 ,1,21
,1,21 2
Effects of Different Root Exudates on Soil N2O Emissions and Isotopic Signature
ZHUANG Shan1, LIN Wei1, DING JunJun1, ZHENG Qian1, KOU XinYue1, LI QiaoZhen1, LI YuZhong ,1,2
,1,2通讯作者:
责任编辑: 李云霞
收稿日期:2019-08-2接受日期:2019-11-6网络出版日期:2020-05-16
| 基金资助: |
Received:2019-08-2Accepted:2019-11-6Online:2020-05-16
作者简介 About authors
庄姗,Tel:18519190629;E-mail:489300802@qq.com。

摘要
关键词:
Abstract
Keywords:
PDF (594KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
庄姗, 林伟, 丁军军, 郑欠, 寇馨月, 李巧珍, 李玉中. 不同根系分泌物对土壤N2O排放及同位素特征值的影响[J]. 中国农业科学, 2020, 53(9): 1860-1873 doi:10.3864/j.issn.0578-1752.2020.09.013
ZHUANG Shan, LIN Wei, DING JunJun, ZHENG Qian, KOU XinYue, LI QiaoZhen, LI YuZhong.
0 引言
【研究意义】N2O是一种重要的温室气体,能够破坏平流层中的臭氧层且参与大气中光化学反应,从而进一步加剧温室效应[1]。土壤是N2O的主要排放源[1,2],约占全球N2O排放总量的65%[3]。自20世纪初合成氨技术发明以来,氮肥被大量的投入到农业生产中,在增加作物产量的同时也使得N2O排放量增加了19%[4,5,6]。而菜地具有高水、高肥、肥料利用率低等特点[7],造成了N2O的大量排放。因此研究菜地N2O排放对减缓全球变暖具有重要的理论和现实意义。【前人研究进展】硝化作用和反硝化作用是N2O产生的主要过程[8]。传统的研究方法主要有乙炔抑制法和同位素标记法等,这些方法在实际操作过程中都有一些局限性,如抑制剂(乙炔)和标记物在系统中扩散不均,易受系统干扰等[9,10]。而自然丰度条件下的N2O同位素位嗜值(SP值)不受N2O前体同位素的影响,进而克服土壤与N作用的环境阻碍而直接评价N2O产生的微生物过程,是估测N2O来源及贡献的一种有效方法[11]。尽管N2O的δ15Nbulk和δ18O值易受底物同位素影响,但也能够在一定程度上反应微生物过程,其与SP值相结合进行分析有助于更加全面了解N2O的排放行为。N2O排放的影响因素很多,其中对土壤孔隙含水量(WFPS)[12,13]、pH[14]、O2浓度[15]、温度[16,17,18]等研究较多。然而,植物通过根系分泌物对N2O的产生也具有重要的影响。植物净光合固定的碳5%—25%通过根系分泌物流失到土壤中[19,20,21],并以有机酸,氨基酸,糖类和其他类型分子的形式释放,是土壤重要的碳源,影响土壤硝化和反硝化作用[22,23];根系分泌物还可以作为电子供体还原NO- 3和NO- 2[24],改变N2O产生的电子传递途径;还可通过改变根际周围微生物的群落结构影响N2O的产生[25]。草酸、丝氨酸、葡萄糖分别是根系分泌物中普遍存在并且含量较多的一种低分子有机酸、氨基酸、糖类[26],它们在N2O产生过程中的作用机理存在一定差异。研究发现草酸显著促进土壤中有机态氮和有机无机复合态氮的矿化速度,促进氮的释放[27]。也有研究表明草酸促进N2O还原基因的复制,对N2O的还原产生影响[28]。葡萄糖也能够促进氮的分解,但这种促进作用明显小于草酸,此外,优先利用葡萄糖的微生物可能导致氮分解率下降[28]。丝氨酸不仅是细胞蛋白质的重要组成部分,也是微生物的能量来源,在土壤中可以矿化为NH+ 4,改变土壤的C/N和pH[29]。【本研究切入点】不同植物根系分泌物的组成和含量不同,大量研究表明N2O的排放与根系分泌物的组成和含量有关[24-25,29-30],但鲜有系统研究不同组分单独作用对N2O产生过程的影响。又由于根系分泌物组成和土壤环境的复杂,在自然条件下很难做到定量提取研究[31],所以通过模拟根系分泌物组分和含量制备根系分泌物溶液添加到土壤中是研究根系分泌物作用下土壤N2O排放特征的重要方法。尽管实验室环境能够控制土壤温度、湿度等环境因子以减少环境差异对N2O排放的影响,但添加根系分泌物后微生物对各根系分泌物的相互作用和响应时间是影响N2O排放量的关键,N2O排放的时空变异性对定量分析不同根系分泌物组分对N2O产生作用的影响仍无法排除。因此研究不同根系分泌物组分对土壤N2O排放的影响,同时确定合理的气体取样时间,对于提高试验效率,深入研究根系分泌物对N2O产生在时间和空间上的作用至关重要。【拟解决的关键问题】通过对根系分泌物中3种主要组分(有机酸、丝氨酸、糖类)的主要成分(草酸、丝氨酸、葡萄糖)作用下土壤N2O排放和同位素特征的综合分析,揭示不同根系分泌物对土壤N2O产生过程的影响及其作用机制,为选择适宜的植物进而减缓土壤N2O排放提供支撑。1 材料与方法
1.1 土壤采集
土壤采集于中国农业科学院农业环境与可持续发展研究所顺义实验基地,东经40°05′2″,北纬116°55′19″。基地为典型的暖温带半湿润大陆性季风气候,年平均日照2 684 h,年平均气温12.5℃,年平均降水量623.5 mm,无霜期195 d左右。土壤类型为潮褐土,土壤密度1.40 g·cm-3,有机质16.48 g·kg-1,全氮1.10 g·kg-1, 全磷0.68 g·kg-1,全钾20.31 g·kg-1,pH 8.7。土壤采集于2018年8月(雨后2 d),取0—20 cm表层土壤于阴凉处自然风干。除去砂石、植物根系等杂质,过2 mm筛,室内保存备用。
1.2 试验方法
选择3种代表性有机物,即草酸代表有机酸,丝氨酸代表氨基酸,葡萄糖代表糖类,以此为研究对象。依据白菜根系分泌速率[26],设置2个添加水平:150 μg C·d-1(低浓度)和300 μg C·d-1(高浓度),另添加蒸馏水为空白对照,共7个处理,每次添加溶液体积为0.85 mL,每种处理设3次重复。用120 mL密封性良好的血清瓶作培养瓶,称取40 g风干土放入120 mL血清瓶中,共21个。用蒸馏水调节土壤孔隙含水量(WFPS:water-filled-pore-space)至75%左右(称重法),放入25℃恒温培养箱黑暗预培养。根据培养前重量每4天补充一次水分(日蒸发量约为0.85 mL),共补充3次。第12天补充水分的同时施入(NH 4)2SO4 溶液(300 mgN·kg-1干土,足量氮使微生物对根系分泌物敏感,且使土壤产生的N2O同位素丰度在仪器测结果最准确的范围内)。在第14天用2.5 mL一次性注射器依次吸取草酸、丝氨酸、葡萄糖溶液和蒸馏水,插入土壤缓慢推动注射器使溶液缓慢送出,作为正式培养第1天,之后每隔24 h添加一次。尽管丝氨酸能够提供N素,但是培养期间添加丝氨酸N素的总量不足土壤总氮的3%,对N2O排放量的影响较小。1.3 主要药品及规格
草酸(H2C2O4·2H2O):分析纯,北京化工厂;D-无水葡萄糖(C6H12O6):>99.9%,MYM Biological Technology;丝氨酸(C3H7NO3):99%,北京百灵威科技有限公司;硫酸铵((NH 4)2SO4):分析纯,天津市福晨化学试剂厂。1.4 气体样品采集
在正式培养的第3天,添加根系分泌物溶液后的第2、4、8、12、16、20和24小时取样,共采集7次。采样前将培养瓶盖上橡胶塞和铝盖,并用密封器压紧密封,培养2 h。其他时间保持培养瓶敞口。用50 mL针筒抽取30 mL氦气注入培养瓶中,来回抽动针筒重复3次,将气体混匀,再将针筒抽到30 mL刻度处,待针筒不回弹后拔出并迅速插入已抽成真空的20 mL血清瓶,做好标记。取样的同时抽取实验室内空气作为N2O的环境背景值(3次重复)。1.5 样品测定与方法:
1.5.1 N2O同位素特征值测定与校正 稳定同位素质谱仪(Delta V Plus‐Precon, Thermo Fisher Scientific, Bremen, Germany)用来测定N2O同位素特征值:δ15Nα、δ15Nbulk、δ18O。计算公式如下[32]:式中,R=15N/14N,R=18O/16O。sa表示样品,st表示标准物。15N和18O的标准分别是大气中的N2和维也纳标准平均海洋水(Vienna Standard Mean Ocean Water),校准用标准参考气体(美国液化空气特种气体有限责任公司(Air Liquide America, Specialty Gases LLC))。
由于测定的N2O为空气中和土壤产生的N2O的混合气,受空气同位素值的影响,各检测结果(δ15Nα、δ15Nbulk、δ18O)需按照如下公式校正[33]:
式中,$\delta_{soil-emitted}$代表土壤排放N2O的同位素值;δmix、cmix分别表示土壤与空气混合气体的同位素值和N2O浓度;δair、cair分别表示空气背景的同位素值和N2O浓度(0.63 mg·m-3。cmix-cair为土壤排放的N2O浓度。
由于土壤N2O排放具有时间变异性,N2O的日平均同位素特征值用通量的加权平均值来表示[34]:
式中,δwa表示同位素的日通量加权平均值。ei表示第i-1取样到i次取样时间内土壤排放N2O的量(i时刻的排放速率×时间间隔(2 h 或4 h))。δi表示i次取样检测的同位素特征值。
1.5.2 N2O来源确定的理论基础 N2O为直线型分子:Nβ-Nα=O。根据两个氮原子与氧原子的相对位置不同,则中间的氮原子命名为α氮原子,末端的氮原子为β氮原子[32]。自然丰度条件下N2O分子中15N原子出现在α位和β位的差异,称为同位素位嗜值(site preference),即SP值,计算公式如下[35,36]:
在不同微生物群落或酶参与下的不同反应过程产生的N2O的SP值不同,这是SP值用于土壤N2O溯源研究的重要理论基础。纯细菌培养下硝化作用和反硝化作用的SP值是已知的,且其值不受底物氮氧同位素的影响。但自然状况下这两种过程往往同时存在[32],为测定气体样品中N2O的同位素值,通常利用同位素二源混合模型计算硝化作用和反硝化作用对N2O的贡献[37]。公式如下:
式中,fN和fD分别表示N2O来自土壤中硝化和反硝化作用的贡献比例,SPN表示纯细菌培养条件下硝化作用的SP值,为33‰[38,39];SPD表示纯细菌培养条件下反硝化作用的SP值,为0‰[40,41];SPsa表示测得样品的SP值,样品SP值大于33‰或小于0‰均按33‰或0‰计算。
1.5.3 N2O排放速率的计算 采集的气体用稳定同位素质谱仪(Delta V Plus-Preco,Thermo Fisher Scientific,Bremen,Germany)结合痕量气体浓缩系统测定N2O气体峰面积,根据N2O在样品中峰面积与环境背景中峰面积的比值,计算样品N2O浓度,根据培养前后瓶内N2O的浓度变化计算单位时间内土壤N2O的排放速率(培养前以及环境背景气体N2O的体积分数为320 nmol·mol-1)。公式如下[42]:
式中,F表示N2O排放速率(μg·kg·h-1),22.4为标准大气压下的摩尔体积(L·mol-1)。M为标准状态下N2O气体的摩尔质量(44 g·mol-1)。1000为单位换算。V表示培养瓶的体积,0.09 L。csa、cair分别表示培养后瓶内气体和空气中N2O浓度(nmol·mol-1)。Δt为培养时间,2 h。T为培养温度,25℃。
