 ,11
,11 2
3
Hemagglutinating Activity of Duck Tembusu Virus
WANG XiaoLei1, LIU YueHuan1, DUAN HuiJuan1, LIU LiXin1, YANG ZhiYuan1, ZHAO JiCheng1, PAN Jie1, LIU RuiHua1, ZHAO WenQi2, TIAN FangJie2, Lü JinBao3, LIN Jian ,1
,1通讯作者:
责任编辑: 林鉴非
收稿日期:2019-04-23接受日期:2019-07-1网络出版日期:2019-12-01
| 基金资助: |
Received:2019-04-23Accepted:2019-07-1Online:2019-12-01
作者简介 About authors
王小蕾,E-mail:wangxl227@126.com

摘要
关键词:
Abstract
Keywords:
PDF (617KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
王小蕾, 刘月焕, 段会娟, 刘立新, 杨志远, 赵际成, 潘洁, 刘瑞华, 赵文奇, 田方杰, 吕金宝, 林健. 鸭坦布苏病毒的血凝性[J]. 中国农业科学, 2019, 52(23): 4415-4422 doi:10.3864/j.issn.0578-1752.2019.23.022
WANG XiaoLei, LIU YueHuan, DUAN HuiJuan, LIU LiXin, YANG ZhiYuan, ZHAO JiCheng, PAN Jie, LIU RuiHua, ZHAO WenQi, TIAN FangJie, Lü JinBao, LIN Jian.
0 引言
【研究意义】2010 年春季开始,在浙江、江苏、山东、河北和北京等地区相继暴发了一种以鸭产蛋下降为主要特征的疫病,经病原学研究证实该病是由黄病毒科黄病毒属中鸭坦布苏病毒(duck Tembusu virus,DTMUV)引起[1,2,3]。北京鸭、樱桃谷鸭、金定鸭、麻鸭、康贝尔鸭和鹅等禽类均可发病,给养鸭业造成了巨大的经济损失[4]。黄病毒属成员多数具有血凝性,如黄热病病毒、流行性乙型脑炎病毒、登革热病毒等可以凝集鸡红细胞[5],利用这种血凝特性可以进行疫病的诊断与抗体检测[6,7,8,9,10],具有操作简单、敏感性高、特异性强、结果易于判定的优点。鸭坦布苏病毒同属于黄病毒属,研究其是否具有血凝特性,进而利用该特性研制鸭坦布苏病毒抗体检测方法,对该病的实验室血清学诊断和免疫监测等将有重要的实际临床应用价值。【前人研究进展】坦布苏病毒首次于1968年在Sarawak地区的蚊子体内分离到,曾一度认为家禽可能是病毒的自然宿主[11]。2000年,研究人员报道了一种新的名为Sitiawan的黄病毒感染鸡后能够引起鸡生长发育受阻[12],Sitiawan病毒与坦布苏病毒核酸的同源性为92%。我国从鸭体内分离到的坦布苏病毒与Bagaza病毒的核酸高度同源[13,14]。在鸭坦布苏病防控上,有多家单位投入了对疫苗及其免疫效果的研究[15,16,17,18],市场上也有了商品化的疫苗,但在鸭坦布苏病毒抗体检测技术方面,姬希文和LI等进行了ELISA 抗体检测试剂盒的研究[19,20],WANG等建立了一种乳胶凝集诊断方法用于抗体的快速检测[21],DENG等研制了检测DTMUV抗体的双抗原夹心式免疫层析试纸条[22],目前还没有商品化的诊断试剂。在鸭坦布苏病毒血凝性研究方面,现有资料表明,普通方法增殖的鸭坦布苏病毒对红细胞无凝集特性[23,24,25,26,27,28],尚无鸭坦布苏病毒血凝抑制试验的研究报道。【本研究切入点】现有资料报道鸭坦布苏病毒通过接种鸭胚、鸡胚、鸭胚成纤维细胞、Vero细胞和BHK-21细胞等增殖[23,25-27,29],本研究通过改进鸭坦布苏病毒增殖工艺,将鸭坦布苏病毒接种乳鼠脑组织,收获含病毒的脑组织,经丙酮处理后暴露病毒的血凝活性。【拟解决的关键问题】对鸭坦布苏病毒是否具有血凝性进行研究,确定其凝集的红细胞种类和血凝反应条件。1 材料与方法
本研究于2015年8月至2016年10月在北京市农林科学院畜牧兽医研究所完成。1.1 材料
1.1.1 病毒 鸭坦布苏病毒HB株F4代,由北京市农林科学院畜牧兽医研究所分离、鉴定和保存。1.1.2 实验动物 1—3日龄的BALB/c乳鼠,由购自北京维通利华实验动物技术有限公司的成年BALB/c鼠繁殖而来。6日龄SPF鸡胚,购自北京梅里亚维通实验动物技术有限公司。
1.1.3 红细胞 鹅、鸭、鸽、猪红细胞分别采自北京周边养殖场的健康动物,鸡红细胞采自SPF鸡,购于北京梅里亚维通实验动物技术有限公司。
1.1.4 96孔U型微量板 购自Greiner公司
1.1.5 主要试剂 丙酮购自国药集团化学试剂北京有限公司;甲醛购自北京益利精细化学品有限公司;β-丙内酯购自Alfa Aesar公司;阿氏液、PBS(0.01 mol·L-1, pH 7.2)、0.5%乳汉液按常规方法配制;pH 9.0 BABS配制方法:1.5 mol·L-1 NaCl溶液80 mL,0.5 mol·L-1 H3BO3溶液100 mL,1 mol·L-1 NaOH溶液24 mL,牛血清白蛋白1 g,加蒸馏水至1 000 mL ;VAD的配制方法参见文献[30],使反应体系的pH为6.0—7.0。
1.2 病毒增殖
将F4代毒种用灭菌PBS 进行1﹕10、1﹕50、1﹕100稀释,以0.025 mL/只的剂量分别经脑内途径接种1—3日龄乳鼠。乳鼠接种后继续由母鼠哺乳,攻毒后4—10日,观察不同稀释度的病毒液接种乳鼠后,乳鼠出现临床症状、死亡时间和死亡数。1.3 病毒提纯
接种后6日,观察到接种的乳鼠出现瘫痪等临床症状,二氧化碳安乐死后收获乳鼠。取鼠脑,参照文献记载的方法[30,31]用蔗糖-丙酮处理脑组织,提取病毒液。1.4 灭活剂和灭活时间的确定
将上述制备的病毒液平均分为6组,其中3组分别加入终浓度为0.05%、0.1%、0.15%的甲醛,另外3组分别加入终浓度为0.0125%(1/8000)、0.025%(1/4000)和0.05%(1/2000)的β-丙内酯溶液,摇匀后置2—8℃条件下灭活。于灭活后24、48、72、96、120 h分别取样进行灭活检,每份样品经卵黄囊途径接种5枚6日龄SPF鸡胚,每胚0.2 mL,置37℃孵育。24 h照胚,在24 h内死亡的鸡胚判为非特异性死亡,非特异性死亡应不超过1枚。24 h后,每日照胚2次,观察至168 h,鸡胚全部存活,判为灭活完全。24—168 h有死胚及时取出,收获胚体,盲传1代,如无鸡胚死亡,判为灭活完全。1.5 红细胞悬液的制备
将采集的各种动物血液,分别与等量阿氏液混合。使用前用PBS洗涤3次,前两次1 500 r/min,离心10 min,最后1次以同样的转速离心15 min,根据试验设计,将沉积的红细胞分别用VAD配制成所需浓度的红细胞(V/V)悬液,置4—8℃备用。1.6 HA测定
96孔U型微量板,从第1孔至第12孔,每孔加入BABS 50 μL,吸取灭活后的病毒液50 μL,依次作2倍系列稀释。按试验设计加入不同种、不同浓度或不同pH值VAD配制的红细胞。设不加样的红细胞对照孔。立即在微量板振摇器上摇匀,放入湿盒,置设定温度下作用50—60 min,观察凝集反应。当对照孔中的红细胞呈显著纽扣状时判定结果。以使红细胞完全凝集的最高稀释度作为判定终点。比较凝集图形、清晰度和稳定程度。2 结果
2.1 病毒增殖
