 ,西南大学/中国农业科学院柑桔研究所国家柑桔品种改良中心,重庆400712
,西南大学/中国农业科学院柑桔研究所国家柑桔品种改良中心,重庆400712Function of Citrus Bacterial Canker Resistance-Related Transcription Factor CitMYB20
YAO LiXiao, FAN HaiFang, ZHANG QingWen, HE YongRui, XU LanZhen, LEI TianGang, PENG AiHong, LI Qiang, ZOU XiuPing, CHEN ShanChun ,National Center for Citrus Variety Improvement, Citrus Research Institute, Southwest University/Chinese Academy of Agricultural Sciences, Chongqing 400712
,National Center for Citrus Variety Improvement, Citrus Research Institute, Southwest University/Chinese Academy of Agricultural Sciences, Chongqing 400712通讯作者:
责任编辑: 岳梅
收稿日期:2019-10-13接受日期:2019-11-26网络出版日期:2020-05-16
| 基金资助: |
Received:2019-10-13Accepted:2019-11-26Online:2020-05-16
作者简介 About authors
姚利晓,E-mail:yaolixiao@cric.cn。
范海芳,E-mail:609925006@qq.com。

摘要
关键词:
Abstract
Keywords:
PDF (1174KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
姚利晓, 范海芳, 张庆雯, 何永睿, 许兰珍, 雷天刚, 彭爱红, 李强, 邹修平, 陈善春. 柑橘溃疡病抗性相关转录因子CitMYB20的功能[J]. 中国农业科学, 2020, 53(10): 1997-2008 doi:10.3864/j.issn.0578-1752.2020.10.007
YAO LiXiao, FAN HaiFang, ZHANG QingWen, HE YongRui, XU LanZhen, LEI TianGang, PENG AiHong, LI Qiang, ZOU XiuPing, CHEN ShanChun.
0 引言
【研究意义】柑橘溃疡病(citrus bacterial canker,CBC)是一种由柑橘黄单胞杆菌柑橘亚种(Xanthomonas citri subsp. citri,Xcc)引起的严重威胁我国柑橘产业的检疫性细菌病害[1]。该病可引起柑橘落叶、落果和枯枝,降低果实的商品价值,给柑橘产业带来巨大的经济损失。筛选抗柑橘抗溃疡病关键基因、利用基因工程培育抗病品种是一种从根本上解决柑橘病害的有效途径[2]。【前人研究进展】柑橘转基因抗溃疡病育种有两种策略,一种策略是通过敲除宿主植物的柑橘溃疡病菌敏感基因以防止病原的侵染[3];另外一种方法是在柑橘中过表达外源抗性基因,使用的外源基因主要有昆虫抗菌肽基因[4]、植物抗性基因[5,6,7]和转录因子[8,9,10]等。转录因子通过激活或抑制其他转录因子和下游基因的表达影响植物对病原的敏感性和抗性。R2R3-MYB转录因子家族含有2个保守的MYB结构域,是植物界最大的转录因子家族之一。植物基因组中含有多个R2R3-MYB成员,如地钱中21个、拟南芥中126个、杨树中192个、苹果中222个[11,12,13,14]。MYB基因在植物抵御有害昆虫、真菌、细菌和病毒侵染的过程中具有重要作用。小麦MYB基因TaMYB19、TaMYB29和TaMYB44在蚜虫取食时表达量显著上升,通过调控韧皮部防卫反应抵抗蚜虫的危害[15];而另一种小麦MYB基因TaPIMP2 通过调控植物抗性基因PR1a、PR2、PR5和PR10的表达,对麦根腐平脐蠕孢(Bipolaris sorokiniana)具有抗性[16];杨树MYB115激活原花青素合成基因,增加次生代谢物含量,从而增强植物对杨树溃疡病菌(Dothiorella grefaria)的抗性[17];甜樱桃PacMYBA在拟南芥中异源表达,可增强转基因株系对丁香假单胞菌番茄致病变种(Pseudomonas syringae pv. tomato,Pst)DC3000的抗性[18];SlMYB28被干扰株系中番茄黄化曲叶病毒(Tomato yellow leaf curl virus,TYLCV)含量降低,表明SlMYB28负调控TYLCV的侵染过程[19]。【本研究切入点】柑橘中存在100多个R2R3-MYB转录因子基因[20,21],对其功能研究结果显示,柑橘MYB转录因子调控花青素、木质素、类黄酮和黄酮醇等次生代谢产物的合成[22,23,24,25],在拟南芥中异源表达可增强转基因植株的抗旱性[26]。但尚未见柑橘MYB基因参与生物学胁迫的相关报道。笔者所在实验室前期转录组测序结果表明,柑橘R2R3-MYB基因CitMYB20(Ciclev10005629m)在感染柑橘溃疡病菌的甜橙中上调表达。【拟解决的关键问题】克隆不同柑橘品种CitMYB20基因序列和启动子序列,分析CitMYB20在柑橘溃疡病菌和不同外源植物激素诱导下的表达水平,并构建过表达载体和干扰载体,对转基因株系进行抗病性评价,以期获得新的抗溃疡病相关候选基因。1 材料与方法