1.5.4 N2O通量和同位素值的校正系数 各采样时刻N2O排放速率和日平均排放速率的校正,各采样时刻测得的同位素值和同位素的日均值之间的校正公式如下[43,44]:
式中,Fi(i=2、4、8、12、16、20、24)表示各时间的平均通量。Ci为校正系数,Fave为N2O日平均排放速率、δ15Nbulk、δ18O和SP的日平均值,其中δ15Nbulk、δ18O和SP的日平均值为各时刻排放速率的加权平均值(根据公式(5)计算)。Fi为实测的第i次实测通量,或第i次取样检测的同位素值。校正系数越接近1,则说明该时刻测量的N2O排放速率和同位素值越接近日平均值,测量结果越具有代表性,即可采用该时刻作为最佳的取样时间[45]。
1.6 数据处理与分析
采用Microsoft Excel·2016对试验数据进行处理和做图。用SPSS·25.0(SPSS Inc., Chicago, IL, USA)进行统计分析。各处理间N2O累计排放量,δ18O,δ15Nbulk和SP用单因素方差分析(One-Way ANOVA),LSD法进行多重比较。双因素方差分析(Two-Way ANOVA)比较根系分泌物组分和浓度及其交互作用对N2O排放量和同位素特征值的影响。用一元线性回归分析最佳采样时间的检测值和日均值间的关系。2 结果
2.1 N2O排放速率的日变化
如图1所示,在取样期间所有处理的N2O排放速率均呈上升趋势,添加根系分泌物处理组均高于对照。在培养时间内添加低浓度、高浓度草酸处理土壤N2O排放速率迅速升高,分别从13.21 μg·kg-1·h-1增加到226.28 μg·kg-1·h-1和从17.84 μg·kg-1·h-1增加到97.21 μg·kg-1·h-1,添加草酸后土壤N2O排放速率,低浓度处理组高于高浓度处理组;葡萄糖处理中低浓度组低于高浓度组,低浓度丝氨酸、高浓度丝氨酸、低浓度葡萄糖和高浓度葡萄糖处理的土壤N2O排放速率分别从70.72 μg·kg-1·h-1增加到125.79 μg·kg-1·h-1、50.82 μg·kg-1·h-1增加到171.71 μg·kg-1·h-1、20.34 μg·kg-1·h-1增加126.69 μg·kg-1·h-1和20.46 μg·kg-1·h-1增加到185.21 μg·kg-1·h-1。图1
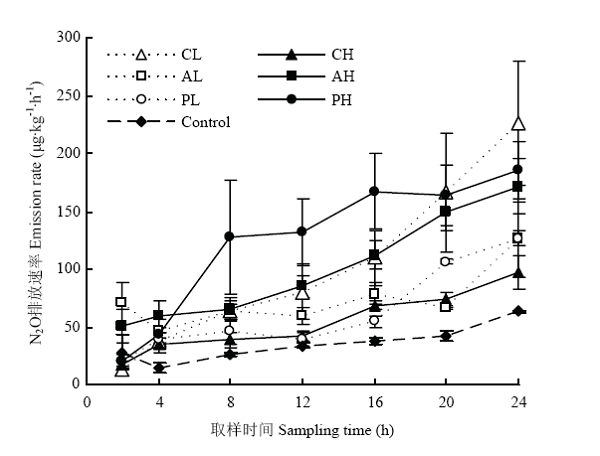 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1添加不同根系分泌物后不同时刻N2O排放速率
误差线表示标准误差(n=3)。下同
Fig. 1N2O emission rates at different observation time after adding root exudates
Error bars represent standard error (n=3). The same as below
双因素方差分析显示N2O累计排放量受根系分泌物和浓度共同作用的影响(P<0.05)(表1)。N2O累计排放量低浓度处理组:草酸处理>丝氨酸处理>葡萄糖处理,而在高浓度处理组:葡萄糖处理>丝氨酸处理>草酸处理。单因素方差分析显示,低浓度草酸、高浓度丝氨酸和高浓度葡萄糖处理土壤N2O累计排放速率显著高于对照(P<0.05)。增加根系分泌物浓度时,仅高浓度葡萄糖处理N2O累计排放量显著高于低浓度葡萄糖处理(P<0.05)。
Table 1
Table 1Cumulative emissions of N2O and daily weighted average of isotope signature
| 处理 Treatment | N2O累计排放量 (mg·kg-1·d-1) | δ18O (‰) | δ15Nbulk (‰) | SP (‰) | |
|---|---|---|---|---|---|
| 草酸 Oxalic acid | CL | 2.68±1.34ab | 24.16±1.55a | -19.82±7.41abc | 17.03±0.40a |
| CH | 1.38±0.24bc | 24.08±1.22a | -20.31±5.82abc | 13.13±2.70b | |
| 丝氨酸 Serine | AL | 1.81±0.45abc | 25.33±0.39a | -24.44±1.95c | 15.71±0.78ab |
| AH | 2.56±0.45ab | 24.68±1.11a | -20.21±2.11abc | 14.35±1.47ab | |
| 葡萄糖 Glucose | PL | 1.61±0.79bc | 25.59±0.25a | -13.12±1.13a | 16.16±1.40a |
| PH | 3.23±1.33a | 24.09±2.30a | -14.6±4.38ab | 15.18±1.04ab | |
| 对照 Control treatment | 0.90±0.09c | 20.06±1.52b | -23.14±3.72c | 13.29±1.40b | |
| 添加根系分泌物 Adding root exudates | <0.001** | 0.196 | 0.089 | 0.032* | |
| 组分 Component | 0.718 | 0.495 | 0.017* | 0.718 | |
| 浓度 Concentration | 0.38 | 0.261 | 0.726 | 0.012* | |
| 组分×浓度 Component×Concentration | 0.0278* | 0.611 | 0.51 | 0.222 | |
The weighted average of δ18O, δ15Nbulk and SP were calculated by equation (5). The same as below
The values followed by different little letters in the same column indicate the significant differences between treatments at P<0.05 level using One-Way ANOVA. * indicate the significant differences between treatments at P<0.05 level using Two-Way ANOVA
δ18O,δ15Nbulk和SP的值为各处理不同采样时间同位素值的加权平均值,由公式(5)计算。下同
同列中小写字母表示单因素方差分析(One-Way ANOVA)显著性P<0.05。*表示双因素方差分析(Two-Way ANOVA)显著性P<0.05
新窗口打开|下载CSV
2.2 N2O同位素值的日变化
不同处理土壤N2O同位素特征值不同(图2)。添加根系分必物处理的δ18O变化基本一致,稳定在23.43‰—25.90‰;而对照则在20.69‰—22.13‰。3种不同根系分泌物处理N2O的δ15Nbulk值不同,葡萄糖处理组δ15Nbulk值最高,变化范围分别是:-15.22‰— 13.55 ‰(低浓度)和-14.82‰—-10.70‰(高浓度)。低浓度丝氨酸处理δ15Nbulk值的范围是-26.56‰— 22.07‰,低于高浓度丝氨酸处理(-22.21‰— -16.66‰)。两浓度草酸处理δ15Nbulk值差异较小,低、高浓度的变化范围分别是-21.89‰—-15.27‰和-23.97‰—-16.75‰。3种根系分泌物浓度越高,SP值越低,低浓度草酸处理SP值从16.27‰增加至17.65‰,高浓度草酸处理SP值从16.69‰降至12.67‰;两浓度的丝氨酸处理SP值逐渐升高,低浓度丝氨酸和高浓度丝氨酸处理变化范围是:11.64‰—18.50‰和11.45‰—14.83‰;两浓度葡萄糖处理SP值先升高后降低,极值分别出现在添加葡萄糖后的第8小时(18.79‰)和第12小时(17.26‰)。图2
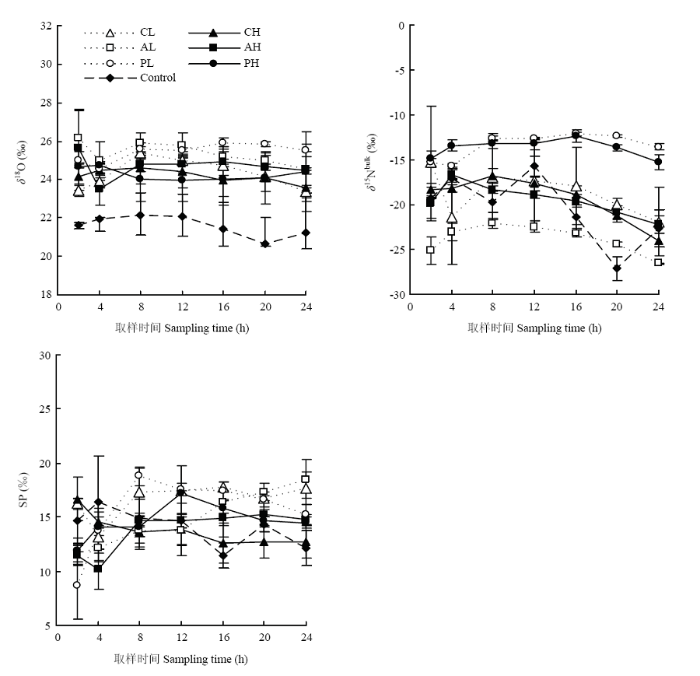 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2添加不同根系分泌物后N2O同位素特征值(δ18O, δ15Nbulk, SP)随时间的变化
Fig. 2Isotope signature (δ18O, δ15Nbulk, SP) of N2O at different observation time after adding root exudates
双因素方差分析显示,N2O的δ15Nbulk受添加根系分泌物成分的影响(P<0.05),其中葡萄糖处理δ15Nbulk值最高((-13.86±1.11)‰)。SP值受添加根系分泌物浓度的影响(P<0.05),浓度越高,SP值越低。单因素方差分析显示添加根系分泌物处理N2O的δ18O值显著高于对照(P<0.05),但不同组分处理间无差异;添加葡萄糖和丝氨酸的不同浓度间N2O的SP值均无明显差异;添加草酸后土壤N2O的SP值,低浓度处理组显著高于高浓度处理组(P<0.05)。
2.3 不同处理反硝化作用对N2O排放的贡献
通过二元混合模型(公式(6),(7))计算各处理反硝化作用和硝化作用对土壤N2O排放的相对贡献率如图3所示。空白对照处理、低浓度草酸、高浓度草酸、低浓度丝氨酸、高浓度丝氨酸、低浓度葡萄糖、高浓度葡萄糖处理反硝化作用的贡献分别是:60%、48%、60%、52%、57%、51%、54%。添加草酸、丝氨酸、葡萄糖的浓度越高,反硝化作用越强。图3
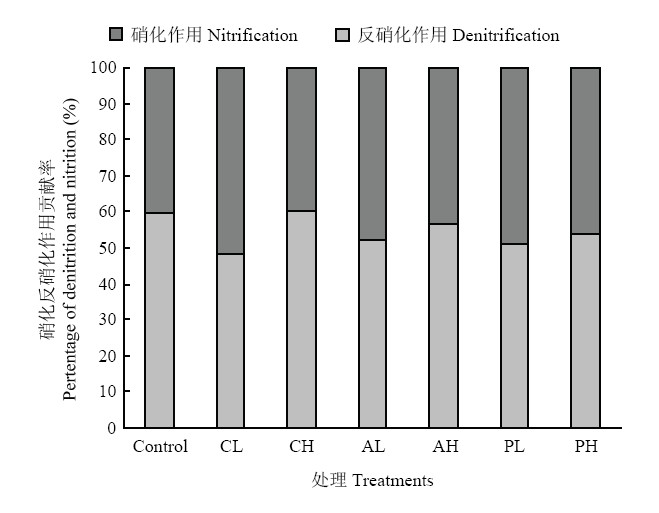 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3不同处理反硝化作用-硝化作用对N2O排放的贡献
Fig. 3Contribution of denitrification-nitrification on N2O
2.4 各时刻N2O排放速率和同位素特征值的校正系数
N2O排放速率和同位素特征值随时间有一定的变化,添加根系分泌物后各时刻的检测值和日平均值的校正系数如图4所示。不同指标的校正系数的波动范围不同,N2O排放速率的校正系数波动较大(0.49—8.46),同位素值的校正系数波动较小(0.79—1.87),其中各指标前8 h校正系数的波动较大,后逐渐减小。N2O排放速率的校正系数为1时的点在添加根系分泌物后的第16 h;N2O的δ18O值24 h内比较稳定,各处理校正系数稳定在1附近;N2O的δ15Nbulk值的校正系数呈先增高后降低的趋势,校正系数为1的点出现在添加根系分泌物后的第2—8小时和第16—20小时;SP值的校正系数为1的点出现在添加根系分泌物后的第16—20小时。图4
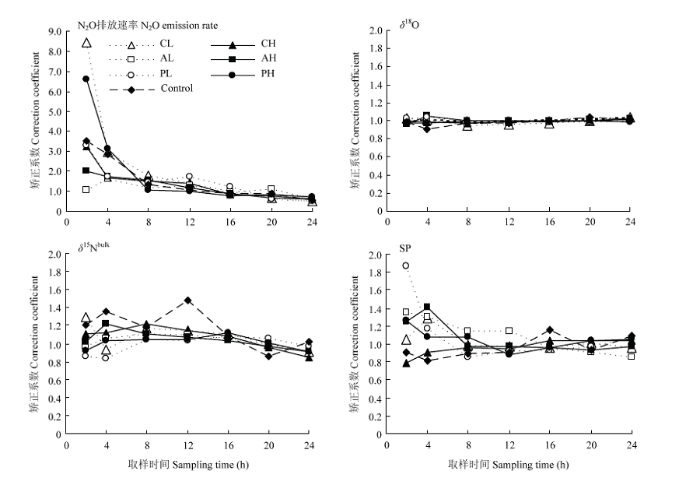 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4添加根系分泌物后各时刻N2O排放速率,稳定同位素值(δ18O, δ15Nbulk,SP)的校正系数
Fig. 4The correction coefficient of emission rate and isotope values (δ18O, δ15Nbulk, SP) at different observation time after adding root exudates
3 讨论
3.1 根系分泌物组分和浓度对N2O排放量及其同位素特征值的影响
