的关系已成为研究的热点,而关于土壤微生物群落结构与马铃薯黄萎病发生之间的关系尚不清晰。研究表明,土传病害的发生与土壤微生物区系的改变存在一定的相关性[11,12,13]。GORISSEN等[11]研究发现,马铃薯青枯病(病原菌Ralstonia solanacearum biovar 2)的病田土壤细菌多样性低于健康土壤,且细菌群落结构发生了变化;祈建军等[14]研究发现,地黄根腐病(病原菌Fusarium solani)发生与其根际土壤微生物呼吸、代谢熵密切相关。近年来,随着测序技术的快速发展,通过高通量测序技术能够从土壤微生物群落水平分析土传病害发生的可能主导因子,可以更好地理解马铃薯健株和病株土样之间真菌的组成及差异,从而为马铃薯黄萎病防治提供更好的思路和解决方法,而另一方面Biolog技术则是从代谢功能角度研究土壤微生物群落功能多样性的变化。【本研究切入点】尽管已有****研究了马铃薯连作对土壤微生物群落结构的影响[15],但黄萎病菌(大丽轮枝菌Verticillium dahliae)侵染马铃薯对根际土壤真菌群落结构产生的影响及其对碳源利用特征的变化情况尚缺乏系统的认识。【拟解决的关键问题】以马铃薯健株和黄萎病株根际土壤为研究对象,采用Illumina MiSeq高通量测序技术研究马铃薯植株根际土壤真菌群落结构的变化,分析真菌群落结构与土壤养分之间的关系,结合Biolog方法对不同根际土壤微生物对碳源的利用能力进行比较,以期揭示黄萎病发生与土壤真菌群落结构的互作关系,为生防菌筛选和利用微生态调控手段防控马铃薯黄萎病提供理论依据。1 材料与方法
1.1 试验区域概况
坝上地区包括河北省围场满族蒙古族自治县部分地区,地处东经116°32′—118°14′,北纬41°35′—42°40′,是全国重点马铃薯种薯繁育和商品薯基地县,全县马铃薯年平均种植面积约2.93万公顷。年平均气温在-0.5—6.0℃,平均降水量为300—560 mm,时空分布上差异较大,70%的降水集中在6—8月,无霜期区域分布差异较大。马铃薯黄萎病主要由土壤和种薯传播,寄主范围广,流行性强,近年来有发生的报道[10]。1.2 材料
供试马铃薯品种荷兰15(感黄萎病品种),由围场满族蒙古族自治县志伟马铃薯种植专业合作社提供,用于田间试验。1.3 根际土壤样品的采集
试验于2019年在河北省围场满族蒙古族自治县牌楼乡红砬子村(东经117°15′,北纬41°56′)进行。马铃薯根际土壤取自连续种植马铃薯5年的地块,土壤类型为沙壤土。具体采集方法如下:在马铃薯黄萎病发生时期(8月上旬),参考赵卫松等[8]马铃薯黄萎病的病害分级标准,选择马铃薯健康植株和黄萎病植株(发病等级为3级以上)作为研究对象,用取样铲分别将马铃薯健株和病株整个根系完整挖出,轻敲根系,与根系结合较松的土壤弃去,将与根系紧密结合的土壤用毛刷清理。健株与病株分别取4个点,每取样点即为1份,取样点间隔30 m,每取样点选择5株马铃薯采集根际土壤混合而成。土壤样品装入低温保温箱中带回实验室,过2 mm筛后一部分保存于4℃冰箱用于Biolog测定,一部分保存于-80℃冰箱,用于真菌群落结构高通量测序分析。1.4 测定项目及方法
1.4.1 土壤真菌群落结构的高通量测序 采用FastDNATM SPIN Kit for Soil(MP Biomedicals,Solon,OH,USA)试剂盒提取土壤基因组DNA,利用NanoDrop 2000分光光度计(Thermo Fisher Scientific Inc.,Waltham,MA,USA)检测DNA的纯度和浓度,DNA于-20℃保存备用。以样品DNA为模板,采用引物ITS1F(5′-CTTGGTCATTTAGAGGAAGTAA-3′)和ITS2R(5′-GCTGCGTTCTTCATCGATGC-3′)对其进行目标片段的ITS可变区扩增[16]。PCR扩增体系为20 μL:5×FastPfu缓冲液4 μL,2.5 mmol·L-1 dNTPs 2 μL,引物(5 μmol·L-1)各0.8 μL,FastPfu聚合酶0.4 μL,DNA模板(20 ng·μL-1)1 μL,最后用灭菌的ddH2O将反应体系补至20 μL。PCR反应程序参数:95℃预变性3 min,27个循环(95℃变性30 s,55℃退火30 s,72℃延伸30 s),最后72℃延伸10 min。PCR扩增产物经2%琼脂糖凝胶电泳检测,并对其目标片段进行切胶回收。纯化后送至上海美吉生物医药科技有限公司利用Illumina MiSeq PE 300平台进行高通量测序。1.4.2 土壤中大丽轮枝菌ITS基因拷贝数量的测定 按照1.3方法采集马铃薯健株和黄萎病株根际土壤,参考赵卫松等[17]方法分别测定土壤中大丽轮枝菌ITS基因的拷贝数量。具体方法如下:以大丽轮枝菌基因组DNA为模板,利用大丽轮枝菌特异性引物DB19(CGGTGACATAATACTGAGAG)/DB20(GACGATGCGGATTGAACGAA)扩增ITS部分序列。将PCR产物与pMD19-T Vector连接、转化于DH5α感受态细胞中,提取重组质粒,并10倍梯度稀释后,将其作为模板进行实时荧光定量PCR扩增。以质粒拷贝数的对数值为横坐标,以循环阈值Ct值为纵坐标建立标准曲线。以不同土壤DNA作为模板进行实时荧光定量PCR反应,得到不同样本的循环阈值,根据建立的标准曲线计算土壤中大丽轮枝菌的拷贝数。
1.4.3 土壤养分的测定 参考WANG等[18]方法分别对马铃薯健株和黄萎病株根际土壤的酸碱度(pH)、硝态氮(NO3--N)、速效磷(AP)、无机磷(IP)和有机质(OM)含量进行测定。