试验于2017年3月至2019年3月在国家柑桔品种改良中心完成。1.1 材料与试剂
供试枣阳小叶枳(Poncirus trifoliata)、金弹金柑(Fortunella japonica)和晚锦橙(Citrus sinensis)均取自国家果树种质重庆柑橘圃。柑橘溃疡病菌由中国农业科学院柑桔研究所胡军华博士提供。根癌农杆菌(Agrobacterium tumefaciens)感受态细胞EHA105、植物表达载体pLGNe、抑制表达中间载体pUCRNAi由笔者实验室制备和保存。大肠杆菌(Escherichia coli)感受态细胞DH5α、T克隆载体pGEM-T Easy、DNaseI(RNase-free)和反转录试剂盒购自TaKaRa公司。植物总RNA提取试剂盒和植物DNA快速提取试剂盒购自北京艾德莱生物科技有限公司。NovoStart? SYBR qPCR SuperMix Plus试剂盒购自NovoProtein公司。1.2 CitMYB20基因序列克隆
按照植物RNA快速提取试剂盒说明书提取晚锦橙、枣阳小叶枳和金弹金柑成熟叶片的总RNA,进行反转录获得cDNA模板。使用CitMYB20-F和CitMYB20-R引物对(表1),在高保真酶PrimeSTAR? Max DNA Polymerase作用下,94℃ 3 min;94℃ 30 s,58℃ 30 s,72℃ 1 min,35个循环;72℃延伸3 min获得PCR产物。经加poly(A)尾,胶纯化回收等操作后,将PCR产物与T载体连接,热击法转化感受态DH5α。挑选菌液PCR检测阳性的克隆送至擎科生物技术有限公司测序。Table 1
表1
表1所用引物序列
Table 1
| 引物名称 Primer name | 用途 Amplification | 引物序列 Primer sequence (5′-3′) | 备注 Remark |
|---|---|---|---|
| CitMYB20-F | 克隆基因开放阅读框 To clone ORF sequence | ATAGGATCCATGGGGAGGGCTCCCTG | BamH I酶切位点 BamH I restriction site |
| CitMYB20-R | CCGGAATTCCTAAAATGGTAATGTTAATGAGTCTGC | EcoR I酶切位点 EcoR I restriction site | |
| CitMYB20-F (g) | 克隆基因抑制表达片段 To clone RNAi fragment sequence | GCTCTAGAGGCGCGCCAAGCAAAACCAGAAGGCC | Xba I和Asc I酶切位点 Xba I and Asc I restriction site |
| CitMYB20-R (g) | CGCGGATCCATTTAAATACCGATCGCAAATTCATTAAG | BamH I和Sma I酶切位点 BamH I and Sma I restriction site | |
| CitMYB20-F (P) | 克隆启动子 To clone promoter sequence | CCAGAACCTTTACTTTAATTTCTATTTTTA | |
| CitMYB20-R (P) | CTTGTTAATTTCTTTCAAAGTAGTGGAG | ||
| CitMYB20-F (q) | 检测CitMYB20的表达量 To detect CitMYB20 expression | CTCCTCGGTCACTACTGGAGA | |
| CitMYB20-R (q) | CATTAAGGCCGCCTCCGAAA | ||
| actin-f | 检测actin的表达量 To detect actin expression | CATCCCTCAGCACCTTCC | |
| actin-r | CCAACCTTAGCACTTCTCC | ||
| 35S-F | 鉴定过表达植株 To identify over-expressed plant | GGAGTCAAAGATTCAAATAGAGGACCTAAC | |
| CitMYB20-R (OE) | TGACCACCTATTCCCCAACATGT | ||
| CitMYB20-F (RNAi) | 鉴定RNAi抑制表达植株 To identify RNAi plant | ATTTGCGATCGGTATTTAAATGTGTAA | |
| CitMYB20-R (RNAi) | GCTAGCCAGGATCCAAATACCTGCAAA |
新窗口打开|下载CSV
1.3 CitMYB20启动子序列克隆
利用植物DNA快速提取试剂盒从枣阳小叶枳和金弹金柑成熟叶片中提取DNA。PCR扩增体系同1.2,所用引物对为CitMYB20-F (P)和CitMYB20-R (P)(表1),退火温度为53℃, 延伸时间为1.5 min。利用PlantCARE(1.4 柑橘溃疡病菌和外源激素对CitMYB20的诱导表达