本研究发现添加根系分泌物处理土壤N2O排放量高于对照,说明根系分泌物的添加会促进土壤N2O的排放,这和以往研究结果一致[46,47]。植物根系分泌物使植物通过光合作用固定的碳外流[48],并以有机碳的形式释放到土壤中。微生物从根系分泌物中获取能量,使土壤酶数量增大,活性增强,导致N2O排放量的增加[49]。植物根系分泌物也可作为碳源改变微生物细胞内N2O产生过程的电子传递途径[50],并促进N2O的产生。微生物对不同根系分泌物的响应机制不同,从而导致N2O产生路径也会有差异,进而造成N2O排放量的差异。本研究发现添加3种根系分泌物处理间土壤N2O的δ18O值无明显差异,且均显著高于对照(P<0.05)。$NO_{3}^{-}$还原为N2O的反硝化过程($NO_{3}^{-}$→$NO_{2}^{-}$→NO→ N2O)可看作O逐渐去除的过程,16O比18O更容易断裂使得产物N2O中富集18O从而产生同位素分馏[38];同时,N2O还原(N2O→N2)过程也会导致剩余N2O的δ18O值升高[51,52]。而$NO_{3}^{-}$在还原过程中产生的中间产物可能会与环境中的H2O发生O交换,从而减弱$NO_{3}^{-}$在还原过程中发生的分馏效应[38]。以上反硝化过程中的每一个还原步骤均是在不同酶的催化下发生,且导致不同的O同位素分馏[53]。因此根系分泌物处理组土壤N2O的δ18O值高于对照组可能是由于根系分泌物的添加促进了反硝化作用的进行,使反硝化还原酶活性改变所致;且3种根系分泌物可能共同作用了某一种对O同位素分馏起关键作用的酶而导致处理间N2O的δ18O值无差异。另外,添加3种根系分泌物可能造成了不同程度的N2O向N2的还原。
各根系分泌物处理N2O的δ15Nbulk值不同,δ15Nbulk值受根系分泌物组分的影响。在肥料$NH_4^+$充足的土壤条件下,N2O的δ15Nbulk值的升高和降低是由多种原因造成的,同位素的分馏可能是重要的原因。硝化作用的产物$NO_3^-$可作为反硝化作用的底物,根据同位素分馏原理,微生物优先利用轻同位素而导致产物发生重同位素贫化[54]使由反硝化过程产生的N2O的δ15Nbulk值降低;其次,N2O还原可能对δ15Nbulk值产生重要影响,N2O还原过程中14N-O键比15N-O键更容易断裂,导致剩余N2O的δ15Nbulk值升高;所以添加根系分泌物通过促进反硝化过程和N2O还原可能会导致N2O的δ15Nbulk值升高[30]。又因为土壤中的不同微生物过程导致的N同位素分馏不同[55],这使得不同根系分泌物处理的土壤N2O的δ15Nbulk值有差异。由于葡萄糖会促进N2O还原过程,该理论已在本课题组其他研究中得到验证[56]。此外,SPEIR等[57]通过15N标记肥料也发现,葡萄糖为易有效碳,而易有效碳的添加会促进反硝化过程进行完全,促进N2O向N2的还原过程,这可能是葡萄糖处理组N2O的δ15Nbulk值显著高于对照的原因(P<0.05)。
SP值表征的是直线型分子N2O(N-N-O)的分子内15N的位置嗜好性差异,该值和微生物过程有关,是一个被国际认可的N2O溯源研究工具[35]。本研究发现同一根系分泌物组分作用下,SP值随根系分泌物浓度的升高而降低。由于纯细菌培养下硝化作用下产生的N2O的SP值为33‰[38,39],反硝化作用下产生的N2O的SP值为0‰[40,41]。本研究所有处理SP值介于两者之间则说明产生的N2O来源于硝化作用和反硝化作用的共同作用。根据硝化和反硝化的二元混合模型可知SP值的升高和降低分别代表土壤微生物过程趋于硝化作用方向和反硝化作用进行,SP值越低,则反硝化作用越强。根系分泌物的添加为土壤提供碳源,从而促进了异养微生物作用下的反硝化过程。在适宜范围内,反硝化作用随着土壤碳源浓度的升高而增强,则产生的N2O的SP值也会因此而降低。
不同浓度处理组各根系分泌物累计排放量的顺序不同,我们发现在低浓度处理组:草酸处理>丝氨酸处理>葡萄糖处理,而在高浓度处理组:葡萄糖处理>丝氨酸处理>草酸处理。通过对土壤N2O排放速率及δ18O、δ15Nbulk、SP值等指标的综合分析,有利于进一步明确不同根系分泌物组分对N2O产生过程的影响。高浓度葡萄糖处理组土壤N2O排放量是低浓度处理组的2倍,但这两种处理土壤产生N2O的δ18O、δ15Nbulk值均无明显差异,在葡萄糖低、高两种浓度处理下,反硝化作用对土壤N2O排放的贡献率分别为51%和54%。说明不同葡萄糖浓度下,硝化作用和反硝化作用对土壤N2O排放的贡献均无显著差异(P>0.05),本研究的这两种葡萄糖浓度并没有导致土壤产生N2O的微生物结构的改变,这两种葡萄糖处理下土壤N2O的N、O同位素分馏也因此无明显变化[55],两种浓度葡萄糖处理下土壤产生的N2O的δ18O、δ15Nbulk值也无明显差异。这和以往研究结果一致[58],即葡萄糖对土壤微生物群落结构的影响较小。葡萄糖的添加对土壤产生N2O的作用机制是增加微生物数量和提高酶活性[58],葡萄糖的浓度越高,越有利于N2O的产生。但也有研究发现,当葡萄糖达到一定浓度后,土壤N2O排放量不会再继续增加反而出现下降,这是由于碳源的添加使土壤中的其他异养微生物大量增殖,并竞争N2O的产生底物,从而使N2O排放量降低[59];同时,碳源会促进无机氮固定为有机氮,抑制有机氮的矿化,微生物死亡后与金属离子结合到超分子聚合体中[60],也是导致N2O排放量降低的因素。由于不同土壤类型[61],微生物对添加的葡萄糖的响应机制不同,从而导致的N2O排放量不同[47]。此外,微生物对葡萄糖的响应还和底物氮源类型有关[62,63,64]。本研究添加较高浓度的铵态氮肥后,导致土壤C/N比值降低,葡萄糖的添加为微生物提供了其限制性的碳源,这是本实验研究结果和其他研究不同的主要原因。丝氨酸在土壤中不仅能够被微生物以碳源的形式直接利用[29],其分子内的N素还能转化为NH+ 4作为硝化作用的底物[65]。但由于丝氨酸提供的N源浓度较低,对N2O排放量的促进作用不明显。土壤排放N2O的SP值随丝氨酸浓度升高而降低,说明添加的丝氨酸主要发挥了为土壤微生物提供碳源的作用,从而促进了反硝化作用。本研究发现草酸和葡萄糖浓度越高,土壤排放的N2O的δ15Nbulk值越低,这主要是高浓度组分的根系分泌物促进反硝化作用,由于同位素分馏而导致N2O的δ15Nbulk值降低。但土壤中由于底物NH+ 4充足,硝化作用为先导反应,这是两种浓度根系分泌物作用下N2O的δ15Nbulk值虽然降低但未达到显著性水平的原因。而丝氨酸未表现出此规律,这可能是丝氨酸N原子参与N2O产生过程的结果。本研究发现低浓度草酸处理组N2O的排放量最高。说明低浓度下草酸对N2O的促进效果最强。草酸会加速有机无机复合体的分解[66,67],还可以增加一些特殊的微生物种类,如,K-战略类型的微生物(在一定环境中具有竞争能力的微生物),并产生大量与氮转化有关的酶,增加矿物态氮的释放[68],促进N2O的排放。本研究利用的土壤为碱性(pH= 8.7),而草酸为酸性,添加后会使土壤微环境的碱性减弱,当草酸达到一定浓度后对土壤碱性环境的削弱程度更强,有利于N2O还原过程的进行,使更多的N2O转化为N2,从而促进N2O向N2的还原过程[69]。这正好验证了本研究高浓度草酸处理组土壤N2O排放量显著低于高浓度处理组的结果。与低浓度草酸处理组(48%)相比,高浓度草酸处理组(60%)反硝化作用对土壤N2O排放的相对贡献率增加了12%,两浓度草酸处理土壤硝化和反硝化过程差异均显著(P<0.05)。这说明草酸处理改变了土壤微生物的结构,且促进了N2O还原基因的复制而导致N2O还原为N2,其他研究中也有类似结果[30],这不同于葡萄糖不改变微生物的群落结构而通过促进反硝化过程的完全进行而使N2O还原为N2,N2O还原与N2O产生同比例增加。高浓度处理组反硝化作用的增强,使通过硝化作用得到的可作为反硝化底物的$NO_3^-$的相对比例增加,进而使高浓度处理组由反硝化过程产生的N2O的相对比例增加,根据同位素分馏效应,产物相对于底物会发生15N贫化[54]。因此,添加高浓度草酸处理组土壤N2O的δ15Nbulk值也会降低,高浓度草酸处理N2O还原导致的δ15Nbulk值升高抵消了反硝化过程产物15N逐渐贫化的效果,使得两浓度处理下δ15Nbulk差异不显著。与此有关的证据还需进一步研究确定。
3.2 基于N2O排放速率及同位素特征值确定最佳取样时间
在采取手动N2O气体观测中,通常采用1次的检测结果而作为1日甚至多日的平均情况,所以采样时间的选择尤为重要。根据研究需要,基于不同指标的取样时间不同,N2O排放速率、N2O的δ15Nbulk及SP值的最佳取样时间分别为添加根系分泌物后的第12—16 h、4—8 h或16—20 h和16 h。由于N2O的δ18O值随着培养时间无明显变化,因此,取样时间对N2O的δ18O值的影响不大。综合N2O的排放速率和各稳定同位素值的取样时间,选择添加根系分泌物后的第16 h为最佳取样时间,从而可以对各个指标进行综合分析,更有利于量化不同微生物过程对土壤N2O排放的贡献率。对各处理最佳取样时间下得到的各个指标的结果分别与日平均值做相关分析显示(图5)。N2O排放速率:R2=0.93(P<0.001),N2O的δ18O值:R2=0.71(P<0.001),N2O的δ15Nbulk值:R2=0.91(P<0.05),SP值:R2=0.91(P<0.001)。因此,确定不同处理组的最佳取样时间是添加根系分泌物后的第16小时。此外,该时刻同位素值相对稳定,说明土壤内微生物过程稳定,利于分析N2O产生的微生物过程,因此结果最具代表性。图5
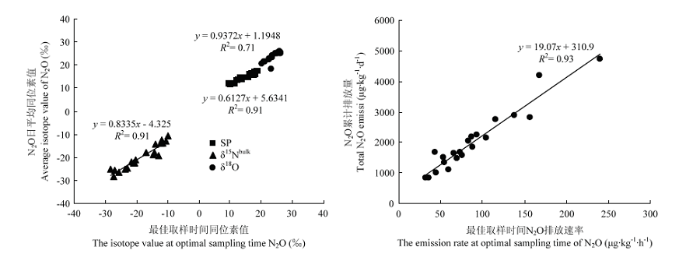 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5最佳取样时间N2O排放速率、稳定同位素值(δ18O, δ15Nbulk, SP)和日平均值的比较
Fig. 5Comparison of emission rate, isotope signature (δ18O, δ15Nbulk, SP) of N2O at optimal sampling time with daily average
3.3 研究展望
鉴于稳定同位素技术在区分N2O产生的微生物过程中存在优势,未来将继续利用同位素技术围绕不同根系分泌物组分及其组合作用下对土壤N2O的贡献规律及其产生的多种微生物途径进行研究。由于N2O还原对同位素值的影响较大,且促进N2O还原为N2是减少农业土壤N2O排放的努力方向,所以未来研究将N2O还原纳入考虑,同时引入国际稳定同位素先进技术和方法,如δ15Nbulk-δ18O模型和δ15Nbulk-SP模型,通过对反应底物浓度、底物同位素和前体同位素图谱的综合分析,进一步阐明植物根系分泌物对土壤N2O产生的作用机制。4 结论
通过外源添加根系分泌物3种主要组分,研究其对N2O排放高峰期的排放速率和稳定同位素特征值的影响。在施加NH+ 4的土壤中添加草酸、丝氨酸、葡萄糖促进土壤N2O的排放,高浓度组处理葡萄糖的促进效果最强,而低浓度组处理草酸的促进效果最强;根系分泌物作用下土壤排放N2O的δ18O的值升高;根系分泌物不同组分作用下N2O的δ15Nbulk值不同,葡萄糖的添加使δ15Nbulk值显著升高;各处理N2O的SP值的范围为13.13‰—15.03‰,根系分泌物浓度越高SP值越低;反硝化作用对N2O的贡献越强。通过对所测指标(N2O排放速率、δ15Nbulk、δ18O和SP值)的校正系数和回归分析显示添加根系分泌物后的最佳取样时间为添加根系分泌物后的第16小时。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 2]
URLPMID:8987358 [本文引用: 1]