接种后6日,1﹕10稀释组的20只乳鼠出现严重的临床症状,表现为四肢抽搐,瘫痪等。除3只乳鼠死亡(3/20)外,其余乳鼠处于濒死状态。1﹕50和1﹕100稀释组的193只和120只乳鼠症状稍轻于1﹕10稀释组乳鼠的症状,分别有6只(6/193)和4只(4/120)乳鼠死亡(表1)。死亡鼠弃去,进行无害化处理,发病鼠收获用于制备抗原。Table 1
表1
表1毒种不同稀释倍数接种后乳鼠发病情况
Table 1
| 稀释度 Dilution | 接种数(只) No. of sucking mice | 接种后不同时间(日)出现临床症状的鼠数 No. of mice appearing clinical symptoms at each day post inoculation | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| 1:10 | 20 | N | N | N | N | A | C |
| 1:50 | 193 | N | N | N | N | B | C |
| 1:100 | 120 | N | N | N | N | B | C |
新窗口打开|下载CSV
2.2 病毒提纯及灭活
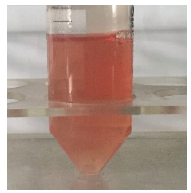
约74 g鼠脑组织匀浆,经蔗糖-丙酮提纯,共提取出408 mL病毒液,呈淡红色澄明液体(图1)。用终浓度为0.05%、0.1%、0.15%的甲醛,分别灭活24、48、72、96和120 h,灭活后的病毒液性状无明显改变,灭活检验接种的5枚鸡胚均存活。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1灭活前的病毒液
Fig. 1Purified viruses before inactivation
其余病毒液原计划用β-丙内酯溶液灭活,由于加入终浓度为0.0125%的β-丙内酯后瞬间病毒液产生大量絮状沉淀,性状发生明显改变(图2),其他2种浓度的试验未继续进行(表2)。
图2
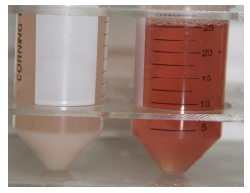 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2β-丙内酯灭活(左)和甲醛灭活(右)的病毒液
Fig. 2Purified viruses inactivation by β-propiolactone (left) and formaldehyde (right)
Table 2
表2
表2病毒液的灭活试验
Table 2
| 灭活剂 Inactivator | 终浓度 Final concentration (%) | 灭活时间Inactivation hours (h) | ||||
|---|---|---|---|---|---|---|
| 24 | 48 | 72 | 96 | 120 | ||
| 甲醛 Formaldehyde | 0.05 | 0A/5B | 0/5 | 0/5 | 0/5 | 0/5 |
| 0.1 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | |
| 0.15 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | |
| β-丙内酯 β-Propiolactone | 0.0125 | ND | ND | ND | ND | ND |
| 0.025 | ND | ND | ND | ND | ND | |
| 0.05 | ND | ND | ND | ND | ND | |
新窗口打开|下载CSV
2.3 HA测定
2.3.1 不同动物红细胞测定灭活病毒液的HA效价和凝集图形 鸡、鹅、鸭、鸽、猪的红细胞用反应终体系为pH 6.2的VAD配制成0.33%的红细胞悬液,分别与甲醛灭活后的病毒液在37℃作用50—60 min。结果显示,病毒液可以凝集上述红细胞,且HA效价基本一致,为1﹕256—1﹕512(表3)。其中,猪红细胞沉降慢,在同一反应时间和条件下,对照孔红细胞不能完全沉降至孔底形成实心点状,而是形成环状,结果不易判定;鸽、鹅、鸭和鸡(雏鸡和育成鸡)的红细胞的凝集图形清晰稳定。Table 3
表3
表3不同动物红细胞的HA效价和凝集图形
Table 3
| 动物 Animal | 日龄 Age (d) | 性别 Gender | HA效价 HA titer | 凝集图形 Agglutination graphics |
|---|---|---|---|---|
| 猪(长白猪) Swine (Landrace) | 60 | 公母混合(3头) Mixed of male and female | 1:256 | 红细胞对照不完全下沉,形成环状 The erythrocyte control sank not completely and formed a ring |
| 鸡(SPF) Chicken (SPF chicken) | 5 | 公(3只)Male | 1:256 | 均一薄膜状凝集,清晰稳定 Clear and stable homogeneous thin-film agglutination |
| 360 | 公(3只)Male | 1:256 | 均一薄膜状凝集,清晰稳定 Clear and stable homogeneous thin-film agglutination | |
| 鸭(北京鸭) Duck (Peking duck) | 60 | 母(3只)Female | 1:512 | 均一薄膜状凝集,清晰稳定 Clear and stable homogeneous thin-film agglutination |
| 鸽(信鸽) Pigeon (Carrier pigeon) | 360 | 公母混合(3只) Mixed of male and female | 1:512 | 均一薄膜状凝集,清晰稳定 Clear and stable homogeneous thin-film agglutination |
| 鹅(白鹅) Goose (White goose) | 180 | 公母混合(3只) Mixed of male and female | 1:512 | 均一薄膜状凝集,清晰稳定 Clear and stable homogeneous thin-film agglutination |
新窗口打开|下载CSV
2.3.2 不同浓度鹅红细胞的HA效价和凝集图形 用反应终体系为pH 6.2的VAD配制成0.2%、0.33%、0.5%、1%的鹅红细胞悬液,分别与甲醛灭活后的病毒液在37℃作用50—60 min。结果显示,0.2%鹅红细胞的HA效价最高,为1:1 024,但血凝图形过小,不清晰,结果不易判定;1%鹅红细胞的HA效价最低,为1:128,出现解凝现象。0.33%鹅红细胞的HA效价为1:512,凝集图形清晰稳定,是比较理想的红细胞浓度(表4)。
Table 4
表4
表4不同浓度鹅红细胞的HA效价和凝集图形
Table 4
| 鹅红细胞浓度 Concentration of goose erythrocyte | HA效价 HA titer | 凝集图形 Agglutination graphics |
|---|---|---|