选取枣阳小叶枳和金弹金柑的成熟叶片,在75%的酒精中消毒3—5 s,再用无菌水清洗3次,无菌脱脂棉将叶片擦干,无菌针在叶片表面刺孔形成伤口,在伤口处接种柑橘溃疡病菌,对照组用水处理,28℃培养0、1、3、5 d,切取接种孔附近的叶片提取RNA。用打孔器在消毒叶片上打孔,取叶圆片分别浸泡在10 μmol·L-1水杨酸溶液、100 μmol·L-1茉莉酸甲酯溶液、10 μmol·L-1乙烯利溶液中,无菌水处理做对照。28℃下处理0、12、24、36、48 h收集叶圆片。
1.5 实时荧光定量PCR(qRT-PCR)分析
qRT-PCR利用NovoStart? SYBR qPCR SuperMix Plus试剂盒,所用引物为CitMYB20-F (q)和CitMYB20-R (q),反应体系为12 μL,在ABI 7500荧光定量PCR仪上进行。反应条件为95℃ 1 min;95℃ 15 s,60℃ 1 min,40个循环。采用2-ΔΔCt(ΔCt=CtCitMYB20- Ctactin)方法计算相对表达量。1.6 过表达载体构建
对连接有CitMYB20的T载体和植物双元表达载体pLGNe进行BamH I和EcoR I双酶切,胶回收酶切产物,16℃连接过夜,将连接产物pLGNe-CitMYB20转化感受态DH5α。随机挑选克隆经PCR、双酶切验证正确后,提取过表达载体pLGNe-CitMYB20,利用电转化法转化感受态农杆菌EHA105备用。1.7 干扰载体构建
利用引物对CitMYB20-F (g)和CitMYB20-R (g)(表1)扩增CitMYB20基因干扰片段。利用限制性内切酶Asc I和Swa I对PCR产物和中间载体pUCRNAi进行双酶切,连接后将干扰片段正向插入中间载体生成pUCRNAi1-CitMYB20。将干扰片段的PCR产物和pUCRNAi1-CitMYB20同时用BamH I和Xba I双酶切,连接后将干扰片段反向插入,构建载体pUCRANi- CitMYB20。将pUCRANi-CitMYB20和pLGNe用Kpn I和Sal I双酶切,将干扰片段插入pLGNe成功构建干扰载体pLGNe-pUCRANi-CitMYB20,并转入根癌农杆菌EHA105备用。1.8 根癌农杆菌法转化和转基因植株检测
在无菌条件下去掉枣阳小叶枳种子表皮,放置于MS固体培养基上28℃暗培养。待外植体生长到一定高度后,将上胚轴切成1 cm左右的茎段。将含有过表达载体pLGNe-CitMYB20的根癌农杆菌菌液和含有干扰载体pLGNe-pUCRANi-CitMYB20的根癌农杆菌菌液及空载体菌液分别侵染上胚轴茎段。用无菌滤纸将茎段擦干,摆放在MS固体共培养基上,置于26℃培养箱中暗培养3 d,转到MS固体筛选培养基中,28℃暗培养7 d左右,随后转移至光照培养箱中28℃培养。待新芽长出后,切取少量组织进行GUS染色。将GUS染色阳性芽嫁接到网室的资阳香橙砧木上。提取转基因植株叶片DNA进行PCR检测,验证CitMYB20是否整合到柑橘基因组中。提取转基因植株叶片RNA进行qRT-PCR,检测CitMYB20的表达量。1.9 柑橘溃疡病抗性评价
按照贾瑞瑞等[27]的描述,采用体外接种法对转基因植株进行柑橘溃疡病抗性评价。从每株转基因植株上选取9张叶片,在75%的酒精中消毒3—5 s,再用无菌水清洗3次,无菌脱脂棉擦干叶片,用无菌针在叶片表面刺14—20个小孔,移液器吸取1 μL柑橘溃疡病菌悬浮液(1×105 cfu/mL)滴加在小孔处。对照组为非转基因植株,28℃条件下培养10 d,拍照,用ImageJ Launcher软件统计病斑面积。Excel处理数据,SPSS 20进行差异显著性分析,P<0.05表示差异显著。2 结果
2.1 不同品种CitMYB20基因序列和启动子序列的克隆与分析
分别从晚锦橙、枣阳小叶枳和金弹金柑中扩增CitMYB20的开放阅读框序列,在GenBank数据登录号为MN689607、MN689608和MN689609。序列分析结果表明,来源于上述3个柑橘品种的CitMYB20的核苷酸序列相似度为98.60%,氨基酸序列相似度为97.64%,表明该基因在不同柑橘品种间序列差异甚微。分别从枣阳小叶枳和金弹金柑中克隆CitMYB20起始密码子上游1 500 bp的启动子序列(GenBank数据登录号为MN689610和MN689611),其核苷酸序列的相似性为95.16%。软件预测结果显示,该基因在抗性品种金弹金柑和易感品种枣阳小叶枳中都含有参与植物激素应答相关的顺式作用元件ABRE(响应脱落酸)、CGTCA-motif(响应茉莉酸甲酯)、TGACG-motif(响应茉莉酸甲酯)。但是金弹金柑中含有TCA-element(响应水杨酸)而枣阳小叶枳中缺少这一元件(表2)。
Table 2
表2
表2CitMYB20启动子顺式作用元件
Table 2
| 元件名称 Motif name | 序列 Sequence | 位置Position | 方向Strand | 功能 Function | ||
|---|---|---|---|---|---|---|
| 枣阳小叶枳 P. trifoliata | 金弹金柑 F. japonica | 枣阳小叶枳 P. trifoliata | 金弹金柑 F. japonica | |||
| ABRE | ACGTG | -1198 | -1190 | + | + | 脱落酸响应 Abscisic acid responsiveness |