Production and consumption processes in soils contribute to the global cycles of many trace gases (CH4, CO, OCS, H2, N2O, and NO) that are relevant for atmospheric chemistry and climate. Soil microbial processes contribute substantially to the budgets of atmospheric trace gases. The flux of trace gases between soil and atmosphere is usually the result of simultaneously operating production and consumption processes in soil: The relevant processes are not yet proven with absolute certainty, but the following are likely for trace gas consumption: H2 oxidation by abiontic soil enzymes; CO cooxidation by the ammonium monooxygenase of nitrifying bacteria; CH4 oxidation by unknown methanotrophic bacteria that utilize CH4 for growth; OCS hydrolysis by bacteria containing carbonic anhydrase; N2O reduction to N2 by denitrifying bacteria; NO consumption by either reduction to N2O in denitrifiers or oxidation to nitrate in heterotrophic bacteria. Wetland soils, in contrast to upland soils are generally anoxic and thus support the production of trace gases (H2, CO, CH4, N2O, and NO) by anaerobic bacteria such as fermenters, methanogens, acetogens, sulfate reducers, and denitrifiers. Methane is the dominant gaseous product of anaerobic degradation of organic matter and is released into the atmosphere, whereas the other trace gases are only intermediates, which are mostly cycled within the anoxic habitat. A significant percentage of the produced methane is oxidized by methanotrophic bacteria at anoxic-oxic interfaces such as the soil surface and the root surface of aquatic plants that serve as conduits for O2 transport into and CH4 transport out of the wetland soils. The dominant production processes in upland soils are different from those in wetland soils and include H2 production by biological N2 fixation, CO production by chemical decomposition of soil organic matter, and NO and N2O production by nitrification and denitrification. The processes responsible for CH4 production in upland soils are completely unclear, as are the OCS production processes in general. A problem for future research is the attribution of trace gas metabolic processes not only to functional groups of microorganisms but also to particular taxa. Thus, it is completely unclear how important microbial diversity is for the control of trace gas flux at the ecosystem level. However, different microbial communities may be part of the reason for differences in trace gas metabolism, e.g., effects of nitrogen fertilizers on CH4 uptake by soil; decrease of CH4 production with decreasing temperature; or different rates and modes of NO and N2O production in different soils and under different conditions.
[本文引用: 1]
DOI:10.1029/97jd00528URLPMID:11541125 [本文引用: 1]