1.4.4 健株与黄萎病株根际土壤的碳源利用特征比较 采用Biolog-ECO技术分析微生物群落对31种碳源(分为6大类,氨基酸类、碳水化合物类、胺类、羧酸类、聚合物类以及双亲化合物类)的代谢特征,即功能多样性。ECO板接种液的制备:将土壤样品在25℃条件下活化24 h,取3 g根际土加入含有27 mL灭菌的NaCl溶液(0.85 mol·L-1)的锥形瓶中180 r/min振荡45 min。取3 mL上清液加入到27 mL NaCl溶液,混匀后再取3 mL上清液加入到27 mL NaCl溶液,最终稀释比例为1﹕1 000,并将制备的接种液转移至储液槽中。利用8孔道排枪向ECO板的各孔中加入150 μL的稀释液,每个土样的稀释液按方法要求设置3组平行。将接种好的微孔板放入25℃的恒温培养箱中,分别于1、2、3、4、5、6、7、8、9和10 d使用ELxS08-Biolog微孔板读数仪(Bio-Tek Instruments Inc,USA)进行测定,测定波长分别为590 nm(颜色+浊度)和750 nm(浊度)。
1.5 数据分析
微生物代谢活性用590 nm下的吸光度值减去750 nm下的吸光度值表示,其中数值<0.06时按0处理。孔的平均颜色变化率(average well color development,AWCD)计算方法如下[19]:AWCD = Σ(C-R)/n。式中,C为每个碳源孔的两波段光密度差值;R为对照孔的光密度值;n为培养基碳源种类数(n=31)。微生物对碳源的相对利用率,以健株和黄萎病株根际土壤处理的6类碳源AWCD值中最大的值为100%,其余以不同处理的各类碳源AWCD值与最大值的比值即为相对利用率。试验数据采用Microsoft Excel 2010进行整理,Origin 8.6进行作图,采用SPSS 17.0软件进行单因素方差分析、独立样本T检验统计分析。采用Canoco 4.5软件对健株和黄萎病株根际土壤真菌群落组成进行主成分分析(PCA)及其与土壤养分的冗余分析(RDA),并利用CanoDraw软件进行作图。
2 结果
2.1 健株和黄萎病株根际土壤养分变化
马铃薯健株和黄萎病株根际土壤养分之间存在不同程度的差异。与健株相比,除了土壤pH不存在显著差异外,黄萎病株根际土壤中的速效磷和无机磷含量显著升高,而硝态氮和有机质含量显著降低(表1)。Table 1
表1
表1健株与黄萎病株根际土壤养分变化
Table 1
| 指标 Index | 健株Healthy plant | 病株Diseased plant |
|---|---|---|
| 硝态氮NO3--N (mg·kg-1) | 13.38±4.48a | 10.03±7.69b |
| pH | 6.89±0.02a | 6.83±0.03a |
| 速效磷AP (mg·kg-1) | 373.51±60.03b | 897.00±20.08a |
| 无机磷IP (μg·g-1) | 99.40±11.71b | 189.84±18.26a |
| 有机质OM (%) | 0.77±0.01a | 0.64±0.01b |
| 数据后不同小写字母表示差异显著(P<0.05)。下同 Different lowercases after the data indicate significant difference (P<0.05). The same as below | ||
新窗口打开|下载CSV
2.2 健株和黄萎病株根际土壤中大丽轮枝菌的数量测定
分别对采集的健株和黄萎病株根际土壤中大丽轮枝菌的数量进行测定,将每克土壤中大丽轮枝菌ITS基因拷贝数量转换为对数,结果表明,黄萎病株根际土壤中大丽轮枝菌ITS基因拷贝数量为8.86,而健株根际土壤中未检测到目标病原菌。2.3 土壤真菌多样性分析
获得的健株根际土壤样本有效序列数目为67 602条,黄萎病株根际土壤样本有效序列条目为62 390条。与健株根际土壤相比,黄萎病株根际土壤真菌多样性指数呈现出下降趋势,但差异不显著(表2)。Table 2
表2
表2健株和黄萎病株根际土壤真菌Alpha多样性
Table 2
| 处理 Treatment | Sobs 指数 Sobs index | 香浓指数 Shannon index | 辛普森指数 Simpson index | ACE 指数 ACE index | Chao I 指数 Chao I index |
|---|---|---|---|---|---|
| 健株Healthy plant | 236.50±21.42a | 2.5066±0.2077a | 0.1753±0.0364a | 291.44±17.11a | 292.95±20.21a |
| 病株Diseased plant | 222.00±15.10a | 2.4829±0.2238a | 0.1730±0.0504a | 280.17±25.83a | 278.20±27.49a |
新窗口打开|下载CSV
2.4 根际土壤真菌群落的特异性分析
通过对健株与黄萎病株根际土壤样品中真菌群落Venn图的比较分析,可以清晰地展现出根际土壤真菌群落OTU数目组成、特异性、重叠或相似性。在OTU分类水平上的特异性分析发现,共有真菌OTU数量为269个,其中健株根际土壤特有109个OTU,黄萎病株根际土壤特有81个OTU(图1-A)。在属分类水平上的特异性分析发现,共有真菌的属为126个,健株根际土壤特有35个属,黄萎病株根际土壤特有18个属(图1-B)。图1
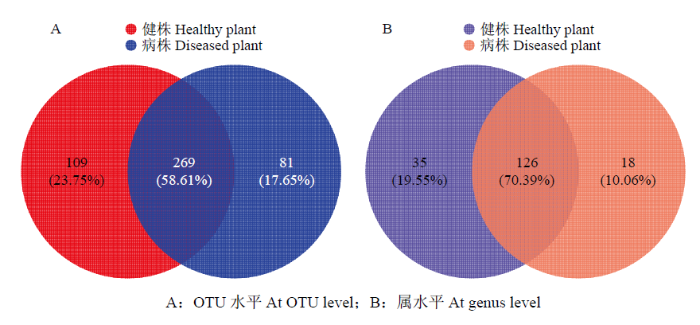 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1健株与黄萎病株根际土壤真菌群落维恩图
A:OTU水平At OTU level;B:属水平At genus level
Fig. 1Venn map of fungal community in rhizosphere soil of healthy and diseased plants
2.5 根际土壤真菌群落结构主成分分析
由图2可知,健株与黄萎病株根际土壤真菌在OTU水平下第一主成分(PCA1)和第二主成分(PCA2)分别可以解释所有变量的85.24%和9.49%,2个主成分方差累积贡献率达到94.73%,说明其能够表征真菌群落组成的特征。同时,相同处理的土壤样品在主坐标中位于不同的象限,表明相同处理植株间存在区别,进一步分析发现健株与黄萎病株根际土壤的真菌群落结构差异显著(R=1.0000,P=0.034)。图2