| 0.2% | 1:1024 | 红细胞对照孔下沉点小,结果不易判定 The erythrocyte control sank as a tiny point so that the results were difficult to interpretation |
| 0.33% | 1:512 | 均一薄膜状凝集,清晰稳定 Clear and stable homogeneous thin-film agglutination |
| 0.5% | 1:256 | 均一薄膜状凝集,清晰,有解凝迹象 Clear homogeneous thin-film agglutination |
| 1% | 1:128 | 均一薄膜状凝集,解凝快,不稳定 Clear homogeneous thin-film agglutination with fast dissolving |
新窗口打开|下载CSV
2.3.3 不同pH值反应体系对HA效价的影响 鹅红细胞用不同VAD配制0.33%的红细胞悬液,分别与甲醛灭活后的病毒液在37℃作用50—60 min。试验结果表明,反应体系的pH值对HA效价和凝集图形有显著的影响,随着pH值的升高,病毒液的HA效价降低,凝集图形变得不稳定(表5)。
Table 5
表5
表5不同pH值反应体系对HA效价的影响
Table 5
| pH值 pH value | HA效价 HA titer | 凝集图形 Agglutination graphics |
|---|---|---|
| 6.0 | 1:512 | 均一薄膜状凝集,清晰稳定 Clear and stable homogeneous thin-film agglutination |
| 6.2 | 1:512 | 均一薄膜状凝集,清晰稳定 Clear and stable homogeneous thin-film agglutination |
| 6.4 | 1:256 | 均一薄膜状凝集,清晰稳定 Clear and stable homogeneous thin-film agglutination |
| 6.6 | 1:256 | 均一薄膜状凝集,清晰稳定 Clear and stable homogeneous thin-film agglutination |
| 6.8 | 1:128 | 均一薄膜状凝集,清晰稳定 Clear and stable homogeneous thin-film agglutination |
| 7.0 | 1:64 | 易解凝,结果不易判定 The thin-film agglutination was easy to dissolve so that the result was difficult to interpretation |
新窗口打开|下载CSV
2.3.4 不同反应温度对HA效价的影响 用反应终体系为pH 6.2的VAD配制成0.33%的鹅红细胞悬液,分别与甲醛灭活后的病毒液在4℃、37℃及室温作用50—60 min,观察反应结果。37℃及室温下反应病毒液的HA效价及凝集图形差别不大;4℃条件下作用60 min后,病毒液与红细胞出现凝集现象,但红细胞对照沉降不完全,无法判定HA效价,过夜作用后,红细胞对照沉降完全,凝集图形清晰,HA效价与37℃反应时相同。这表明鸭坦布苏病毒在4℃时仍具有血凝性(表6)。
Table 6
表6
表6不同反应温度对HA效价的影响
Table 6
| 反应温度 Reaction temperature | HA效价 HA titer | 凝集图形 Agglutination graphics |
|---|---|---|
| 37℃ | 1:512 | 均一薄膜状凝集,清晰稳定 Clear and stable homogeneous thin-film agglutination |
| 室温Room temperature 4℃(60 min) | 1: 512 / | 均一薄膜状凝集,清晰稳定 Clear and stable homogeneous thin-film agglutination 红细胞对照沉降不完全,结果无法判定 The erythrocyte control sedimentated slowly so that the result was difficult to interpretation |
| 4℃(过夜作用)(overnight) | 1: 512 | 均一薄膜状凝集,清晰稳定 Clear and stable homogeneous thin-film agglutination |
新窗口打开|下载CSV
3 讨论
据文献报道,黄病毒大多能够凝集鹅、鸽和新生雏鸡的红细胞,但其血凝素易于破坏,而且血凝反应要求比较严格的pH域[32]。鸭坦布苏病毒属于黄病毒属Ntaya病毒群,但在前期的报道中,用鸡胚、鸭胚、鸭胚成纤维细胞、Vero细胞和BHK–21增殖的鸭坦布苏病毒不能凝集鸡、鸭、鹅的红细胞[23,24,25,26,27,28]。笔者参考流行性乙型脑炎病毒血凝抑制试验抗原的制备方法,用乳鼠脑组织增殖鸭坦布苏病毒并用丙酮处理提纯病毒液,进行了血凝性的研究。为保证生物安全,对病毒液先进行灭活。β-丙内酯和甲醛是两种常用的病毒灭活剂,出人意料的是,提纯后澄明的病毒液加入β-丙内酯很快就有异物产生,该现象表明,提纯的病毒液中可能存在与β-丙内酯起化学反应的物质或者病毒可直接与β-丙内酯发生化学反应,因此,β-丙内酯不能用于灭活提纯的鸭坦布苏病毒液。终浓度为0.05%的甲醛2—8℃作用24h即可完全灭活病毒,且后续的试验结果表明,灭活后的病毒液仍具有血凝性。
提纯的病毒液可与鸡、鸭、鹅、鸽等禽红细胞发生凝集反应,也可以凝集哺乳动物猪的红细胞,相比之下与禽红细胞的血凝反应更稳定。我们曾采取同样的方法处理鸭胚增殖的病毒液(尿囊液),但病毒没有暴露出对鹅红细胞的凝集特性(内部资料),可能与尿囊液中抑制物的化学结构和性质不同(对丙酮的敏感性)有关。
红细胞浓度会影响血凝图形。红细胞浓度过低,红细胞对照孔的沉淀图形过小,不利于观察判定。本研究中,采用0.33%或0.5%的红细胞与鸭坦布病毒进行血凝反应易于观察到血凝现象,因采用0.5%红细胞悬液时有解凝迹象,0.33%红细胞悬液更为合适。黄病毒对血凝反应的pH要求比较严格,如东方马脑炎病毒、西方马脑炎病毒要求pH 6.4左右,登革热病毒1型和2型、流行性乙型脑炎病毒、西尼罗河病毒等要求pH 7.0[5]。通过研究发现,鸭坦布苏病毒血凝反应适宜的pH为6.0—6.8,当pH为6.0—6.2时,HA效价最高,且凝集图形清晰稳定。某些病毒的血凝作用具有温度依赖性,只能在某个温度范围内才出现血凝现象;但另一些病毒可以在4℃、室温和37℃呈现统一的血凝作用[32]。试验结果表明鸭坦布苏病毒可以在4℃、室温和37℃与鹅红细胞凝集。钮慧敏等用蔗糖丙酮法提纯的鼠脑抗原也能凝集0.5%鹅红细胞,HA效价为1:32[29],可能与具体操作方法和血凝条件不同有关。
以乳鼠增殖的鸭坦布苏病毒具有血凝性,HA效价较高,为后续建立检测鸭坦布苏病毒抗体的血凝抑制试验奠定了基础,这将有助于对该病的实验室血清学诊断和免疫监测。
4 结论
乳鼠增殖的鸭坦布苏病毒提纯后具有广泛的血凝性,可以凝集鸡、鸭、鹅、鸽和猪的红细胞,血凝性稳定,在4℃、室温和37℃下均可以与鹅红细胞凝集,以0.33%浓度的红细胞悬液血凝为宜,反应最适pH为6.0—6.2。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1016/j.virol.2011.06.003URL [本文引用: 1]