| CGTCA-motif | CGTCA | -321 | -321 | - | - | 茉莉酸甲酯响应 Methyl jasmonate responsiveness |
| -316 | + | |||||
| TGACG-motif | TGACG | -321 | -321 | + | + | |
| -316 | - | |||||
| TCA-element | CCATCTTTTT | -1452 | - | 水杨酸响应 Salicylic acid responsiveness | ||
| -1390 | + | |||||
新窗口打开|下载CSV
2.2 柑橘溃疡病菌对CitMYB20的诱导表达
取易感品种枣阳小叶枳和抗性品种金弹金柑的成熟离体叶片接种柑橘溃疡病菌菌液。qRT-PCR结果表明易感品种枣阳小叶枳在接种柑橘溃疡病菌0、1、3和5 d时,CitMYB20的表达量与对照相比无明显变化;而抗性品种金弹金柑CitMYB20的相对表达量则上调幅度明显,尤其是在接种柑橘溃疡病菌5 d时表达量为对照的2.5倍(图1)。图1
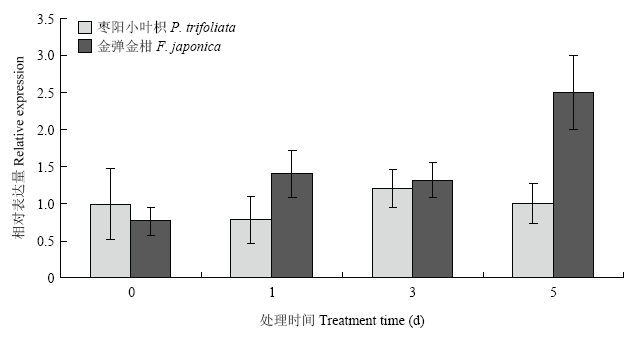 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1柑橘溃疡病菌对CitMYB20的诱导表达
Fig. 1The expression of CitMYB20 induced by Xcc
2.3 激素对CitMYB20的诱导表达
CitMYB20对不同外源激素的应答反应不尽相同。其中,水杨酸和茉莉酸甲酯对CitMYB20诱导表达结果相似,在两种激素处理后,金弹金柑中CitMYB20的相对表达量均呈现出先升高后降低的趋势,处理24 h相对表达量最高;而枣阳小叶枳中该基因的相对表达量均为先降低后升高,处理24 h相对表达量最低。乙烯利处理后,CitMYB20的表达量在枣阳小叶枳中12 h达到最高值,之后表达量降低;而在金弹金柑中其表达量呈现缓慢下降的趋势(图2)。图2
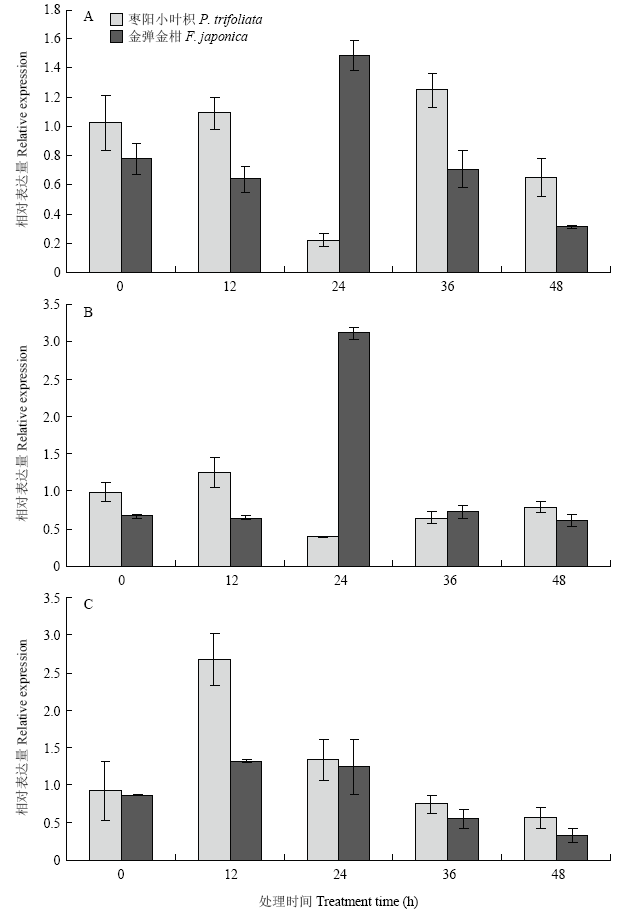 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2不同激素对CitMYB20的诱导表达分析
A:10 μmol·L-1水杨酸Salicylic acid;B:100 μmol·L-1 茉莉酸甲酯Methyl jasmonate;C:10 μmol·L-1 乙烯利Ethephon
Fig. 2Expression analysis of CitMYB20 induced by different hormones
2.4 CitMYB20过表达和干扰植株抗性分析
经菌液PCR检测、质粒BamH I/EcoR I双酶切鉴定和测序结果分析,确认CitMYB20的开放阅读框成功插入pLGNe载体,获得长度为13.47 kb的pLGNe- CitMYB20植物过表达载体。另外选取CitMYB20的特异性片段,将抑制表达片段分别正、反向连接到pUCRNAi上得到中间载体pUCRNAi-CitMYB20,随后连接到pLGNe载体上,经Kpn I/Sal I双酶切验证和测序分析,获得长度为14.80 kb的pLGNe-pUCRNAi- CitMYB20干扰载体。利用根癌农杆菌法转化枣阳小叶枳上胚轴,将GUS染色呈蓝色的阳性芽嫁接到无菌的晚锦橙实生苗上。待嫁接芽抽出新芽后,将试管苗嫁接到网室中两年生的资阳香橙砧木上。提取嫁接苗叶片DNA,PCR法再次检测转基因阳性植株,共获得7株CitMYB20过表达株系,分别命名为OE-1、OE-4、OE-5、OE-6、OE-8、OE-9、OE-10;5株CitMYB20干扰株系,分别命名为R-3、R-8、R-9、R-10和R-11。qRT-PCR结果显示,在过表达植株中,CitMYB20显著上调表达,最高表达倍数达207倍(图3);在干扰植株中,CitMYB20的表达显著下调,最低仅为对照表达量的12%(图4)。
图3
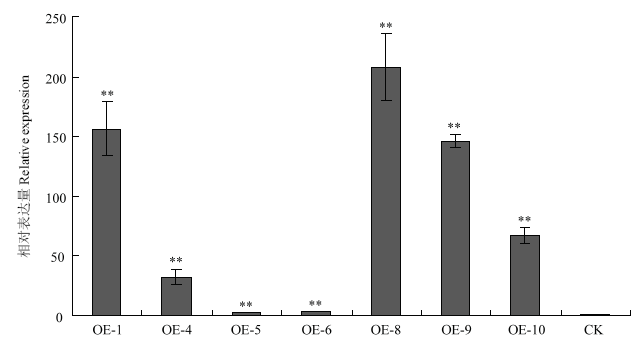 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3过表达植株的CitMYB20相对表达量检测
CK:非转基因植株Non-transgenic plant;OE:过表达植株Over-expressed plant;**:P<0.01。下同The same as below
Fig. 3Detection of CitMYB20 relative expression level in over-expressed plants
图4
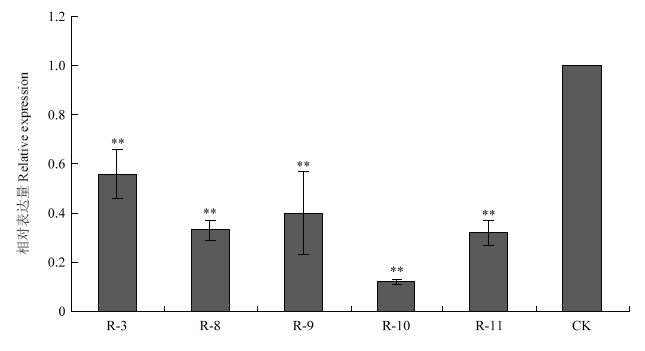 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4RNAi植株的CitMYB20相对表达量检测
R:RNAi植株RNAi plant。下同The same as below
Fig. 4Detection of CitMYB20 relative expression level in RNAi plants