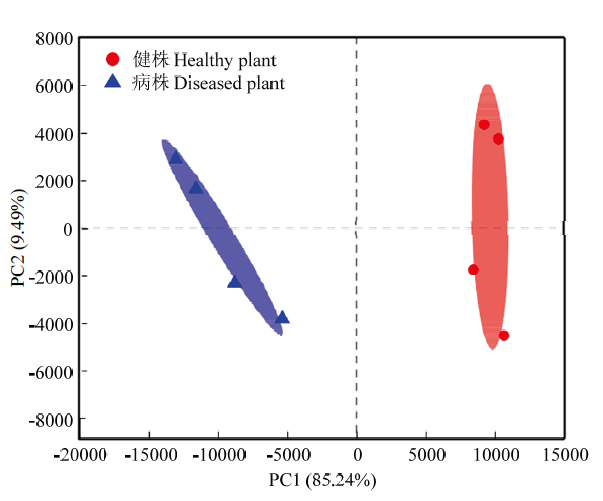 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2健株与黄萎病株根际土壤真菌群落结构的主成分分析
Fig. 2Principal co-ordinates analysis of fungal community structure in rhizosphere soil of healthy and diseased plants
2.6 根际土壤真菌群落结构变化
2.6.1 门水平根际土壤真菌群落结构变化 将丰度<1%的归为其他,门水平下健株与黄萎病株根际土壤真菌群落组成的研究结果表明(图3),获得的优势菌群包括子囊菌门(Ascomycota)、担子菌门(Basidiomycota)和丝孢菌门(Mortierellomycota),其平均相对丰度分别介于68.43%—82.58%、13.75%—28.30%和2.83%—3.28%。与健株根际土壤相比,黄萎病株的子囊菌门和丝孢菌门的相对丰度上升,上升幅度分别为20.68%和16.16%;而担子菌门的相对丰度下降,下降幅度为51.43%。图3
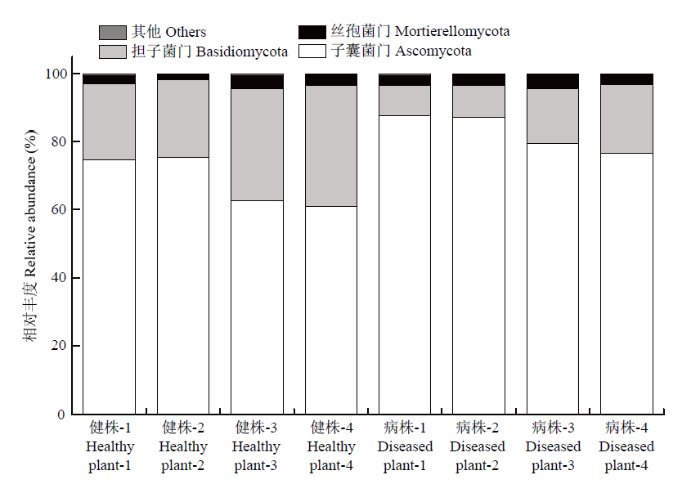 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3门水平真菌群落组成相对丰度变化
Fig. 3Relative abundance of fungal community composition at phylum level
2.6.2 属水平根际土壤真菌群落结构变化 将处理中至少1组相对丰度>1%进行统计,丰度<1%、未能鉴定或未培养的菌群归为其他,属水平下健株与黄萎病株根际土壤真菌群落组成的研究结果表明(图4),获得的优势菌群为小不整球壳属(Plectosphaerella)、Guehomyces、轮枝菌属(Verticillium)、镰孢菌属(Fusarium)、链格孢属(Alternaria)、刺盘孢属(Colletotrichum)、被孢霉属(Mortierella)、葡萄穗霉属(Stachybotrys)、腐质霉属(Humicola)、赤霉属(Gibberella)、曲霉属(Aspergillus)、青霉属(Penicillium)、维希尼克氏酵母属(Vishniacozyma)、红酵母属(Rhodotorula)和芽枝霉属(Cladosporium),其平均相对丰度分别介于15.40%—28.05%、10.59%—27.46%、0.47%—37.42%、10.49%—10.75%、2.37%—6.10%、2.24%—3.89%、2.83%—3.28%、0.14%—4.45%、1.14%—1.61%、0.86%—1.59%、0.71%—1.27%、0.30%—1.38%、0.19%—1.37%、0.07%—1.12%和0.14%—1.00%。与健株根际土壤相比,黄萎病株根际土壤中轮枝菌属、链格孢属、刺盘孢属、被孢霉属、腐质霉属、青霉属、维希尼克氏酵母属、红酵母属和芽枝霉属的相对丰度呈上升趋势,增加倍数分别为71.96、1.57、0.74、0.16、0.42、3.62、6.11、15.38和6.24倍;而小不整球壳属、Guehomyces、葡萄穗霉属、赤霉属、曲霉属菌群的相对丰度呈下降趋势,下降幅度分别为45.10%、61.41%、96.87%、45.85%和44.39%。
图4
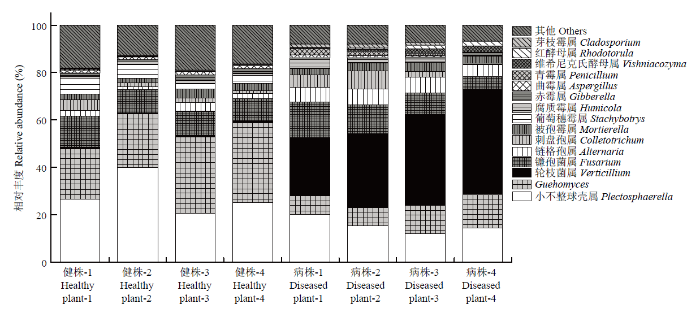 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4属水平真菌群落组成相对丰度变化
Fig. 4Relative abundance of fungal community composition at genus level
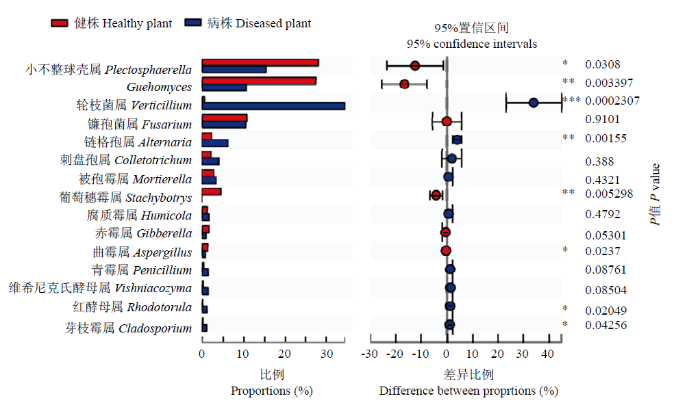
采用独立样本T检验进一步对健株和黄萎病株根际土壤真菌属水平物种差异进行分析(图5),结果表明健株和黄萎病株根际土壤真菌平均相对丰度较高的前15个菌群中,存在显著差异的菌群包括小不整球壳属(P=0.0308)、Guehomyces(P=0.0034)、轮枝菌属(P=0.0002)、链格孢属(P=0.0016)、葡萄穗霉属(P=0.0053)、曲霉属(P=0.0237)、红酵母属(P=0.0205)、芽枝霉属(P=0.0426);不存在显著差异的菌群包括赤霉属(P=0.0530)、镰孢菌属(P=0.9101)、刺盘孢属(P=0.3880)、被孢霉属(P=0.4321)、腐质霉属(P=0.4792)、青霉属(P=0.0876)、维希尼克氏酵母属(P=0.0850)。
图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5健株和黄萎病株根际土壤真菌属水平菌群差异分析(前15个)
X轴代表不同的分组,不同颜色的箱子表示不同的分组,Y轴代表某一物种在不同分组中的平均相对丰度
Fig. 5Analysis of the differences of fungi species in rhizosphere soil of healthy and diseased plants at genus level (top 15)
X axis represents different groups, boxes of different colors represent different groups. Y axis represents average relative abundance of a species in different groups