During investigations into an outbreak of egg production decline, retarded growth, and even death among ducks in Southeast China, a novel Tembusu virus strain named Tembusu virus Fengxian 2010 (FX2010) was isolated. This virus replicated in embryonated chicken eggs and caused embryo death. In cross-neutralization tests, antiserum to the partial E protein of Tembusu virus Mm1775 strain neutralized FX2010, whereas antiserum to Japanese encephalitis virus did not. FX2010 is an enveloped RNA virus of approximately 4550 nm in diameter. Sequence analysis of its E and NS5 genes showed that both genes share up to 99.6% nucleotide sequence identity with Baiyangdian virus, and up to 88% nucleotide sequence identity with their counterparts in Tembusu virus. FX2010 was transmitted without mosquito, and caused systemic infection and lesions in experimentally infected ducks. These results indicate that FX2010 and BYD virus are newly emerged Tembusu virus strains that cause an infectious disease in ducks. (C) 2011 Elsevier Inc.
DOI:10.1371/journal.pone.0018106URLPMID:21455312 [本文引用: 1]

Since April 2010, a severe outbreak of duck viral infection, with egg drop, feed uptake decline and ovary-oviduct disease, has spread around the major duck-producing regions in China. A new virus, named BYD virus, was isolated in different areas, and a similar disease was reproduced in healthy egg-producing ducks, infecting with the isolated virus. The virus was re-isolated from the affected ducks and replicated well in primary duck embryo fibroblasts and Vero cells, causing the cytopathic effect. The virus was identified as an enveloped positive-stranded RNA virus with a size of approximately 55 nm in diameter. Genomic sequencing of the isolated virus revealed that it is closely related to Tembusu virus (a mosquito-borne Ntaya group flavivirus), with 87-91% nucleotide identity of the partial E (envelope) proteins to that of Tembusu virus and 72% of the entire genome coding sequence with Bagaza virus, the most closely related flavivirus with an entirely sequenced genome. Collectively our systematic studies fulfill Koch's postulates, and therefore, the causative agent of the duck egg drop syndrome occurring in China is a new flavivirus. Flavivirus is an emerging and re-emerging zoonotic pathogen and BYD virus that causes severe egg-drop, could be disastrous for the duck industry. More importantly its public health concerns should also be evaluated, and its epidemiology should be closely watched due to the zoonotic nature of flaviviruses.
DOI:10.3201/eid1710.101890URL [本文引用: 1]

In China in 2010, a disease outbreak in egg-laying ducks was associated with a flavivirus. The virus was isolated and partially sequenced. The isolate exhibited 87%-91% identity with strains of Tembusu virus, a mosquito-borne flavivirus of the Ntaya virus group. These findings demonstrate emergence of Tembusu virus in ducks.
[本文引用: 1]
[本文引用: 1]
DOI:10.1084/jem.99.5.429URLPMID:13163320 [本文引用: 2]

Through the use of acetone and ether extraction of brain tissue from newborn mice infected with certain arthropod-borne viruses, it has been possible to demonstrate hemagglutinins for chick erythrocytes associated with the following viruses: dengue Type 1, dengue Type 2, Eastern equine encephalitis, Ilhéus, Japanese B, Ntaya, St. Louis, Sindbis, Uganda S, Venezuelan equine encephalitis, West Nile (Egypt 101 strain), Western equine encephalitis, and yellow fever (viscerotropic and neurotropic strains). On the basis of the temperature and pH required for reaction, the viruses can be assembled in two groups: A-those that require 37 degrees C. and a pH of about 6.4, comprising Eastern, Venezuelan, and Western equine encephalitis and Sindbis viruses; and B-those that require either 4 degrees or 22 degrees C. and a pH of about 7.0, comprising dengue Types 1 and 2, Ilhéus, Japanese B, Ntaya, St. Louis, Uganda S, West Nile, and yellow fever viruses. A method of eliminating non-specific inhibitory substances present in sera was developed. The method consists essentially of filtration through Seitz pads. Extensive serological crossings were found among viruses of each group, while antisera of one group failed consistently to cross-react with antigens of the other. Antisera deriving from animals immunized with certain viruses for which no hemagglutinins could be developed by the present method, reacted with members of either one or the other group. Thus Semliki Forest virus would appear to belong to Group A, and Russian Far Eastern and louping ill viruses to Group B.
DOI:10.1155/2016/5253842URLPMID:27446953 [本文引用: 1]

Secondary dengue infection by heterotypic serotypes is associated with severe manifestations of disease, that is, dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS). The World Health Organization (WHO) has recommended criteria based on the hemagglutination inhibition (HI) test to distinguish between primary and secondary dengue infections. Since the HI test has practical limitations and disadvantages, we evaluated the accuracy of WHO HI criteria and compared it with criteria based on an IgG enzyme-linked immunosorbent assay (ELISA) using a plaque reduction neutralization test (PRNT) as the gold standard. Both WHO HI criteria and IgG ELISA criteria performed strongly (16/16) in determining primary infection. However, to determine secondary infection, the IgG ELISA criteria performed better (72/73) compared to the WHO HI criteria (23/73).
DOI:10.1016/j.phrp.2014.08.003URLPMID:25389515 [本文引用: 1]

Several different methods are currently used to detect antibodies to Japanese encephalitis virus (JEV) in serum samples or cerebrospinal fluid. These methods include the plaque reduction neutralization test (PRNT), the hemagglutination inhibition (HI) test, indirect immunofluorescence assay (IFA), and enzyme-linked immunosorbent assay (ELISA). The purpose of this study was to compare the performance of each method in detecting vaccine-induced antibodies to JEV.
DOI:10.1371/journal.pntd.0002184URLPMID:23638205 [本文引用: 1]

In recent decades, sporadic cases and outbreaks in humans of West Nile virus (WNV) infection have increased. Serological diagnosis of WNV infection can be performed by enzyme-linked immunosorbent assay (ELISA), immunofluorescence assay (IFA) neutralization test (NT) and by hemagglutination-inhibition assay. The aim of this study is to collect updated information regarding the performance accuracy of WNV serological diagnostics.
[本文引用: 1]
[本文引用: 1]
URLPMID:13788307 [本文引用: 1]

Haemagglutinating antigens for various strains of tick-borne and Japanese B encephalitis viruses and for certain mutants of the virus of American equine encephalomyelitis have been prepared at the D. I. Ivanovski Institute of Virology of the USSR Academy of Medical Sciences, Moscow, and used in cross haemagglutination-inhibition tests with the corresponding immune sera. The results of these experiments suggest that the haemagglutination-inhibition test using goose red cells constitutes a simple, rapid and accurate method for the serological diagnosis of diseases caused by the neuroviruses mentioned above and for the identification of isolated strains of these viruses, and would be of considerable value in epidemiological surveys.
DOI:10.1080/00034983.1975.11686984URLPMID:235907 [本文引用: 1]