与非转基因植株的表型相比,枣阳小叶枳CitMYB20过表达植株和干扰植株的株高和叶片大小未观测到明显的变化。选取生长状态一致的过表达植株叶片,采用针刺法接种柑橘溃疡病病菌,10 d后对其拍照统计病斑面积。结果显示,过表达植株叶片的病斑面积均小于非转基因植株叶片的病斑面积,其中OE-1、OE-6、OE-10这3株转基因材料的病斑面积与非转基因植株相比存在显著差异,病斑面积减少25%左右(图5)。RNAi抑制CitMYB20表达植株叶片的病斑面积均大于非转基因植株叶片的病斑面积,其中R-9转基因植株的病斑面积与非转基因植株相比存在显著差异,病斑面积增加约40%(图6)。
图5
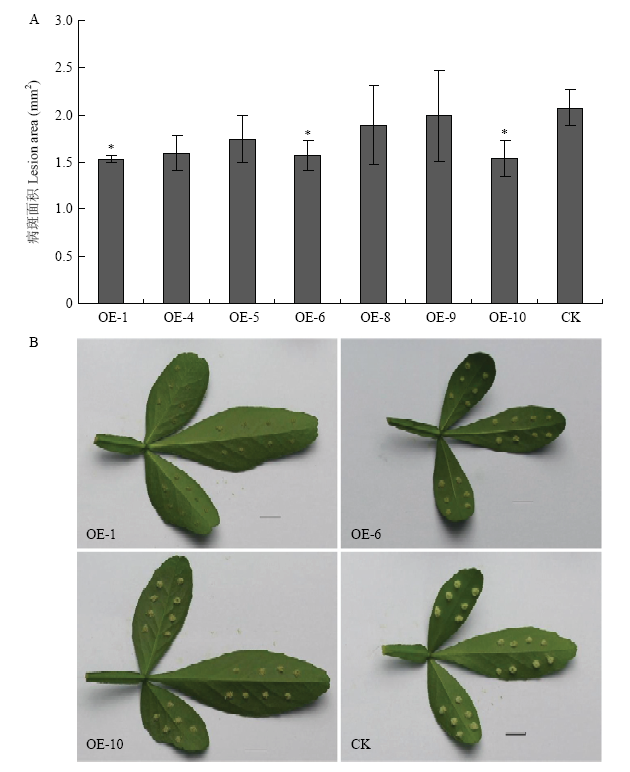 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5过表达CitMYB20植株的柑橘溃疡病抗性评价
A:病斑面积(*:P<0.05)Lesion area (*: P<0.05);B:病斑观察Lesion observation
Fig. 5Evaluation of citrus bacterial canker resistance in over-expressed CitMYB20 plants
图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6 CitMYB20抑制表达植株的柑橘溃疡病抗性评价
A:病斑面积(*:P<0.05)Lesion area (*: P<0.05);B:病斑观察Lesion observation
Fig. 6Evaluation of citrus bacterial canker resistance in plants inhibiting CitMYB20 expression
3 讨论
CitMYB20(Ciclev10005629m)是XIE等[20]从柑橘基因组数据库中鉴定出的众多R2R3-MYB类转录因子基因之一。范海芳等[28]曾从晚锦橙、酸柚、纽荷尔脐橙和四季橘中克隆CitMYB20基因序列,其序列相似度在核酸水平达到99%。本试验克隆了枣阳小叶枳和金弹金柑CitMYB20基因序列,与晚锦橙CitMYB20相比,在核苷酸水平上相似度也达99%,表明CitMYB20在柑橘不同品种间高度保守。受柑橘溃疡病菌侵染后,CitMYB20在抗性品种四季橘中上调表达3倍,在易感品种纽荷尔脐橙中无差异表达[28]。本研究以柑橘溃疡病抗性品种金弹金柑和易感品种枣阳小叶枳作为材料,分析柑橘溃疡病菌侵染不同时间基因表达量的变化,也发现CitMYB20在感染柑橘溃疡病菌的抗性品种中显著上调表达,同样在感染柑橘溃疡病菌的易感品种中未发现显著差异表达。本研究中,CitMYB20过表达植株在接种柑橘溃疡病菌10 d后的病斑面积与对照植株相比明显减小(图5),抑制表达植株在接种柑橘溃疡病病菌后病斑面积与对照植株相比明显增加(图6),表明CitMYB20能够抵抗柑橘溃疡病菌侵染,在抗溃疡病途径中起到正调控作用。然而,转基因植株对柑橘溃疡病的抗性与CitMYB20的表达量之间没有观测到明显的相关性。在已报道的转基因植物研究中也有类似现象,即基因的过表达或抑制表达程度与转基因植株的抗性和敏感性之间不存在直接关系,或具有相同或相似表达水平的转基因植株对病原的抗性程度不等[8,29-30]。这可能与外源基因在植物基因组上插入位点相关,影响基因组上其他基因的表达从而影响其抗性;也可能与外源基因在翻译和翻译后水平的调控相关,虽然外源基因表达水平甚高,但在转基因植物中其蛋白的表达并不一定很高,因而外源基因的过表达量达到抗性水平时,更高的基因表达量也不会进一步增加转基因植物的抗性。
R2R3-MYB转录因子通过调控植物体内多种途径抵抗细菌病原的危害。研究显示AtMYB30是过敏性细胞死亡的正向调控因子,在拟南芥和烟草中过表达AtMYB30,转基因植株对不同的病原细菌表现过敏反应(hypersensitive response,HR)或类似过敏反应,并能增强对丁香假单胞杆菌番茄致病变种的抗性;在反义表达AtMYB30的拟南芥株系中,能强烈抑制对病原细菌的抗性和HR细胞死亡反应,HR和防卫反应相关基因的表达也发生改变[31]。偃麦草R2R3-MYB基因TiMYB2R-1的异源表达可增强转基因小麦对全蚀病菌(Gaeumannomyces graminis)的抗性[32]。小麦R2R3-MYB基因TaPIMP1在烟草中的异源表达则表现出对青枯病菌(Ralstonia solanacearum)的抗性[33]。CitMYB20与拟南芥同源基因的进化树分析显示,该基因与拟南芥R2R3-MYB类转录因子AtMYB15的同源性最高[28],前人研究表明AtMYB15能够参与木质素的生物合成和植物的基础免疫调节,增强对丁香假单胞杆菌番茄致病变种DC3000的抗性[34],由此推测CitMYB20可能通过植物次生代谢物的合成在柑橘溃疡病菌侵染过程中发挥抵抗作用。
水杨酸[35]、茉莉酸[36]等植物激素能够参与植物抗病途径的信号传导,在植物抵御病菌侵染的过程中发挥重要作用。目前关于R2R3-MYB转录因子通过激素信号通路参与病菌响应的研究也有报道,如AtMYB44能够通过水杨酸信号通路抵抗丁香假单胞杆菌番茄致病变种的侵染[37]。拟南芥茉莉酸合成突变体coi1受灰霉病菌(Botrytis cinerea)侵染后,拟南芥R2R3-MYB基因BOS1的诱导表达被延迟且表达增幅减少,提示病菌对BOS1的诱导表达与茉莉酸信号通路之间存在相关性[38]。本研究使用外源激素水杨酸、茉莉酸甲酯和乙烯利分别对CitMYB20进行诱导表达,结果显示该基因在金弹金柑和枣阳小叶枳中对水杨酸和茉莉酸甲酯呈现出相反的诱导表达方式(图2),推测该基因的抗病途径可能受到水杨酸和茉莉酸甲酯的诱导。另外,笔者在分析启动子顺式作用元件时发现,与金弹金柑相比,枣阳小叶枳CitMYB20的启动子序列中缺少水杨酸响应的功能元件TCA-element(表2)。有研究显示启动子序列的缺失会严重影响基因表达的变化[39]。笔者的研究结果也显示,在受外源水杨酸诱导时,枣阳小叶枳中CitMYB20在一定时期表达量不增反降(图2)。枣阳小叶枳对柑橘溃疡病抗性的丧失是否因为TCA-element元件的缺失从而失去水杨酸诱导抗性基因表达的能力,有待于进一步研究。