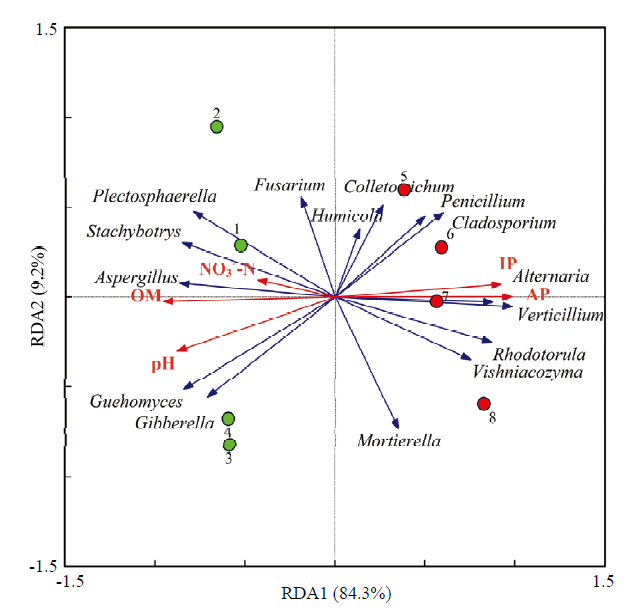
2.7 真菌属水平群落结构与土壤养分含量的冗余分析
属水平土壤优势真菌群落结构与土壤养分含量的相关性分析表明(图 6),2个坐标轴总共解释93.5%的真菌群落结构与土壤养分含量的关系,能够较好地反映土壤养分含量对土壤真菌群落结构的影响。进一步分析表明,健株与黄萎病株根际土壤优势群落的相对丰度对土壤养分的响应不同。其中,健株根际土壤优势群落的相对丰度(如小不整球壳属、Guehomyces、葡萄穗霉属、赤霉属、曲霉属)与硝态氮、有机质和pH呈正相关,而黄萎病株根际土壤优势群落的相对丰度(如轮枝菌属、链格孢属、刺盘孢属、被孢霉属、腐质霉属、青霉属、维希尼克氏酵母属、红酵母属和芽枝霉属)与无机磷和速效磷呈正相关。2.8 健株与黄萎病株根际土壤微生物AWCD变化
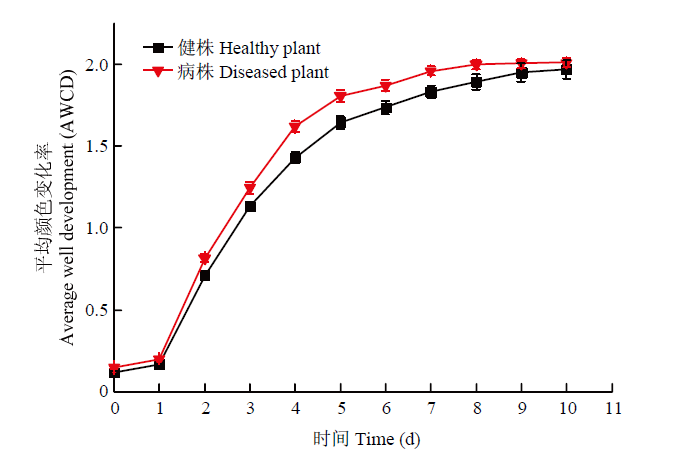
AWCD 值提供了不同根际土壤微生物对相同碳源利用的差异性,反映了微生物对碳源的利用能力,从功能代谢方面表示微生物群落结构功能多样性。由图7可知,随着培养时间的延长,健株和黄萎病株根际土壤微生物活性不断升高,自1 d起AWCD值迅速升高,7 d后趋于稳定状态。进一步对健株和黄萎病株根际土壤微生物的AWCD值分析发现,黄萎病株根际土壤微生物的AWCD值在不同时间均高于健株根际土壤。图7
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7健株与黄萎病株根际土壤微生物AWCD变化
Fig. 7AWCD variation of rhizosphere soil microorganisms of healthy and diseased plants
2.9 健株和黄萎病株根际土壤微生物对碳源利用特征
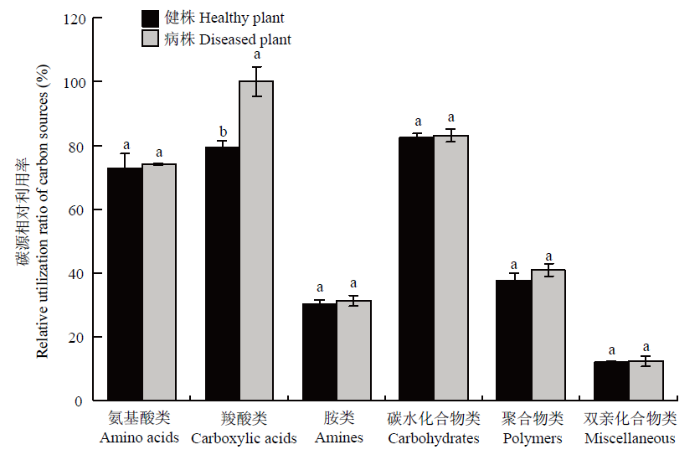
由图8可知,健株和黄萎病株根际土壤微生物对氨基酸类、羧酸类和碳水化合物类物质具有较高的利用率,达到70%以上,其次为聚合物类和胺类,对双亲类化合物的利用率最低。健株根际土壤微生物对氨基酸类、羧酸类、胺类、碳水化合物类、聚合物类和双亲化合物类的相对利用率分别为72.94%、79.63%、30.32%、82.44%、37.66%和12.02%;而黄萎病株根际土壤微生物对上述6类碳源的相对利用率分别为74.09%、100%、31.28%、83.24%、40.99%和12.35%。差异显著性分析表明,黄萎病株根际土壤微生物对羧酸类相对利用率显著高于健株(P<0.05),而对其他5类碳源的相对利用率差异不显著。图8
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图8健株和黄萎病株根际土壤微生物对6类碳源的相对利用率
Fig. 8Relative utilization ratio for six groups of carbon sources by rhizosphere soil microorganisms of healthy and diseased plants
3 讨论
3.1 作物病害发生与根际土壤微生物群落结构的关系
土壤微生物群落不仅是植株根际的重要组成部分,而且在植物生长发育及病害防治方面具有重要作用[20]。土壤微生物多样性是维持土壤生态功能的基础,其对维持土壤健康和抑制植物病害至关重要。吴照祥等[21]认为土壤健康状况不能简单地以真菌群落生物多样性来表示,其他指标(如真菌的组成及丰度)也能够用于指示土壤健康状况。许多研究表明土壤微生物群落多样性和组成与土传病害的发生存在一定的联系,丰富的微生物群落会提高土壤对土传病害的抑制能力,但二者关系比较复杂[22,23]。目前,关于土传病害导致的土壤微生物群落多样性发生改变存在不同的观点。一部分****认为,健株根际土壤中真菌多样性显著高于感病土壤,群落组成发生改变[24,25]。刘海洋等[26]对棉花黄萎病不同发病程度根际土壤真菌群落的研究表明,重病棉田土壤中真菌多样性低于轻病或健康棉田;李忠奎等[27]研究表明,烟草黑胫病和根结线虫病的土壤真菌多样性低于健康烟田,感病烟田土壤病原菌数量增加是导致多样性下降的主要因素之一,并且在根际土壤微生物群落结构方面存在明显差异;SHANG等[28]研究发现,健康兰州百合根际土壤真菌多样性高于根腐病发病土壤,发病根际土壤中除了镰孢菌属丰度较高外,轮枝菌属、丝核菌属、青霉属和Ilyonectria的相对丰度也较高。
另一部分****认为,健株和感病植株的根际土壤真菌多样性差异不显著,但真菌群落组成存在差异[21,29-30]。宋旭红等[29]研究表明,黄连根腐病株及健株根际土壤真菌多样性差异不显著,在病株土壤中子囊菌门、担子菌门和壶菌门的相对丰度显著高于健株土壤,而接合菌门、球囊菌门和Neocallimastigomycota的相对丰度则显著低于健株土壤;在属水平上,病株土壤中镰孢菌属的相对丰度显著高于健株土壤。吴照祥等[21]研究认为,健株和感病植株的根际土壤真菌多样性差异不显著,健株根际土壤中火丝菌属(Pyronema)和被孢霉属的相对丰度显著高于感病植株根际土壤,尖镰孢和周刺座霉属的Volutella colletotrichoides相对丰度则显著低于感病植株土壤。本研究结果表明,马铃薯健株根际和黄萎病发病植株根际土壤真菌多样性差异不显著,健株根际土壤的小不整球壳属、Guehomyces、葡萄穗霉属和曲霉属的相对丰度显著高于感病植株根际土壤,而感病植株根际土壤中轮枝菌属、链格孢属、红酵母属和芽枝霉属的相对丰度显著高于健株土壤。LUAN等[30]报道棉花枯萎病和黄萎病株根际土壤中镰孢菌属和轮枝菌属数量较多,而在健康棉花根际土中木霉属(Trichoderma)等有益菌的数量显著高于发病棉花。本研究通过实时荧光定量PCR和高通量测序发现,马铃薯黄萎病株根际土壤轮枝菌属含量(或相对丰度)显著高于健株根际土壤,相对丰度是健株根际土壤的71.96倍,表明马铃薯黄萎病的发生与轮枝菌属含量的大幅度增加密切相关,获得了与LUAN等[30]相似的研究观点。此外,链格孢属菌群是引起马铃薯早疫病的病原菌,在病株根际土壤中相对丰度显著高于健株土壤;刺盘孢属和镰孢菌属分别是引起马铃薯炭疽病和枯萎病的病原菌,其相对丰度在马铃薯健株与病株根际土壤中差异不显著;而在健株根际土壤中曲霉属的相对丰度显著高于病株根际土壤。由此推测,在拮抗微生物资源筛选方面,可以通过有针对性地筛选曲霉属菌群作为候选拮抗微生物菌群来抑制黄萎病。