Thirty isolations of Tembusu virus and four of Sindbis virus were obtained from approximately 280 000 mosquitoes collected between October 1968 and February 1970 in Sarawak, particularly from K. Tijirak, a Land Dyak village 19 miles South of Kuching. Twenty-two isolations of Tembusu virus and two of Sindbis virus were from Culex tritaeniorhynchus; two of Tembusu virus and two of Sindbis virus came from Culex gelidus. Tembusu virus was active throughout the year at K. Tijirak, the highest infection rates in C. tritaeniorhynchus being in January-March and May-August, when the C. tritaeniorhynchus population was declining and ageing. These results confirm that C. tritaeniorhynchus is the principal arthopod host of Tembusu virus in Sarawak. Antibody studies suggest that birds, particularly domestic fowl, are probably vertebrate maintenance hosts of Tembusu and Sindbis viruses in Sarawak.
DOI:10.4269/ajtmh.2000.63.94URLPMID:11358004 [本文引用: 1]

A new virus named Sitiawan virus (SV) was isolated from sick broiler chicks in chicken embryos. The virus replicated well with cytopathogenic effect (CPE) in the chicken B-lymphocyte cell line LSCC-BK3. The virus was an enveloped RNA virus of approximately 41 nm in size with hemagglutinating activity (HA) to goose erythrocytes. It was cross-reactive with Japanese encephalitis virus (JEV), a member of flaviviruses by HA inhibition tests but not by cross-virus neutralization tests. The cDNA fragment of NS5 gene was amplified with primers corresponding to NS5 gene of flaviviruses. The nucleotide sequences were 92% homologous to Tembusu virus, a member of the mosquito-borne virus cluster of the genus Flavivirus. In cross-neutralization tests with Tembusu virus, antiserum to SV did not neutralize Tembusu virus, and antiserum to Tembusu virus neutralized more weakly to SV than against homologous virus. These results indicate that SV is a new virus which can be differentiated serologically from Tembusu virus but is otherwise similar with respect to nucleotide sequence. The virus causes encephalitis, growth retardation, and increased blood glucose levels in inoculated chicks.
DOI:10.1128/JVI.07132-11URL [本文引用: 1]

Duck tembusu virus (DTMUV) is an emerging agent that causes a severe disease in ducks. We report herein the first complete genome sequences of duck tembusu virus strains YY5, ZJ-407, and GH-2, isolated from Shaoxing ducks, breeder ducks, and geese, respectively, in China. The genomes of YY5, ZJ-407, and GH-2 are all 10,990 nucleotides (nt) in length and encode a putative polyprotein of 3,426 amino acids. It is flanked by a 5' and a 3' noncoding region (NCR) of 94 and 618 nt, respectively. Knowledge of the whole sequence of DTMUV will be useful for further studies of the mechanisms of virus replication and pathogenesis.
DOI:10.1111/j.1865-1682.2012.01328.xURLPMID:22515847 [本文引用: 1]

The house sparrow (Passer domesticus) is one of the most widely distributed wild birds in China. Tembusu virus (TMUV) strain, TMUV-SDHS, was isolated from house sparrows living around the poultry farms in Shandong Province, Northern China. Genetic analysis of E and NS5 genes showed that it had a close relationship with that of the YY5 strain, which can cause severe egg drop in ducks. Pathogenicity studies showed that the virus is highly virulent when experimentally inoculated into the ducks. These findings show that house sparrows carrying the Tembusu virus may play an important role in transmitting the virus among other species.
DOI:10.1637/10960-101514-RegURLPMID:26473674 [本文引用: 1]

To evaluate the potential use of an inactivated virus-based vaccine for the control and prevention of the newly emerged duck Tembusu virus infection in China, a duck Tembusu virus isolate, Tembusu-HB, was propagated in 12-day-old duck embryos and inactivated by treatment with formaldehyde. The inactivated viral antigen was emulsified with mineral oil, and five batches of the vaccine were manufactured. The immunogenicity and protection efficacy of the vaccine were evaluated in Beijing ducks and Beijing white geese. Results showed that more than 80% of immunized ducks were protected against virulent virus challenge after two intramuscular or subcutaneous injections of the inactivated vaccine, as evidenced by the negative virus isolation results. The protection is also correlated with a positive virus-specific antibody response as detected by ELISA. In contrast, none of the control ducks and geese had any detectable antibody response. Virus was isolated from all control ducks and geese after virulent virus challenge. Interestingly, a variable level of protection (20%-80%) was observed in Beijing white geese immunized twice with the same batches of vaccine, suggesting a species-specific effect of the vaccine. Overall, the results clearly suggest that the inactivated duck Tembusu virus vaccine is immunogenic and provides protection against virulent virus challenge.
DOI:10.1016/j.virol.2013.12.028URLPMID:24503086 [本文引用: 1]

Duck Tembusu virus (DTMUV) is a newly emerging pathogenic flavivirus that is causing massive economic loss in the Chinese duck industry. To obtain a live vaccine candidate against the disease, the DTMUV isolate FX2010 was passaged serially in chicken embryo fibroblasts (CEFs). Characterization of FX2010-180P revealed that it was unable to replicate efficiently in chicken embryonated eggs, nor intranasally infect mice or shelducks at high doses of 5.5log10 tissue culture infectious doses (TCID50). FX2010-180P did not induce clinical symptoms, or pathological lesions in ducks at a dose of 5.5log10TCID50. The attenuation of FX2010-180P was due to 19 amino acid changes and 15 synonymous mutations. Importantly, FX2010-180P elicited good immune responses in ducks inoculated at low doses (3.5log10TCID50) and provided complete protection against challenge with a virulent strain. These results indicate that FX2010-180P is a promising candidate live vaccine for prevention of duck Tembusu viral disease.
DOI:10.3864/j.issn.0578-1752.2016.14.016URL [本文引用: 1]

【Objective】 Tembusu virus BZ-2010 strain was continuously passaged in specific-pathogen-free embryonic (SPF) eggs in order to select a live attenuated vaccine candidate of good safety and immunogenicity properties. 【Method】 Tembusu virus BZ-2010 strain was cultured for 120 passages in SPF eggs. The safety of the 120th passage viral strain was evaluated with 1-day-old SPF ducklings and 30-week-old egg-laying ducks. The property of virulent return of VC2 viral strain was evaluated with 1-day-old SPF ducklings. The neutralizing antibodies were detected after the 18-week-old breeding ducks were immunized with VC2 strain. The protective effects were evaluated after the 25-week-old breeding ducks were immunized with VC2 strain. E gene and NS4A gene of BZ_2010 and VC2 strains were amplified by RT-PCR and sequenced. 【Result】 The average death time of SPF eggs was shortened by passage virus and viral titer was increased with the escalation of passage times in SPF chicken embryonic eggs. ELD50 of the 20th virus was 10-5.3/0.1mL and ELD50 of the 120th virus was 10-5.8/0.1mL.The viral titer reached the plateau at passage 80 and remained unchanged further passages. The experimental ducks showed no clinical symptoms after 1-day-old ducklings and 30-week-old breeding ducks were immunized with VC2 strain by subcutaneous injection and by intramuscular injection, respectively. The results showed that VC2 strain had a good safety. No symptoms appeared in 1-day-old ducklings in which VC2 strain were cultured for 5 passages. 1-day-old ducklings were infected with the 5th tissue suspension and no symptoms were observed in liver pathological section by microscope. The results showed that VC2 strain had a good stability. Sequence analysis revealed that the E protein of Tembusu VC2 evolved amino acid changes in positions 86, 157, 189, 301, and 302, respectively. The NS4A protein of Tembusu VC2 only had one amino acid change in position 54 in that phenylalanine was replaced by Leucine. The level of antibodies rose very quickly, reached the plateau at the 4th week and remained a long time. Ducks were challenged by TMUV virulent strain at 2 and 50 weeks after immunization with VC2 strain in the experimental group. There was no symptom, normal stool, and regular egg production in the vaccinated group after challenge of virulent strain. The results showed that VC2 strain could provide complete protection for the challenge of TMUV virulent strain.【Conclusions】 An attenuated strain of TMUV with good immunogenicity and highsafety was acquired through serial passages of SPF chicken embryos. The level of antibodies rose very quickly and remained a long time after immunization of the VC2 attenuatedstrain. The toxicity attack experiments showed that VC2 could provide complete protection for the challenge of TMUV virulent strain.
DOI:10.3864/j.issn.0578-1752.2016.14.016URL [本文引用: 1]