4 结论
CitMYB20编码序列在不同柑橘品种间非常保守,但启动子序列在柑橘溃疡病抗性品种金弹金柑和敏感品种枣阳小叶枳之间存在差异。CitMYB20在金弹金柑中受柑橘溃疡病菌和外源激素水杨酸、茉莉酸甲酯的诱导表达。过表达CitMYB20柑橘株系对柑橘溃疡病的抗性得到明显提高,抑制CitMYB20表达的植株则降低了对柑橘溃疡病的抗性,推测该基因在柑橘抗溃疡病途径中具有重要作用。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.plaphy.2017.01.015URL [本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.plaphy.2018.06.034URL [本文引用: 1]
DOI:10.3864/j.issn.0578-1752.2017.13.008URL [本文引用: 1]

【Objective】 The objective of this study is to annotate the BZIP family and clone the citrus canker related transcription factor CsBZIP40. It is also aimed to confirm the subcellular localization and the expression profile reduced by exogenous hormone and mechanical damage and the relations between CsBZIP40 and Xanthomonas citri subsp. citri (Xcc) infection. 【Method】Based on the public genome databases, the BZIP gene family was expertly and comprehensively annotated and named based on the chromosomal localization of all the members of BZIP; the motifs of the BZIPs were analyzed by MEME online tool. the phylogenetic tree of BZIPs in Citrus and Arabidopsis thaliana was constructed using software Mega 6.0 based on which the category of BZIP family was obtained. Canker-related transitional factor CsBZIP40 obtained from transcriptome data was also detected by qRT-PCR. Elements in the promoter and the nuclear localization signal were analyzed with database plantCARE and online tool cNLSmapper, respectively. And then the subcellular localization was confirmed by GFP fusion experiments in onion to confirm the prediction of nuclear localization analyzed with softwares. Expression profiles induced by salicylic acid (SA), jasmonic acid methyl ester (MeJA), ethylene (ET) and mechanical damage of CsBZIP40 were checked with qRT-PCR. 