此外,少数****研究发现,病株根际土壤真菌多样性高于健株土壤,且真菌群落组成发生改变。脐橙黄龙病株根际土壤真菌多样性高于健康土壤,且菌群中子囊菌门、担子菌门和球囊菌门与病害发生存在显著的相关性[31]。综合分析认为,作物病害发生与根际土壤微生物群落结构关系非常复杂,但均显著改变土壤微生物群落组成,尤其是病株根际土壤中增加了病原菌的数量或相对丰度。
3.2 健株、病株根际土壤养分含量与植物病害发生的关系
陈杰等[15]研究表明,马铃薯连作种植的病株土壤中速效磷和速效钾含量均低于健株土壤,铵态氮含量在健株土壤低于病株土壤,推测可以通过增加磷、钾肥的施用量及同时适当减少氮肥用量来提高马铃薯植株的抗病性,减少土传病害的发生;李忠奎等[27]研究表明,健康烟株根际土壤的有机质、全氮、碱解氮、全磷、有效磷、全钾、速效钾含量显著高于易感病烟田;白霜等[32]研究发现,新疆棉花黄萎病株土壤有机质、可溶性总盐含量均高于健株,棉花病株与健株土壤pH无明显差异,棉花黄萎病病害发生与棉花土壤有机质、可溶性总盐含量密切相关;段春梅等[33]对黄瓜枯萎病株与健株土壤养分进行比较,结果表明病株土壤中的速效磷、速效钾含量分别较健株降低了16.3%和16.8%,病株和健株土壤中速效氮、有机质、水溶性盐分含量及pH无差异,说明土壤速效磷、速效钾含量与黄瓜枯萎病发生有一定关系。本研究结果表明,马铃薯黄萎病的发生与根际土壤速效磷和无机磷含量存在正相关性,病株与健株根际土壤pH无明显差异。进一步推测,健株与病株根际土壤养分与植物病害发生的关系可能因病害和作物种类的不同而存在区别。综合分析健株和病株根际土壤真菌群落组成与土壤养分之间的关系,可为合理施肥改善土壤微生物的群落结构,进而降低土传病害的发生提供科学依据。土壤有机质在维持土壤质量中起着关键作用[34]。SHEN等[35]研究表明,土壤微生物组成与土壤有机质相关性最高,并且土壤有机质与香蕉枯萎病的发生和镰孢菌属的相对丰度存在显著的负相关关系,表明土壤有机质对病害具有潜在抑制作用;LIU等[36]研究表明,土壤中镰孢菌对马铃薯枯萎病及其产量造成影响,镰孢菌属的相对丰度与土壤有机质和总氮量呈负相关关系;LEON等[37]研究表明,土壤有机质含量与菜豆根腐病发生呈负相关关系;DAVIS等[38]研究发现,土壤中有机质含量高能够使马铃薯黄萎病发病率下降。本研究结果表明,马铃薯健株根际土壤真菌群落结构和丰度与有机质、硝态氮含量存在正相关性,而与速效磷和无机磷含量存在负相关性(图6)。因此,通过人工施肥等措施改变根际土壤硝态氮和有机质含量、降低速效磷和无机磷的含量,从而调整病原菌和有益真菌的比例,对今后预防或降低马铃薯黄萎病的发生具有重要意义。
图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6土壤养分含量与真菌属水平群落结构的冗余分析
绿色圆形代表健株根际土壤(1—4),红色圆形代表黄萎病株根际土壤(5—8)
Plectosphaerella:小不整球壳属;Verticillium:轮枝菌属;Fusarium:镰孢菌属;Alternaria:链格孢属;Colletotrichum:刺盘孢属;Mortierella:被孢霉属;Stachybotrys:葡萄穗霉属;Humicola:腐质霉属;Gibberella:赤霉属;Aspergillus:曲霉属;Penicillium:青霉属;Vishniacozyma:维希尼克氏酵母属;Rhodotorula:红酵母属;Cladosporium:芽枝霉属
Fig. 6RDA analysis of nutrient content of rhizosphere soil and fungal communities structure at genus level
The green circle represents rhizosphere soil of healthy plants, the red circle represents rhizosphere soil of diseased plants
3.3 健株与病株根际土壤微生物对碳源利用能力分析
Biolog微生物鉴定方法是测定土壤微生物群落功能代谢多样性的一种有效手段,通过测定不同碳源的平均吸光值,能够反映微生物对各类碳源的利用情况。国内外利用该方法在研究土壤微生物代谢功能多样性方面开展了大量的工作[39,40,41,42]。张丽娟等[43]研究表明,大蒜根腐病株和健株根际土壤微生物对碳源有不同的利用强度,健康大蒜根际土壤微生物对碳水化合物和聚合物类碳源利用能力较高,病株根际土壤微生物对胺类和羧酸类碳源利用程度较高;顾美英等[44]研究核桃健株和腐烂病株根际土壤微生物对碳源的利用情况,结果表明健株土壤微生物对碳水化合物类和氨基酸类碳源有较强的利用能力,而腐烂病株根际土壤微生物利用碳水化合物类、氨基酸类和羧酸类碳源能力下降;吴照祥等[21]研究发现,三七健株和根腐病株根际土壤微生物对碳源利用能力无显著差异。本研究结果表明,与马铃薯健株根际土壤相比,黄萎病株的根际土壤微生物显著提高了对羧酸类碳源的利用能力,而对碳水化合物类、氨基酸类等碳源的利用能力差异不显著。在10个羧酸类碳源方面,健株与病株对碳源利用能力的强弱和种类发生改变,健株根际土壤微生物利用强弱顺序表现为4-羟基苯甲酸>γ-羟基丁酸>D-葡萄胺酸>衣康酸>D-半乳糖内酯>D-半乳糖醛酸>丙酮酸甲酯>D-苹果酸>2-羟基苯甲酸>α-丁酮酸,而病株根际土壤微生物利用强弱顺序表现为4-羟基苯甲酸>γ-羟基丁酸>D-苹果酸>D-半乳糖醛酸>D-葡萄胺酸>2-羟基苯甲酸>D-半乳糖内酯>衣康酸>α-丁酮酸>丙酮酸甲酯,且病株对相同碳源的利用能力强于健株。