【Objective】 Tembusu virus BZ-2010 strain was continuously passaged in specific-pathogen-free embryonic (SPF) eggs in order to select a live attenuated vaccine candidate of good safety and immunogenicity properties. 【Method】 Tembusu virus BZ-2010 strain was cultured for 120 passages in SPF eggs. The safety of the 120th passage viral strain was evaluated with 1-day-old SPF ducklings and 30-week-old egg-laying ducks. The property of virulent return of VC2 viral strain was evaluated with 1-day-old SPF ducklings. The neutralizing antibodies were detected after the 18-week-old breeding ducks were immunized with VC2 strain. The protective effects were evaluated after the 25-week-old breeding ducks were immunized with VC2 strain. E gene and NS4A gene of BZ_2010 and VC2 strains were amplified by RT-PCR and sequenced. 【Result】 The average death time of SPF eggs was shortened by passage virus and viral titer was increased with the escalation of passage times in SPF chicken embryonic eggs. ELD50 of the 20th virus was 10-5.3/0.1mL and ELD50 of the 120th virus was 10-5.8/0.1mL.The viral titer reached the plateau at passage 80 and remained unchanged further passages. The experimental ducks showed no clinical symptoms after 1-day-old ducklings and 30-week-old breeding ducks were immunized with VC2 strain by subcutaneous injection and by intramuscular injection, respectively. The results showed that VC2 strain had a good safety. No symptoms appeared in 1-day-old ducklings in which VC2 strain were cultured for 5 passages. 1-day-old ducklings were infected with the 5th tissue suspension and no symptoms were observed in liver pathological section by microscope. The results showed that VC2 strain had a good stability. Sequence analysis revealed that the E protein of Tembusu VC2 evolved amino acid changes in positions 86, 157, 189, 301, and 302, respectively. The NS4A protein of Tembusu VC2 only had one amino acid change in position 54 in that phenylalanine was replaced by Leucine. The level of antibodies rose very quickly, reached the plateau at the 4th week and remained a long time. Ducks were challenged by TMUV virulent strain at 2 and 50 weeks after immunization with VC2 strain in the experimental group. There was no symptom, normal stool, and regular egg production in the vaccinated group after challenge of virulent strain. The results showed that VC2 strain could provide complete protection for the challenge of TMUV virulent strain.【Conclusions】 An attenuated strain of TMUV with good immunogenicity and highsafety was acquired through serial passages of SPF chicken embryos. The level of antibodies rose very quickly and remained a long time after immunization of the VC2 attenuatedstrain. The toxicity attack experiments showed that VC2 could provide complete protection for the challenge of TMUV virulent strain.
DOI:10.3864/j.issn.0578-1752.2016.14.018URL [本文引用: 1]

【Objective】The objective of this study is to evaluate the efficacy of maternal antibodies induced by Duck Tembusu Virus Disease Inactivated Vaccine and to determine the age of optimal initial immunity.【Method】Fertilized eggs were collected at random from the Cherry Valley Duck farm which was 135 days post-vaccination with Duck Tembusu Virus Disease Inactivated Vaccine (HB strain),ten progeny ducklings from the immunized breed ducks and 5 progeny ducklings from non-immunized breed ducks wererandomly selected when they were 5 ,7 ,10, and 15 days old. Serum samples were collected from all ducks for the detection of maternal antibody, then the ducks were challenged with Duck Tembusu virus (HB strain) at 0.1ml(100DID50)/duck intramuscularly. Clinical symptoms of the challenged ducks were observed within 10 days, such as food intake, feces, abnormal clinical sighs and death. Serum samples were collected from all ducks for virus isolation via jugular vein on 2 days post inoculation (DPI). Each serum sample was inoculated into five 6-day-old SPF chicken embryos at the inoculum of 0.1 ml per embryo. Then they were hatched at 37℃ for 168h. The chicken embryos died within 24h were discarded. If more than one (including one) death chicken embryos were obsearved, then it was concluded that virus isolation was positive. The rate of protection of ducklings with maternal antibody and the morbidity of ducklings without maternal antibody were calculated. On 5 dpi, all ducklings were weighed respectively, and the average daily gain was calculated. The effect of maternal antibody on the weight gain of ducklings were analyzed by T test for paired samples. The efficacy of maternal antibodies was evaluated by neutralizing antibody titer, body weight changes and virus isolation.【Result】 (1) The number of positive maternal antibody titers peaked in 1 day old ducklings was 56.1%(37/66), then fell to 40% (4/10) in ducklings on day 5, 50% (5/10) on day 7, 30% (3/10) on day 10, and 0% (0/10) on day 15. (2)On viral challenge, the control group showed signs of depression (20/20), neurologic disturbances (6/20) and death (2/20). Ducklings with positive maternal antibody titers showed mild depression. (3) On 5 dpi, the average daily gain of 5-, 7-, 10- and 15-day old ducklings with maternal antibody were 115.5, 142.8, 177.8 and 162.2g, respectively, and that of the ducklings without maternal antibody were 54.5, 91, 165 and 118.8g, respectively. (4) The rate of protection against challenge with DTMUV of 5-, 7-, 10- and 15-day old ducklings with maternal antibody were 50%(5/10), 60%(6/10), 20%(2/10) and 0%(0/10), respectively. The morbidity of 5-, 7-, 10- and 15-day old ducklings without maternal antibody were all 100%. (5) The average weight gain and efficacy reached a peak in 5-day old and 7-day old ducklings, which were 50% (5/10) and 60% (6/10), respectively. Although the maternal antibodies decreased between 10 days old and 15 days old ducklings (20% and 0%), it still has protective effect compared with the control group. 【Conclusion】(1) Duck Tembusu Virus Disease killed vaccine maternal antibodies, so it play an important role in the protection of 10-day-old ducklings against virus infection; (2) Vaccination age is optimized between 7 to 10 days of age.
DOI:10.3864/j.issn.0578-1752.2016.14.018URL [本文引用: 1]