Atmospheric heavy ozone is enriched in the isotopes 18O and 17O. The magnitude of this enhancement, of the order of 100%, is very large compared with that commonly known in atmospheric chemistry and geochemistry. The heavy oxygen atom in heavy ozone is therefore useful as a tracer of chemical species and pathways that involve ozone or its derived products. As a test of the isotopic exchange reactions, we successfully carry out a series of numerical experiments to simulate the results of the laboratory experiments performed by Wen and Thiemens [1993] on ozone and CO2. A small discrepancy between the experimental and the model values for 17O exchange is also revealed. The results are used to compute the magnitude of isotopic exchange between ozone and carbon dioxide via the excited atom O(1D) in the middle atmosphere. The model for 18O is in good agreement with the observed values.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1038/ismej.2013.205URLPMID:24225887 [本文引用: 1]

N2O gas is involved in global warming and ozone depletion. The major sources of N2O are soil microbial processes. Anthropogenic inputs into the nitrogen cycle have exacerbated these microbial processes, including nitrification. Ammonia-oxidizing archaea (AOA) are major members of the pool of soil ammonia-oxidizing microorganisms. This study investigated the isotopic signatures of N2O produced by soil AOA and associated N2O production processes. All five AOA strains (I.1a, I.1a-associated and I.1b clades of Thaumarchaeota) from soil produced N2O and their yields were comparable to those of ammonia-oxidizing bacteria (AOB). The levels of site preference (SP), δ(15)N(bulk) and δ(18)O -N2O of soil AOA strains were 13-30%, -13 to -35% and 22-36%, respectively, and strains MY1-3 and other soil AOA strains had distinct isotopic signatures. A (15)N-NH4(+)-labeling experiment indicated that N2O originated from two different production pathways (that is, ammonia oxidation and nitrifier denitrification), which suggests that the isotopic signatures of N2O from AOA may be attributable to the relative contributions of these two processes. The highest N2O production yield and lowest site preference of acidophilic strain CS may be related to enhanced nitrifier denitrification for detoxifying nitrite. Previously, it was not possible to detect N2O from soil AOA because of similarities between its isotopic signatures and those from AOB. Given the predominance of AOA over AOB in most soils, a significant proportion of the total N2O emissions from soil nitrification may be attributable to AOA.
DOI:10.1128/aem.69.10.5950-5956.2003URLPMID:14532049 [本文引用: 1]

Characterizing denitrification rates in aquatic ecosystems is essential to understanding how systems may respond to increased nutrient loading. Thus, it is important to ensure the precision and accuracy of the methods employed for measuring denitrification rates. The acetylene (C2H2) inhibition method is a simple technique for estimating denitrification. However, potential problems, such as inhibition of nitrification and incomplete inhibition of nitrous oxide reduction, may influence rate estimates. Recently, membrane inlet mass spectrometry (MIMS) has been used to measure denitrification in aquatic systems. Comparable results were obtained with MIMS and C2H2 inhibition methods when chloramphenicol was added to C2H2 inhibition assay mixtures to inhibit new synthesis of denitrifying enzymes. Dissolved-oxygen profiles indicated that surface layers of sediment cores subjected to the MIMS flowthrough incubation remained oxic whereas cores incubated using the C2H2 inhibition methods did not. Analysis of the microbial assemblages before and after incubations indicated significant changes in the sediment surface populations during the long flowthrough incubation for MIMS analysis but not during the shorter incubation used for the C2H2 inhibition method. However, bacterial community changes were also small in MIMS cores at the oxygen transition zone where denitrification occurs. The C2H2 inhibition method with chloramphenicol addition, conducted over short incubation intervals, provides a cost-effective method for estimating denitrification, and rate estimates are comparable to those obtained by the MIMS method.
DOI:10.1128/AEM.72.1.638-644.2006URLPMID:16391101 [本文引用: 1]

The intramolecular distribution of nitrogen isotopes in N2O is an emerging tool for defining the relative importance of microbial sources of this greenhouse gas. The application of intramolecular isotopic distributions to evaluate the origins of N2O, however, requires a foundation in laboratory experiments in which individual production pathways can be isolated. Here we evaluate the site preferences of N2O produced during hydroxylamine oxidation by ammonia oxidizers and by a methanotroph, ammonia oxidation by a nitrifier, nitrite reduction during nitrifier denitrification, and nitrate and nitrite reduction by denitrifiers. The site preferences produced during hydroxylamine oxidation were 33.5 +/- 1.2 per thousand, 32.5 +/- 0.6 per thousand, and 35.6 +/- 1.4 per thousand for Nitrosomonas europaea, Nitrosospira multiformis, and Methylosinus trichosporium, respectively, indicating similar site preferences for methane and ammonia oxidizers. The site preference of N2O from ammonia oxidation by N. europaea (31.4 +/- 4.2 per thousand) was similar to that produced during hydroxylamine oxidation (33.5 +/- 1.2 per thousand) and distinct from that produced during nitrifier denitrification by N. multiformis (0.1 +/- 1.7 per thousand), indicating that isotopomers differentiate between nitrification and nitrifier denitrification. The site preferences of N2O produced during nitrite reduction by the denitrifiers Pseudomonas chlororaphis and Pseudomonas aureofaciens (-0.6 +/- 1.9 per thousand and -0.5 +/- 1.9 per thousand, respectively) were similar to those during nitrate reduction (-0.5 +/- 1.9 per thousand and -0.5 +/- 0.6 per thousand, respectively), indicating no influence of either substrate on site preference. Site preferences of approximately 33 per thousand and approximately 0 per thousand are characteristic of nitrification and denitrification, respectively, and provide a basis to quantitatively apportion N2O.
DOI:10.1002/rcm.3456URLPMID:18435506 [本文引用: 1]

Nitrous oxide is produced in soil during several processes, which may occur simultaneously within different micro-sites of the same soil. Stable isotope techniques have a crucial role to play in the attribution of N(2)O emissions to different microbial processes, through estimation (natural abundance, site preference) or quantification (enrichment) of processes based on the (15)N and (18)O signatures of N(2)O determined by isotope ratio mass spectrometry. These approaches have the potential to become even more powerful when linked with recent developments in secondary isotope mass spectrometry, with microbial ecology, and with modelling approaches, enabling sources of N(2)O to be considered at a wide range of scales and related to the underlying microbiology. Such source partitioning of N(2)O is inherently challenging, but is vital to close the N(2)O budget and to better understand controls on the different processes, with a view to developing appropriate management practices for mitigation of N(2)O. In this respect, it is essential that as many of the contributing processes as possible are considered in any study aimed at source attribution, as mitigation strategies for one process may not be appropriate for another. To aid such an approach, here the current state of the art is critically examined, remaining challenges are highlighted, and recommendations are made for future direction.
DOI:10.13287/j.1001-9332.201707.026URLPMID:29741059 [本文引用: 1]

To understand the mechanisms of agricultural N2O emission, we investigated the N2O emission dynamics, the N2O isotope signatures, and the site preference value under different soil water conditions in the vegetable farmland of North China, by using the stable isotope technique and the acetylene inhibition method. The results demonstrated that N2O emission was significantly affec-ted by the water condition, and N2O emissions from soil with water-filled pore space (WFPS) of 70% were significantly higher than that with 50% WFPS. N2O emission occurred mostly in the early stage of fertilization, and decreased rapidly in the later stage of fertilization. At 50% WFPS, nitrification was the major process generating N2O during the early fertilization stage, accounting for approximately 90% of the N2O emission. However, the contribution of nitrification decreased sharply, whereas denitrification became the dominant process, accounting for 80% of the N2O emission 7 days after the fertilization. On the other hand, at 70% WFPS, denitrification was the main process releasing N2O during the early fertilization stage, decreasing from 70% to 40% and then gradually increasing to 80% 10 days after the fertilization. Overall, N2O emission was mainly dominated by the denitrification. The effect of different water treatments on soil nitrification and denitrification took place mainly in the early stage of fertilization, and N2O emission was gradually dominated by the denitrification at the later stage. These results suggested we could reduce N2O emission by approp-riately reducing the amount of irrigation in the vegetable farmland of North China.
DOI:10.13287/j.1001-9332.201707.026URLPMID:29741059 [本文引用: 1]