在6个氨基酸类碳源方面,健株与病株对碳源利用的强弱顺序均表现为L-天冬酰胺酸>L-苯基丙氨酸>L-精氨酸>L-丝氨酸>甘氨酰-L-谷氨酸>L-苏氨酸,两者利用能力差异不显著。在3个胺类碳源方面,健株与病株的利用强弱顺序均表现为N-乙酰-D-葡萄糖胺>苯乙基胺>腐胺,病株利用能力高于健株,但差异不显著。在7个碳水化合物类碳源方面,健株根际土壤微生物利用强弱顺序表现为α-D-乳糖>肝糖>D-纤维二糖>D-甘露醇>i-赤藻糖醇>β-甲基-D-葡萄糖苷>D-木糖,而病株根际土壤微生物利用强弱顺序为D-纤维二糖>α-D-乳糖>肝糖>D-甘露醇>i-赤藻糖醇>D-木糖>β-甲基-D-葡萄糖苷。在3个聚合物类碳源方面,健株根际土壤微生物利用强弱顺序表现为吐温40>吐温80>α-环式糊精,而病株根际土壤微生物利用强弱顺序表现为吐温80>吐温40>α-环式糊精,病株对相同碳源的利用能力强于健株。在2个双亲化合物类碳源方面,健株与病株对碳源利用的强弱顺序均表现为葡萄糖-1-磷酸盐>D, L-α-磷酸甘油。Biolog-ECO技术能快速反映微生物群落功能多样性的变化特征。Biolog-ECO生态板中的31种碳源物质可分为氨基酸类、碳水化合物类、羧酸类、胺类、聚合物类以及双亲化合物类,其中碳水化合物类、氨基酸类和羧酸类物质是植物根系分泌物的主要成分。因此,在借助Biolog方法研究健康与发病植株根际土壤微生物功能多样性时,应将碳源代谢利用情况与植物根系分泌物联系起来,以完善该技术在微生物多样性研究中的应用。已有研究表明,病原菌侵入寄主后会改变植株生理代谢,导致根系分泌物中一些成分及含量发生变化,从而可能导致健康植株与发病植株根际微生物数量、种类以及对碳源的利用能力明显不同[45,46]。本研究采用Biolog技术探明了健株与黄萎病株根际土壤微生物代谢活动的差异,从微生物群落功能多样性方面探索黄萎病发生的可能原因。
此外,已有****发现,通过研究根际土壤微生物群落,为探索土壤中存在拮抗微生物提供了新的线索[47]。本研究通过高通量测序技术分别获得了健株与黄萎病株根际土壤特异的真菌菌群。将不能分离鉴定或平均相对丰度<1%的归为其他,在健株根际土壤特异的菌群包括,Apiotrichum(2.13%)、Cyphellophora(2.66%)、瓣菌属(Exidia,2.66%)、Wardomyces(3.19%)、Setophaeosphaeria(4.26%)、盘菌属(Peziza,4.26%)。在黄萎病株根际土壤特异的菌群包括,Rhizophlyctis(83.83%)和Cystofilobasidium(1.14%)。因此,通过高通量测序了解健株与黄萎病株根际土壤真菌群落结构,从马铃薯健株根际土壤特异菌群中筛选马铃薯黄萎病新的拮抗菌,是筛选生防菌并构建具备拮抗功能的健康马铃薯微生物菌群的潜在资源。
4 结论
黄萎病株根际土壤真菌多样性指数降低,且大丽轮枝菌的数量大于健株根际土壤。健株与黄萎病株根际土壤的优势真菌群落相对丰度对土壤养分的响应不同,健株根际土壤优势群落的相对丰度(如小不整球壳属、Guehomyces、葡萄穗霉属、赤霉属、曲霉属)与硝态氮、有机质和pH呈正相关,黄萎病株根际土壤优势群落的相对丰度(如轮枝菌属、链格孢属、刺盘孢属、被孢霉属、腐质霉属、青霉属、维希尼克氏酵母属、红酵母属和芽枝霉属)与土壤无机磷和速效磷含量呈正相关。健株与病株根际土壤优势群落的相对丰度与土壤养分含量表现相反的趋势。此外,与健株相比,病株根际土壤微生物对羧酸类碳源的利用能力显著提高,最终揭示黄萎病发生与土壤真菌群落结构的互作关系。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
URL [本文引用: 1]
URL [本文引用: 1]
DOI:10.1094/PDIS.2002.86.11.1184URLPMID:30818465 [本文引用: 1]
URLPMID:18943988 [本文引用: 1]
URL [本文引用: 2]
URL [本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 3]
[本文引用: 3]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.3724/SP.J.1006.2013.02055URL [本文引用: 2]
DOI:10.3724/SP.J.1006.2013.02055URL [本文引用: 2]
[本文引用: 1]
URL [本文引用: 1]
URL [本文引用: 1]
[本文引用: 1]
DOI:10.1128/AEM.57.8.2351-2359.1991URLPMID:16348543 [本文引用: 1]

The BLOLOG redox technology based on tetrazolium dye reduction as an indicator of sole-carbon-source utilization was evaluated as a rapid, community-level method to characterize and classify heterotrophic microbial communities. Direct incubation of whole environmental samples (aquatic, soil, and rhizosphere) in BIOLOG plates containing 95 separate carbon sources produced community-dependent patterns of sole-carbon-source utilization. Principal-component analysis of color