【Objective】The objective of this study is to evaluate the efficacy of maternal antibodies induced by Duck Tembusu Virus Disease Inactivated Vaccine and to determine the age of optimal initial immunity.【Method】Fertilized eggs were collected at random from the Cherry Valley Duck farm which was 135 days post-vaccination with Duck Tembusu Virus Disease Inactivated Vaccine (HB strain),ten progeny ducklings from the immunized breed ducks and 5 progeny ducklings from non-immunized breed ducks wererandomly selected when they were 5 ,7 ,10, and 15 days old. Serum samples were collected from all ducks for the detection of maternal antibody, then the ducks were challenged with Duck Tembusu virus (HB strain) at 0.1ml(100DID50)/duck intramuscularly. Clinical symptoms of the challenged ducks were observed within 10 days, such as food intake, feces, abnormal clinical sighs and death. Serum samples were collected from all ducks for virus isolation via jugular vein on 2 days post inoculation (DPI). Each serum sample was inoculated into five 6-day-old SPF chicken embryos at the inoculum of 0.1 ml per embryo. Then they were hatched at 37℃ for 168h. The chicken embryos died within 24h were discarded. If more than one (including one) death chicken embryos were obsearved, then it was concluded that virus isolation was positive. The rate of protection of ducklings with maternal antibody and the morbidity of ducklings without maternal antibody were calculated. On 5 dpi, all ducklings were weighed respectively, and the average daily gain was calculated. The effect of maternal antibody on the weight gain of ducklings were analyzed by T test for paired samples. The efficacy of maternal antibodies was evaluated by neutralizing antibody titer, body weight changes and virus isolation.【Result】 (1) The number of positive maternal antibody titers peaked in 1 day old ducklings was 56.1%(37/66), then fell to 40% (4/10) in ducklings on day 5, 50% (5/10) on day 7, 30% (3/10) on day 10, and 0% (0/10) on day 15. (2)On viral challenge, the control group showed signs of depression (20/20), neurologic disturbances (6/20) and death (2/20). Ducklings with positive maternal antibody titers showed mild depression. (3) On 5 dpi, the average daily gain of 5-, 7-, 10- and 15-day old ducklings with maternal antibody were 115.5, 142.8, 177.8 and 162.2g, respectively, and that of the ducklings without maternal antibody were 54.5, 91, 165 and 118.8g, respectively. (4) The rate of protection against challenge with DTMUV of 5-, 7-, 10- and 15-day old ducklings with maternal antibody were 50%(5/10), 60%(6/10), 20%(2/10) and 0%(0/10), respectively. The morbidity of 5-, 7-, 10- and 15-day old ducklings without maternal antibody were all 100%. (5) The average weight gain and efficacy reached a peak in 5-day old and 7-day old ducklings, which were 50% (5/10) and 60% (6/10), respectively. Although the maternal antibodies decreased between 10 days old and 15 days old ducklings (20% and 0%), it still has protective effect compared with the control group. 【Conclusion】(1) Duck Tembusu Virus Disease killed vaccine maternal antibodies, so it play an important role in the protection of 10-day-old ducklings against virus infection; (2) Vaccination age is optimized between 7 to 10 days of age.
[本文引用: 1]
[本文引用: 1]
DOI:10.1371/journal.pone.0053026URLPMID:23300851 [本文引用: 1]

Since April 2010, domesticated ducks in China have been suffering from an emerging infectious disease characterized by retarded growth, high fever, loss of appetite, decline in egg production, and death. The causative agent was identified as a duck Tembusu virus (DTMUV), a member of the Ntaya virus (NTAV) group within the genus Flavivirus, family Flaviviridae. DTMUV is highly contagious and spreads rapidly in many species of ducks. More than 10 million shelducks have been infected and approximately 1 million died in 2010. The disease remains a constant threat to the duck industry; however, it is not known whether DTMUV can infect humans or other mammalians, despite the fact that the virus has spread widely in southeast China, one of the most densely populated areas in the world. The lack of reliable methods to detect the serum antibodies against DTMUV has limited our ability to conduct epidemiological investigations in various natural hosts and to evaluate the efficiency of vaccines to DTMUV.
URL [本文引用: 1]

An outbreak of egg-drop syndrome occurred on a Sheldrake duck farm in Longhai in Fujian Province, China, in 2012. The main clinical symptoms were sharply reduced egg production, crooked necks, and death. We isolated the virus from the sick ducks, identified it, and observed the histopathologic changes after viral infection. We detected viral RNA in the blood and feces of the infected ducks and developed a latex-agglutination diagnostic method to detect anti-Tembusu-virus antibodies. Our results show that the pathogenic virus is a Tembusu virus. The histopathologic changes included follicular cell degeneration and necrosis, follicular cavity filled with blood cells, massive necrosis in the brain, and degeneration and necrosis of the nerve and glial cells. When the transmission of the virus in the infected ducks was studied, the duck blood was positive for viral nucleic acid for up to 29 days, and the feces were positive for viral nucleic acid for up to 13 days. We successfully established a simple, rapid, and easy-to-use latex-agglutination diagnostic method for the detection of antibodies against duck Tembusu virus.
DOI:10.1016/j.jviromet.2017.08.022URLPMID:28864043 [本文引用: 1]

Duck Tembusu virus (DTMUV), a novel flavivirus, causes severe disease in ducks. There is an urgent need for a rapid and effective diagnostic method to control the spread of DTMUV. We chose the envelope (E) protein from DTMUV as an antigen and combined it with colloidal gold particles as tracers to specifically detect anti-DTMUV antibodies. Based on the double-antigen sandwich format, an immunochromatographic strip (ICS) for the rapid detection of anti-DTMUV antibodies was developed. The ICS showed a high specificity and no cross-reactivity with other sera. By detecting a serially diluted duck anti-DTMUV serum, the sensitivity of the ICS was 16-fold higher than that of the agar gel double diffusion test. Moreover, the ICS was both stable and reproducible, maintaining the same performance at 4°C for at least 6 months. To evaluate the effectiveness of the ICS, 217 duck serum samples were tested with the ICS and an indirect enzyme-linked immunosorbent assay (iELISA). The consistency ratio of positive and negative results between the two methods was 97.87% and 97.06%, respectively. The agreement between the ICS and the ELISA was 97.24%. The ICS developed in this study offers a specific, sensitive, and rapid method to detect anti-DTMUV antibodies.
URL [本文引用: 3]

从以产蛋下降为主的樱桃谷种鸭以及出现神经症状的雏鸭各分离出1株病毒,分别命名为BZ株和LC株。该2株病毒对SPF鸡胚和健康鸭胚均能产生相同的病变,分离病毒不能凝集鸡、鸭、鹅、鸽等的红细胞,在鸭胚成纤维细胞(DEF)能够产生典型的细胞病变(CPE),电镜下观察到约50 nm的病毒粒子。病理组织学研究表明,二者在临床上均可导致脑组织危害,表现为脑膜水肿、血管充血和皮质层神经胶质细胞增生等。血清学检测表明,分离病毒与禽流感病毒(AIV)、鸭瘟病毒(DEV)、新城疫病毒(NDV)等病原无交叉。生物学特性鉴定该病原为有囊膜单股RNA病毒。利用不同禽病的特异性引物分别进行PCR或RTPCR,均未扩增出特异条带。设计随机引物进行RTPCR,扩增出基因片段,利用GenBank进行Blast同源比较,结果发现,分离病毒与以色列火鸡脑膜脑炎病毒(Israel turkey meningoencephalitis virus,TMEV)和在马来西亚发现的Tembumu 病毒至少在2段基因上具有较高的同源性,属于黄病毒属。测序表明,分离病毒与Tembumu 病毒的非结构蛋白(NS5基因)和囊膜蛋白(E基因)的核苷酸同源性为86.7%~90.2% 和87.0%~91.8%,与TMEV的NS5基因和E基因的同源性为72.4%~73.2%和72.7%~72.8%。2分离株之间E基因和NS5基因的核苷酸同源性均为99.5%。血清中和试验表明,BZ株阳性血清可以中和LC病毒,因此证实二者可能是同一种病毒。综合以上研究,建议将该病命名为“鸭病毒性脑炎”(Duck viral encephalitis disease)。
URL [本文引用: 3]