To understand the mechanisms of agricultural N2O emission, we investigated the N2O emission dynamics, the N2O isotope signatures, and the site preference value under different soil water conditions in the vegetable farmland of North China, by using the stable isotope technique and the acetylene inhibition method. The results demonstrated that N2O emission was significantly affec-ted by the water condition, and N2O emissions from soil with water-filled pore space (WFPS) of 70% were significantly higher than that with 50% WFPS. N2O emission occurred mostly in the early stage of fertilization, and decreased rapidly in the later stage of fertilization. At 50% WFPS, nitrification was the major process generating N2O during the early fertilization stage, accounting for approximately 90% of the N2O emission. However, the contribution of nitrification decreased sharply, whereas denitrification became the dominant process, accounting for 80% of the N2O emission 7 days after the fertilization. On the other hand, at 70% WFPS, denitrification was the main process releasing N2O during the early fertilization stage, decreasing from 70% to 40% and then gradually increasing to 80% 10 days after the fertilization. Overall, N2O emission was mainly dominated by the denitrification. The effect of different water treatments on soil nitrification and denitrification took place mainly in the early stage of fertilization, and N2O emission was gradually dominated by the denitrification at the later stage. These results suggested we could reduce N2O emission by approp-riately reducing the amount of irrigation in the vegetable farmland of North China.
[本文引用: 1]
[本文引用: 1]
DOI:10.1128/AEM.02484-09URLPMID:20118356 [本文引用: 1]

The objective of this study was to investigate how changes in soil pH affect the N(2)O and N(2) emissions, denitrification activity, and size of a denitrifier community. We established a field experiment, situated in a grassland area, which consisted of three treatments which were repeatedly amended with a KOH solution (alkaline soil), an H(2)SO(4) solution (acidic soil), or water (natural pH soil) over 10 months. At the site, we determined field N(2)O and N(2) emissions using the (15)N gas flux method and collected soil samples for the measurement of potential denitrification activity and quantification of the size of the denitrifying community by quantitative PCR of the narG, napA, nirS, nirK, and nosZ denitrification genes. Overall, our results indicate that soil pH is of importance in determining the nature of denitrification end products. Thus, we found that the N(2)O/(N(2)O + N(2)) ratio increased with decreasing pH due to changes in the total denitrification activity, while no changes in N(2)O production were observed. Denitrification activity and N(2)O emissions measured under laboratory conditions were correlated with N fluxes in situ and therefore reflected treatment differences in the field. The size of the denitrifying community was uncoupled from in situ N fluxes, but potential denitrification was correlated with the count of NirS denitrifiers. Significant relationships were observed between nirS, napA, and narG gene copy numbers and the N(2)O/(N(2)O + N(2)) ratio, which are difficult to explain. However, this highlights the need for further studies combining analysis of denitrifier ecology and quantification of denitrification end products for a comprehensive understanding of the regulation of N fluxes by denitrification.
DOI:10.1093/femsre/fuv021URLPMID:25934121 [本文引用: 1]

The continuous increase of the greenhouse gas nitrous oxide (N2O) in the atmosphere due to increasing anthropogenic nitrogen input in agriculture has become a global concern. In recent years, identification of the microbial assemblages responsible for soil N2O production has substantially advanced with the development of molecular technologies and the discoveries of novel functional guilds and new types of metabolism. However, few practical tools are available to effectively reduce in situ soil N2O flux. Combating the negative impacts of increasing N2O fluxes poses considerable challenges and will be ineffective without successfully incorporating microbially regulated N2O processes into ecosystem modeling and mitigation strategies. Here, we synthesize the latest knowledge of (i) the key microbial pathways regulating N2O production and consumption processes in terrestrial ecosystems and the critical environmental factors influencing their occurrence, and (ii) the relative contributions of major biological pathways to soil N2O emissions by analyzing available natural isotopic signatures of N2O and by using stable isotope enrichment and inhibition techniques. We argue that it is urgently necessary to incorporate microbial traits into biogeochemical ecosystem modeling in order to increase the estimation reliability of N2O emissions. We further propose a molecular methodology oriented framework from gene to ecosystem scales for more robust prediction and mitigation of future N2O emissions.
DOI:10.1016/j.scitotenv.2015.02.076URLPMID:25747359 [本文引用: 1]

This study examines the effects of temperature on nitrous oxide (N2O) emissions in a bench-scale intensive aquaculture system rearing Koi fish. The water temperature varied from 15 to 24 °C at interval of 3 °C. Both volumetric and specific rate for nitrification and denitrification declined as the temperature decreased. The concentrations of ammonia and nitrite, however, were lower than the inhibitory level for Koi fish regardless of temperature. The effects of temperature on N2O emissions were significant, with the emission rate and emission factor increasing from 1.11 to 1.82 mg N2O-N/d and 0.49 to 0.94 mg N2O-N/kg fish as the temperature decreased from 24 to 15 °C. A global map of N2O emission from aquaculture was established by using the N2O emission factor depending on temperature. This study demonstrates that N2O emission from aquaculture is strongly dependent on regional water temperatures as well as on fish production.
[本文引用: 1]
DOI:10.1098/rstb.2013.0112URLPMID:23713114 [本文引用: 1]

Soil nitrogen (N) budgets are used in a global, distributed flow-path model with 0.5° × 0.5° resolution, representing denitrification and N2O emissions from soils, groundwater and riparian zones for the period 1900-2000 and scenarios for the period 2000-2050 based on the Millennium Ecosystem Assessment. Total agricultural and natural N inputs from N fertilizers, animal manure, biological N2 fixation and atmospheric N deposition increased from 155 to 345 Tg N yr(-1) (Tg = teragram; 1 Tg = 10(12) g) between 1900 and 2000. Depending on the scenario, inputs are estimated to further increase to 408-510 Tg N yr(-1) by 2050. In the period 1900-2000, the soil N budget surplus (inputs minus withdrawal by plants) increased from 118 to 202 Tg yr(-1), and this may remain stable or further increase to 275 Tg yr(-1) by 2050, depending on the scenario. N2 production from denitrification increased from 52 to 96 Tg yr(-1) between 1900 and 2000, and N2O-N emissions from 10 to 12 Tg N yr(-1). The scenarios foresee a further increase to 142 Tg N2-N and 16 Tg N2O-N yr(-1) by 2050. Our results indicate that riparian buffer zones are an important source of N2O contributing an estimated 0.9 Tg N2O-N yr(-1) in 2000. Soils are key sites for denitrification and are much more important than groundwater and riparian zones in controlling the N flow to rivers and the oceans.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.scitotenv.2017.04.150URLPMID:28456121 [本文引用: 1]

In micro-polluted constructed wetland (CW), the low pollutant concentrations and the low COD/N ratios (chemical oxygen demand: total nitrogen in influent), make the biological treatment more difficult. It is expected that root exudates drive microbial-based transformations within plant rhizosphere. In this research, the roles of root exudates of three aquatic plants (Phragmites australis, Typha angustifolia and Cyperus alternifolius) in improving the growth of heterotrophic denitrifying bacteria were determined in a micro-polluted CW. In studied root rhizospheres, the total organic carbon (TOC) released from the plant roots varied significantly among plant species and seasons; the average TOC ranged from 0.1715 to 0.9221mgg-1rootDMd-1, which could fuel a denitrification rate of approximately 156-841kgNO3--Nha-1year-1 if all were used by the denitrifying bacteria; the abundances of nirK- and nirS-encoding bacteria were significantly influenced by the concentration of sucrose and glucose (0.869≤r≤0.933, p<0.05), and microbial community richness and diversity had response to root exudates. The results revealed that root exudates can act as endogenous carbon sources for heterotrophic denitrifying bacteria and ultimately determine the microbe distribution patterns in micro-polluted CW.
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
DOI:10.1002/rcm.1519URLPMID:15282780 [本文引用: 2]

The influence of flooding on N2O fluxes, denitrification rates, dual isotope (delta18O and delta15N) and isotopomer (1delta15N and 2delta15N) ratios of emitted N2O from estuarine intertidal zones was examined in a laboratory study using tidal flooding incubation chambers. Five replicate soil cores were collected from two differently managed intertidal zones in the estuary of the River Torridge (North Devon, UK): (1) a natural salt marsh fringing the estuary, and 2 a managed retreat site, previous agricultural land to which flooding was restored in summer 2001. Gas samples from the incubated soil cores were collected from the tidal chamber headspaces over a range of flooding conditions, and analysed for the delta18O, delta15N, 1delta15N and 2delta15N values of the emitted N2O. Isotope signals did not differ between the two sites, and nitrate addition to the flooding water did not change the isotopic content of emitted N2O. Under non-flooded conditions, the isotopic composition of the emitted N2O displayed a moderate variability in delta18O and 2delta15N delta values that was expected for microbial activity associated with denitrification. However, under flooded conditions, half of the samples showed strong and simultaneous depletions in 1delta15N and delta18O values, but not in 2delta15N. Such an isotope signal has not been reported in the literature, and it could point towards an unidentified N2O production pathway. Its signature differed from denitrification, which was generally the N2O production pathway in the salt marsh and the managed retreat site.
[本文引用: 3]
DOI:10.1111/j.1462-2920.2008.01599.xURLPMID:18393993 [本文引用: 3]

To determine to which extent root-derived carbon contributes to the effects of plants on nitrate reducers and denitrifiers, four solutions containing different proportions of sugar, organic acids and amino acids mimicking maize root exudates were added daily to soil microcosms at a concentration of 150 microg C g(-1) of soil. Water-amended soils were used as controls. After 1 month, the size and structure of the nitrate reducer and denitrifier communities were analysed using the narG and napA, and the nirK, nirS and nosZ genes as molecular markers respectively. Addition of artificial root exudates (ARE) did not strongly affect the structure or the density of nitrate reducer and denitrifier communities whereas potential nitrate reductase and denitrification activities were stimulated by the addition of root exudates. An effect of ARE composition was also observed on N(2)O production with an N(2)O:(N(2)O + N(2)) ratio of 0.3 in microcosms amended with ARE containing 80% of sugar and of 1 in microcosms amended with ARE containing 40% of sugar. Our study indicated that ARE stimulated nitrate reduction or denitrification activity with increases in the range of those observed with the whole plant. Furthermore, we demonstrated that the composition of the ARE affected the nature of the end-product of denitrification and could thus have a putative impact on greenhouse gas emissions.
[本文引用: 1]
[本文引用: 3]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 1]
DOI:10.1038/srep29257URLPMID:27387280 [本文引用: 1]