responses quantified from digitized images of plates revealed distinctive patterns among microbial habitats and spatial gradients within soil and estuarine sites. Correlation of the original carbon source variables to the principal components gives a functional basis to distinctions among communities. Intensive spatial and temporal analysis of microbial communities with this technique can produce ecologically relevant classifications of heterotrophic microbial communities.
[本文引用: 1]
URL [本文引用: 4]
URL [本文引用: 4]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URL [本文引用: 1]
URL [本文引用: 1]
[本文引用: 2]
[本文引用: 2]
DOI:10.1007/s11274-016-2051-2URLPMID:27116961 [本文引用: 1]

Lanzhou lily (Liliumdavidii var. unicolor) is the best edible lily as well as a traditional medicinal plant in China. The microbes associated with plant roots play crucial roles in plant growth and health. However, little is known about the differences of rhizosphere microbes between healthy and wilted Lanzhou lily (Lilium davidii var. unicolor) plants. The objective of this study was to compare the rhizosphere microbial community and functional diversity of healthy and wilted plants, and to identify potential biocontrol agents with significant effect. Paired end Illumina Mi-Seq sequencing of 16S rRNA and ITS gene amplicons was employed to study the bacterial and fungal communities in the rhizosphere soil of Lanzhou lily plants. BIOLOG technology was adopted to investigate the microbial functional diversity. Our results indicated that there were major differences in the rhizosphere microbial composition and functional diversity of wilted samples compared with healthy samples. Healthy Lanzhou lily plants exhibited lower rhizosphere-associated bacterial diversity than diseased plants, whereas fungi exhibited the opposite trend. The dominant phyla in both the healthy and wilted samples were Proteobacteria and Ascomycota, i.e., 34.45 and 64.01 %, respectively. The microbial functional diversity was suppressed in wilted soil samples. Besides Fusarium, the higher relative abundances of Rhizoctonia, Verticillium, Penicillium, and Ilyonectria (Neonectria) in the wilted samples suggest they may pathogenetic root rot fungi. The high relative abundances of Bacillus in Firmicutes in healthy samples may have significant roles as biological control agents against soilborne pathogens. This is the first study to find evidence of major differences between the microbial communities in the rhizospheric soil of healthy and wilted Lanzhou lily, which may be linked to the health status of plants.