【Result】A total of 47 BZIP genes were annotated from the whole genome of Citrus sinensis and all the BZIPs are located on every chromosome except the 9th one. The BZIP concentration on chromosome 3 are 4.5×10-7/Mb which is the highest while chromosome 2 is the lowest, contains only 2% of all BZIPs in citrus. There were fewer gene duplication events detected from BZIP family of citrus compared with other plants, such as Arabidopsis, grapevine and so on. That is why citrus has a smaller BZIP family size. The full-length of CsBZIP40 is 5 756 bp with a 1 530 bp open reading frame which codes a protein containing 509 amino acids. It is closely related to AT1g08320 in A. thaliana based on the evolutionary analysis. In citrus, the BZIPs have been annotated which can be divided into 10 different sub-families. CsBZIP40 belongs to sub-family D, which is always take part in the pathogen resistance in plants. The gene promoter contains multiple cis involved in plant adversity or hormone response, such as Box-W1, HSE, ERE and so on. Subcellular localization results confirmed the prediction of protein localization in nucleus. Based on the qPCR data, the exogenous salicylic acid cannot induce the different expression of CsBZIP40, in contrast, jasmonic acid methyl ester, mechanical damage and ethylene can induce significant differences in gene expression level. Xcc attack can significantly increase the expression level of CsBZIP40 in Calamondin but no difference in Newhall navel orange. 【Conclusion】CsBZIP40 would be an important transcription factor which is closely associated with the resistance of citrus canker. This gene should be a potential candidate in the molecular breeding to improve the canker resistance of citrus.
DOI:10.3864/j.issn.0578-1752.2017.13.008URL [本文引用: 1]

【Objective】 The objective of this study is to annotate the BZIP family and clone the citrus canker related transcription factor CsBZIP40. It is also aimed to confirm the subcellular localization and the expression profile reduced by exogenous hormone and mechanical damage and the relations between CsBZIP40 and Xanthomonas citri subsp. citri (Xcc) infection. 【Method】Based on the public genome databases, the BZIP gene family was expertly and comprehensively annotated and named based on the chromosomal localization of all the members of BZIP; the motifs of the BZIPs were analyzed by MEME online tool. the phylogenetic tree of BZIPs in Citrus and Arabidopsis thaliana was constructed using software Mega 6.0 based on which the category of BZIP family was obtained. Canker-related transitional factor CsBZIP40 obtained from transcriptome data was also