Nitrous oxide (N2O) is a potent greenhouse gas. In North China, vegetable fields are amended with high levels of N fertilizer and irrigation water, which causes massive N2O flux. The aim of this study was to determine the contribution of microbial processes to N2O production and characterize isotopic signature effects on N2O source partitioning. We conducted a microcosm study that combined naturally abundant isotopologues and gas inhibitor techniques to analyze N2O flux and its isotopomer signatures [δ(15)N(bulk), δ(18)O, and SP (intramolecular (15)N site preference)] that emitted from vegetable soil after the addition of NH4(+) fertilizers. The results show that ammonia oxidation is the predominant process under high water content (70% water-filled pore space), and nitrifier denitrification contribution increases with increasing N content. δ(15)N(bulk) and δ(18)O of N2O may not provide information about microbial processes due to great shifts in precursor signatures and atom exchange, especially for soil treated with NH4(+) fertilizer. SP and associated two end-member mixing model are useful to distinguish N2O source and contribution. Further work is needed to explore isotopomer signature stability to improve N2O microbial process identification.
[本文引用: 4]
DOI:10.1038/ismej.2013.205URLPMID:24225887 [本文引用: 2]

N2O gas is involved in global warming and ozone depletion. The major sources of N2O are soil microbial processes. Anthropogenic inputs into the nitrogen cycle have exacerbated these microbial processes, including nitrification. Ammonia-oxidizing archaea (AOA) are major members of the pool of soil ammonia-oxidizing microorganisms. This study investigated the isotopic signatures of N2O produced by soil AOA and associated N2O production processes. All five AOA strains (I.1a, I.1a-associated and I.1b clades of Thaumarchaeota) from soil produced N2O and their yields were comparable to those of ammonia-oxidizing bacteria (AOB). The levels of site preference (SP), δ(15)N(bulk) and δ(18)O -N2O of soil AOA strains were 13-30%, -13 to -35% and 22-36%, respectively, and strains MY1-3 and other soil AOA strains had distinct isotopic signatures. A (15)N-NH4(+)-labeling experiment indicated that N2O originated from two different production pathways (that is, ammonia oxidation and nitrifier denitrification), which suggests that the isotopic signatures of N2O from AOA may be attributable to the relative contributions of these two processes. The highest N2O production yield and lowest site preference of acidophilic strain CS may be related to enhanced nitrifier denitrification for detoxifying nitrite. Previously, it was not possible to detect N2O from soil AOA because of similarities between its isotopic signatures and those from AOB. Given the predominance of AOA over AOB in most soils, a significant proportion of the total N2O emissions from soil nitrification may be attributable to AOA.
DOI:10.5194/bg-11-2679-2014URL [本文引用: 2]
DOI:10.1002/rcm.6975URLPMID:25088133 [本文引用: 2]

The contribution of fungal denitrification to the emission of the greenhouse gas nitrous oxide (N2O) from soil has not yet been sufficiently investigated. The intramolecular (15)N site preference (SP) of N2O could provide a tool to distinguish between N2O produced by bacteria or fungi, since in previous studies fungi exhibited much higher SP values than bacteria.
URL [本文引用: 1]

To identify the characteristics of N2O emission from protected vegetable land in Beijing, and to seek a way that decreases N2O emission and increase or keep cucumber yield, with the method of static chamber-gas chromatograph technique, N2O emission was monitored in cucumber field from protected vegetable land in Beijing. The effects of different amounts of fertilization on N2O emission, vegetable yields and economic benefit were analyzed. The results showed that significant temporal variability of N2O flux from all treatments was observed in different growing stages of cucumber. Larger emission happened at the initial stage of the experiment. N2O emission decreased and remained stable with time. At the late stage, a peak emission happened and continued for a long time because of larger amount of top dressing. The order of total N2O emission was: T4 (conventional fertilization + chicken dug,in short “CF+CD”) >T3 (3/4CF+CD) > T1 (1/4CF+CD) > T2 (1/2CF+CD) > Tn (CD) > T0 (Control treatment), and there existed significant difference between treatments. By considering fertilizer rates, N2O emission and cucumber yield, it was concluded that the fertilization rate of T3 (3/4CF+CD) was very reasonable, which could provide basis for applying fertilizer rationally, reducing farm production costs, estimating greenhouse gas emissions from cropland and compiling national greenhouse gases emission inventory
URL [本文引用: 1]

To identify the characteristics of N2O emission from protected vegetable land in Beijing, and to seek a way that decreases N2O emission and increase or keep cucumber yield, with the method of static chamber-gas chromatograph technique, N2O emission was monitored in cucumber field from protected vegetable land in Beijing. The effects of different amounts of fertilization on N2O emission, vegetable yields and economic benefit were analyzed. The results showed that significant temporal variability of N2O flux from all treatments was observed in different growing stages of cucumber. Larger emission happened at the initial stage of the experiment. N2O emission decreased and remained stable with time. At the late stage, a peak emission happened and continued for a long time because of larger amount of top dressing. The order of total N2O emission was: T4 (conventional fertilization + chicken dug,in short “CF+CD”) >T3 (3/4CF+CD) > T1 (1/4CF+CD) > T2 (1/2CF+CD) > Tn (CD) > T0 (Control treatment), and there existed significant difference between treatments. By considering fertilizer rates, N2O emission and cucumber yield, it was concluded that the fertilization rate of T3 (3/4CF+CD) was very reasonable, which could provide basis for applying fertilizer rationally, reducing farm production costs, estimating greenhouse gas emissions from cropland and compiling national greenhouse gases emission inventory
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
DOI:10.1111/j.1462-2920.2008.01599.xURLPMID:18393993 [本文引用: 1]

To determine to which extent root-derived carbon contributes to the effects of plants on nitrate reducers and denitrifiers, four solutions containing different proportions of sugar, organic acids and amino acids mimicking maize root exudates were added daily to soil microcosms at a concentration of 150 microg C g(-1) of soil. Water-amended soils were used as controls. After 1 month, the size and structure of the nitrate reducer and denitrifier communities were analysed using the narG and napA, and the nirK, nirS and nosZ genes as molecular markers respectively. Addition of artificial root exudates (ARE) did not strongly affect the structure or the density of nitrate reducer and denitrifier communities whereas potential nitrate reductase and denitrification activities were stimulated by the addition of root exudates. An effect of ARE composition was also observed on N(2)O production with an N(2)O:(N(2)O + N(2)) ratio of 0.3 in microcosms amended with ARE containing 80% of sugar and of 1 in microcosms amended with ARE containing 40% of sugar. Our study indicated that ARE stimulated nitrate reduction or denitrification activity with increases in the range of those observed with the whole plant. Furthermore, we demonstrated that the composition of the ARE affected the nature of the end-product of denitrification and could thus have a putative impact on greenhouse gas emissions.
DOI:10.1016/j.soilbio.2015.02.001URL [本文引用: 1]
DOI:10.1002/rcm.3249URLPMID:17935120 [本文引用: 1]

Stable isotope analysis of oxygen (O) is increasingly used to determine the origin of nitrate (NO(3)-) and nitrous oxide (N(2)O) in the environment. The assumption underlying these studies is that the (18)O signature of NO(3)- and N(2)O provides information on the different O sources (O(2) and H(2)O) during the production of these compounds by various biochemical pathways. However, exchange of O atoms between H(2)O and intermediates of the (de)nitrification pathways may change the isotopic signal and thereby bias its interpretation for source determination. Chemical exchange of O between H(2)O and various nitrogenous oxides has been reported, but the probability and extent of its occurrence in terrestrial ecosystems remain unclear. Biochemical O exchange between H(2)O and nitrogenous oxides, NO(2)- in particular, has been reported for monocultures of many nitrifiers and denitrifiers that are abundant in nature, with exchange rates of up to 100%. Therefore, biochemical O exchange is likely to be important in most soil ecosystems, and should be taken into account in source determination studies. Failing to do so might lead to (i) an overestimation of nitrification as NO(3)- source, and (ii) an overestimation of nitrifier denitrification and nitrification-coupled denitrification as N(2)O production pathways. A method to quantify the rate and controls of biochemical O exchange in ecosystems is needed, and we argue this can only be done reliably with artificially enriched (18)O compounds. We conclude that in N source determination studies, the O isotopic signature of especially N(2)O should only be used with extreme caution.
DOI:10.1021/ac020113wURLPMID:12380811 [本文引用: 1]

We report a novel method for measurement of the oxygen isotopic composition (18O/16O) of nitrate (NO3-) from both seawater and freshwater. The denitrifier method, based on the isotope ratio analysis of nitrous oxide generated from sample nitrate by cultured denitrifying bacteria, has been described elsewhere for its use in nitrogen isotope ratio (15N/14N) analysis of nitrate. (1) Here, we address the additional issues associated with 18O/16O analysis of nitrate by this approach, which include (1) the oxygen isotopic difference between the nitrate sample and the N20 analyte due to isotopic fractionation associated with the loss of oxygen atoms from nitrate and (2) the exchange of oxygen atoms with water during the conversion of nitrate to N2O. Experiments with 18O-labeled water indicate that water exchange contributes less than 10%, and frequently less than 3%, of the oxygen atoms in the N20 product for Pseudomonas aureofaciens. In addition, both oxygen isotope fractionation and oxygen atom exchange are consistent within a given batch of analyses. The analysis of appropriate isotopic reference materials can thus be used to correct the measured 18O/16O ratios of samples for both effects. This is the first method tested for 18O/16O analysis of nitrate in seawater. Benefits of this method, relative to published freshwater methods, include higher sensitivity (tested down to 10 nmol and 1 microM NO3-), lack of interference by other solutes, and ease of sample preparation.
DOI:10.1002/rcm.7909URLPMID:28556299 [本文引用: 1]

Fungal denitrifiers can contribute substantially to N2 O emissions from arable soil and show a distinct site preference for N2 O (SP(N2 O)). This study sought to identify another process-specific isotopic tool to improve precise identification of N2 O of fungal origin by mass spectrometric analysis of the N2 O produced.
[本文引用: 2]
DOI:10.1002/rcm.8305URLPMID:30304571 [本文引用: 2]