[本文引用: 2]
[本文引用: 2]
[本文引用: 3]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URL [本文引用: 1]
[本文引用: 1]
DOI:10.1371/journal.pone.0086610URLPMID:24497959 [本文引用: 1]

BACKGROUND: Previous studies have focused on linking soil community structure, diversity, or specific taxa to disturbances. Relatively little attention has been directed to crop monoculture soils, particularly potato monoculture. Information about microbial community changes over time between monoculture and non-monoculture treatments is lacking. Furthermore, few studies have examined microbial communities in potato monoculture soils using a high throughput pyrosequencing approach. METHODOLOGY/PRINCIPAL FINDINGS: Soils along a seven-year gradient of potato monoculture were collected and microbial communities were characterized using high throughput pyrosequencing approach. Principal findings are as follows. First, diversity (H(Shannon)) and richness (S(Chao1)) indices of bacterial community, but not of fungal community, were linearly decreased over time and corresponded to a decline of soil sustainability represented by yield decline and disease incidence increase. Second, Fusarium, the only soilborne pathogen-associated fungal genus substantially detected, was linearly increased over time in abundance and was closely associated with yield decline. Third, Fusarium abundance was negatively correlated with soil organic matter (OM) and total nitrogen (TN) but positively with electrical conductivity (EC). Fourth, Fusarium was correlated in abundances with 6 bacterial taxa over time. CONCLUSIONS: Soil bacterial and fungal communities exhibited differential responses to the potato monoculture. The overall soil bacterial communities were shaped by potato monoculture. Fusarium was the only soilborne pathogen-associated genus associated with disease incidence increase and yield decline. The changes of soil OM, TN and EC were responsible for Fusarium enrichment, in addition to selections by the monoculture crop. Acidobacteria and Nitrospirae were linearly decreased over time in abundance, corresponding to the decrease of OM, suggesting their similar ecophysiologial trait. Correlations between abundance of Fusarium with several other bacterial taxa suggested their similar behaviors in responses to potato monoculture and/or soil variables, providing insights into the ecological behaviors of these taxa in the environment.
[本文引用: 1]
URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URL [本文引用: 1]
URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URL [本文引用: 1]