detected by qRT-PCR. Elements in the promoter and the nuclear localization signal were analyzed with database plantCARE and online tool cNLSmapper, respectively. And then the subcellular localization was confirmed by GFP fusion experiments in onion to confirm the prediction of nuclear localization analyzed with softwares. Expression profiles induced by salicylic acid (SA), jasmonic acid methyl ester (MeJA), ethylene (ET) and mechanical damage of CsBZIP40 were checked with qRT-PCR. 【Result】A total of 47 BZIP genes were annotated from the whole genome of Citrus sinensis and all the BZIPs are located on every chromosome except the 9th one. The BZIP concentration on chromosome 3 are 4.5×10-7/Mb which is the highest while chromosome 2 is the lowest, contains only 2% of all BZIPs in citrus. There were fewer gene duplication events detected from BZIP family of citrus compared with other plants, such as Arabidopsis, grapevine and so on. That is why citrus has a smaller BZIP family size. The full-length of CsBZIP40 is 5 756 bp with a 1 530 bp open reading frame which codes a protein containing 509 amino acids. It is closely related to AT1g08320 in A. thaliana based on the evolutionary analysis. In citrus, the BZIPs have been annotated which can be divided into 10 different sub-families. CsBZIP40 belongs to sub-family D, which is always take part in the pathogen resistance in plants. The gene promoter contains multiple cis involved in plant adversity or hormone response, such as Box-W1, HSE, ERE and so on. Subcellular localization results confirmed the prediction of protein localization in nucleus. Based on the qPCR data, the exogenous salicylic acid cannot induce the different expression of CsBZIP40, in contrast, jasmonic acid methyl ester, mechanical damage and ethylene can induce significant differences in gene expression level. Xcc attack can significantly increase the expression level of CsBZIP40 in Calamondin but no difference in Newhall navel orange. 【Conclusion】CsBZIP40 would be an important transcription factor which is closely associated with the resistance of citrus canker. This gene should be a potential candidate in the molecular breeding to improve the canker resistance of citrus.
[本文引用: 3]
[本文引用: 3]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1105/tpc.014167URL [本文引用: 1]
[本文引用: 1]