Biochar amendments often decrease N2 O gas production from soil, but the mechanisms and magnitudes are still not well characterized since N2 O can be produced via several different microbial pathways. We evaluated the influence of biochar amendment on N2 O emissions and N2 O isotopic composition, including 15 N site preference (SP) under anaerobic conditions.
[D].
[本文引用: 1]
[D].
[本文引用: 1]
[本文引用: 1]
DOI:10.1128/AEM.02993-09URLPMID:20154105 [本文引用: 2]

In agricultural cropping systems, crop residues are sources of organic carbon (C), an important factor influencing denitrification. The effects of red clover, soybean, and barley plant residues and of glucose on denitrifier abundance, denitrification gene mRNA levels, nitrous oxide (N(2)O) emissions, and denitrification rates were quantified in anoxic soil microcosms for 72 h. nosZ gene abundances and mRNA levels significantly increased in response to all organic carbon treatments over time. In contrast, the abundance and mRNA levels of Pseudomonas mandelii and closely related species (nirS(P)) increased only in glucose-amended soil: the nirS(P) guild abundance increased 5-fold over the 72-h incubation period (P &lt; 0.001), while the mRNA level significantly increased more than 15-fold at 12 h (P &lt; 0.001) and then subsequently decreased. The nosZ gene abundance was greater in plant residue-amended soil than in glucose-amended soil. Although plant residue carbon-to-nitrogen (C:N) ratios varied from 15:1 to 30:1, nosZ gene and mRNA levels were not significantly different among plant residue treatments, with an average of 3.5 x 10(7) gene copies and 6.9 x 10(7) transcripts g(-1) dry soil. Cumulative N(2)O emissions and denitrification rates increased over 72 h in both glucose- and plant-tissue-C-treated soil. The nirS(P) and nosZ communities responded differently to glucose and plant residue amendments. However, the targeted denitrifier communities responded similarly to the different plant residues under the conditions tested despite changes in the quality of organic C and different C:N ratios.
URL [本文引用: 1]

【Objective】 In order to understand the mechanisms of N transformation and manage N fertilization better, the effect of glucose addition on N transformation in paddy soils with a gradient of organic C content were determined. 【Method】 Changes of mineralization, nitrification and denitrification in paddy soils, as well as their response to glucose addition were measured by incubation experiment. 【Result】 Intensity of mineralization and denitrification were: soils with high fertility > soils with middle fertility > soils with low fertility. During the first week of incubation, net mineralization and denitrification rate in paddy soils with high fertility were 1.9 and 1.1 times of that in soil with middle fertility and 5.3 and 2.9 times of that in soils with low fertility, respectively. Addition of glucose decreased approx. 78.8%, 109.2%, and 177.4% of net mineralization in soils with high, middle, and low fertility, respectively. However, denitrification rate in soils with middle and low fertility increased by 14.4% and 166.2%. The highest nitrate content in all the tested soils was 0.62 mg•kg-1 and the highest nitrification ratio was 0.33%. Addition of glucose had no obvious effects on nitrate content and nitrification ratio. 【Conclusion】 It was suggested that the intensity of mineralization and denitrification were quite different in soils with different fertility. The intensity was increased with the increase of soil organic C content. Addition of glucose decreased mineralization, but increased denitrification. The effects of glucose addition were significantly different in different soils. The effect of glucose addition on soils with low organic C content were greater than that on soils with high organic C content. Neither addition of glucose nor inherent soil organic C had obvious effects on nitrification of test paddy soils.
URL [本文引用: 1]

【Objective】 In order to understand the mechanisms of N transformation and manage N fertilization better, the effect of glucose addition on N transformation in paddy soils with a gradient of organic C content were determined. 【Method】 Changes of mineralization, nitrification and denitrification in paddy soils, as well as their response to glucose addition were measured by incubation experiment. 【Result】 Intensity of mineralization and denitrification were: soils with high fertility > soils with middle fertility > soils with low fertility. During the first week of incubation, net mineralization and denitrification rate in paddy soils with high fertility were 1.9 and 1.1 times of that in soil with middle fertility and 5.3 and 2.9 times of that in soils with low fertility, respectively. Addition of glucose decreased approx. 78.8%, 109.2%, and 177.4% of net mineralization in soils with high, middle, and low fertility, respectively. However, denitrification rate in soils with middle and low fertility increased by 14.4% and 166.2%. The highest nitrate content in all the tested soils was 0.62 mg•kg-1 and the highest nitrification ratio was 0.33%. Addition of glucose had no obvious effects on nitrate content and nitrification ratio. 【Conclusion】 It was suggested that the intensity of mineralization and denitrification were quite different in soils with different fertility. The intensity was increased with the increase of soil organic C content. Addition of glucose decreased mineralization, but increased denitrification. The effects of glucose addition were significantly different in different soils. The effect of glucose addition on soils with low organic C content were greater than that on soils with high organic C content. Neither addition of glucose nor inherent soil organic C had obvious effects on nitrification of test paddy soils.
URL [本文引用: 1]

Effect of addition of dissolved organic matter (DOM) on nitrification in fluvo-aquic soil was studied.The soil used in the experiment was collected from Suqian,Jiangsu.The experiment had three treatments different in DOM addition rate,220,440 and 880 mg·L-1,respectively.Results show that addition of DOM inhibited nitrification to a certain extent.After 16 days of incubation,nearly 100% of NH+4-N in CK had changed into NO-3-N.Compared with CK,the three treatments,DOC220,DOC440 and DOC880,decreased by 7.83%,13.60% and 19.12%,respectively in nitrification,and hence the decrease in NH+4-N concentration in the treatments was much slower than in CK.The addition of DOM,however,increased nitrite accumulation significantly.In CK,NO-2-N concentration peaked on D 12 in incubation,up to 67.83(mg·L-1),whereas in Treatment DOC220,DOC440 and DOC880,it did on D 12,14 and 16,and 21.17%,33.91% and 59.90% higher,respectively.The addition of DOM also decreased the production rate of NO-3-N.The maximum concentration of NO-3-N,143.61 mg·L-1,in CK was found on D 16,while only 41.97,78.09 and 91.30 mg·L-1 in Treatment DOC220,DOC440 and DOC880 respectively,much lower than in CK.In order to eliminate the effect of extraneous nitrogen brought in with the addition of DOM,comparison was made of effects of DOM addition and application of(NH4)2SO4 as the sole nitrogen source on nitrification in soils with same initial nitrogen concentration.Results also show increase in NO-2-N accumulation and decrease in NO-3-N production,which indicate that the organic carbon and low molecular organic compounds in DOM affected the process of nitrification.The findings of the experiment implied a high risk of NO-2-N accumulation existed in environments high in organic matter content,which may have some bad effects on the health of the terrestrial and aquatic systems.
URL [本文引用: 1]

Effect of addition of dissolved organic matter (DOM) on nitrification in fluvo-aquic soil was studied.The soil used in the experiment was collected from Suqian,Jiangsu.The experiment had three treatments different in DOM addition rate,220,440 and 880 mg·L-1,respectively.Results show that addition of DOM inhibited nitrification to a certain extent.After 16 days of incubation,nearly 100% of NH+4-N in CK had changed into NO-3-N.Compared with CK,the three treatments,DOC220,DOC440 and DOC880,decreased by 7.83%,13.60% and 19.12%,respectively in nitrification,and hence the decrease in NH+4-N concentration in the treatments was much slower than in CK.The addition of DOM,however,increased nitrite accumulation significantly.In CK,NO-2-N concentration peaked on D 12 in incubation,up to 67.83(mg·L-1),whereas in Treatment DOC220,DOC440 and DOC880,it did on D 12,14 and 16,and 21.17%,33.91% and 59.90% higher,respectively.The addition of DOM also decreased the production rate of NO-3-N.The maximum concentration of NO-3-N,143.61 mg·L-1,in CK was found on D 16,while only 41.97,78.09 and 91.30 mg·L-1 in Treatment DOC220,DOC440 and DOC880 respectively,much lower than in CK.In order to eliminate the effect of extraneous nitrogen brought in with the addition of DOM,comparison was made of effects of DOM addition and application of(NH4)2SO4 as the sole nitrogen source on nitrification in soils with same initial nitrogen concentration.Results also show increase in NO-2-N accumulation and decrease in NO-3-N production,which indicate that the organic carbon and low molecular organic compounds in DOM affected the process of nitrification.The findings of the experiment implied a high risk of NO-2-N accumulation existed in environments high in organic matter content,which may have some bad effects on the health of the terrestrial and aquatic systems.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.soilbio.2015.02.019URL [本文引用: 1]
DOI:10.1038/nclimate2580URL [本文引用: 1]
[本文引用: 1]
DOI:10.1111/j.1574-6941.2010.00856.xURLPMID:20370831 [本文引用: 1]

The N(2)O : N(2) product ratio of denitrification is negatively correlated with soil pH, but the mechanisms involved are not clear. We compared soils from field experiments where the pH had been maintained at different levels (pH 4.0-8.0) by liming (&gt; or = 20 years), and quantified functional gene pools (nirS, nirK and nosZ), their transcription and gas kinetics (NO, N(2)O and N(2)) of denitrification as induced by anoxic incubation with and without a carbon substrate (glutamate). Denitrification in unamended soil appeared to be based largely on the activation of a pre-existing denitrification proteome, because constant rates of N(2) and N(2)O production were observed, and the transcription of functional genes was below the detection level. In contrast, glutamate-amended soils showed sharp peaks in the transcripts of nirS and nosZ, increasing the rates of denitrification and pH-dependent transient accumulation of N(2)O. The results indicate that the high N(2)O : N(2) product ratio at low pH is a post-transcriptional phenomenon, because the transcription rate of nosZ relative to that of nirS was higher at pH 6.1 than at pH 8.0. The most plausible explanation is that the translation/assembly of N(2)O reductase is more sensitive to low pH than that of the other reductases involved in denitrification.