从以产蛋下降为主的樱桃谷种鸭以及出现神经症状的雏鸭各分离出1株病毒,分别命名为BZ株和LC株。该2株病毒对SPF鸡胚和健康鸭胚均能产生相同的病变,分离病毒不能凝集鸡、鸭、鹅、鸽等的红细胞,在鸭胚成纤维细胞(DEF)能够产生典型的细胞病变(CPE),电镜下观察到约50 nm的病毒粒子。病理组织学研究表明,二者在临床上均可导致脑组织危害,表现为脑膜水肿、血管充血和皮质层神经胶质细胞增生等。血清学检测表明,分离病毒与禽流感病毒(AIV)、鸭瘟病毒(DEV)、新城疫病毒(NDV)等病原无交叉。生物学特性鉴定该病原为有囊膜单股RNA病毒。利用不同禽病的特异性引物分别进行PCR或RTPCR,均未扩增出特异条带。设计随机引物进行RTPCR,扩增出基因片段,利用GenBank进行Blast同源比较,结果发现,分离病毒与以色列火鸡脑膜脑炎病毒(Israel turkey meningoencephalitis virus,TMEV)和在马来西亚发现的Tembumu 病毒至少在2段基因上具有较高的同源性,属于黄病毒属。测序表明,分离病毒与Tembumu 病毒的非结构蛋白(NS5基因)和囊膜蛋白(E基因)的核苷酸同源性为86.7%~90.2% 和87.0%~91.8%,与TMEV的NS5基因和E基因的同源性为72.4%~73.2%和72.7%~72.8%。2分离株之间E基因和NS5基因的核苷酸同源性均为99.5%。血清中和试验表明,BZ株阳性血清可以中和LC病毒,因此证实二者可能是同一种病毒。综合以上研究,建议将该病命名为“鸭病毒性脑炎”(Duck viral encephalitis disease)。
[本文引用: 2]
[本文引用: 2]
DOI:10.3864/j.issn.0578-1752.2013.05.020URL [本文引用: 3]

【Objective】 The objective of the study is to isolate Tembusu virus from goose and explore its pathogenicity to young goose.【Method】RT-PCR was applied to detect Tembusu virus in sick geese samples collected from three farms in Jiangsu, in which the positive samples were used to isolate Tembusu virus. The biological characteristics and sequence of Envelope (E) gene of isolated virus were determined.【Result】These samples were all PCR positive for Tembusu virus. One strain was isolated from a positive sample and named as SHYG. Sequence analysis based on E gene suggested that its sequence was homologous highly with that of Tembusu viruses isolated from ducks in China. The SHYG strain induced cytopathic effect in Vero cell. The results of animal experiment revealed that the SHYG strain caused depression, diarrhea, neural symptom, and even death when it was inoculated to 2-week-old geese, but no pathogenic to mice. The histological observation showed congestion, bile capillary expansion and steatosis in liver, hemorrhage, inflammatory exudate and hemosiderin pigmentation in lung, reticuloendothelial cell vacuolization in spleen, hemorrhage in kidney, and hyperemia and proliferation of gliocyte in brain. 【Conclusion】 The Tembusu virus strain SHYG is pathogenic to young goose.
DOI:10.3864/j.issn.0578-1752.2013.05.020URL [本文引用: 3]

【Objective】 The objective of the study is to isolate Tembusu virus from goose and explore its pathogenicity to young goose.【Method】RT-PCR was applied to detect Tembusu virus in sick geese samples collected from three farms in Jiangsu, in which the positive samples were used to isolate Tembusu virus. The biological characteristics and sequence of Envelope (E) gene of isolated virus were determined.【Result】These samples were all PCR positive for Tembusu virus. One strain was isolated from a positive sample and named as SHYG. Sequence analysis based on E gene suggested that its sequence was homologous highly with that of Tembusu viruses isolated from ducks in China. The SHYG strain induced cytopathic effect in Vero cell. The results of animal experiment revealed that the SHYG strain caused depression, diarrhea, neural symptom, and even death when it was inoculated to 2-week-old geese, but no pathogenic to mice. The histological observation showed congestion, bile capillary expansion and steatosis in liver, hemorrhage, inflammatory exudate and hemosiderin pigmentation in lung, reticuloendothelial cell vacuolization in spleen, hemorrhage in kidney, and hyperemia and proliferation of gliocyte in brain. 【Conclusion】 The Tembusu virus strain SHYG is pathogenic to young goose.
[本文引用: 2]
[本文引用: 2]
[本文引用: 3]
[本文引用: 3]
DOI:10.1016/j.saa.2019.117908URLPMID:31841672 [本文引用: 2]

A new probe (SRh) which based on dual-binding benzene and rhodamine B conjugate derivatives for hypochlorite detection was developed. By desulfurization effect, probe SRh displayed"Off-On" switching in its fluorogenic and chromogenic responses to hypochlorite. The detection limit of ClO- was at a low level (up to 2.43?nM). Moreover, probe SRh has been applied in bioimaging with good biocompatibility and low cytotoxicity.
DOI:10.1016/j.saa.2019.117908URLPMID:31841672 [本文引用: 2]

A new probe (SRh) which based on dual-binding benzene and rhodamine B conjugate derivatives for hypochlorite detection was developed. By desulfurization effect, probe SRh displayed"Off-On" switching in its fluorogenic and chromogenic responses to hypochlorite. The detection limit of ClO- was at a low level (up to 2.43?nM). Moreover, probe SRh has been applied in bioimaging with good biocompatibility and low cytotoxicity.
[本文引用: 2]
[本文引用: 2]
DOI:10.20506/rst.36.3.2713URLPMID:30160702 [本文引用: 2]

The Biological Standards Commission of the World Organisation for Animal Health (OIE) oversees the preparation and validation of OIE-approved International Reference Standards for use in serological assays for detecting infectious diseases of animals or the adequacy of their immune response following vaccination against those diseases. The principal use of OIE-approved International Reference Standards is to harmonise serological testing and to promote the mutual recognition of test results for international trade. In the OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, the organisation recommends the use of the OIE anti-rabies positive reference serum of dog origin to titrate serum samples in international units (IU)/ml for use in rabies serological tests. The first batch of OIE reference serum of dog origin was produced in1991 and was used internationally until the beginning of 2010. The preparation of the new batch began in 2012 and, in contrast to the previous batch, three commercial inactivated rabies vaccines based on the most frequently used vaccine strains (Pasteur Virus and Flury Low Egg Passage) were selected for the immunisation of dogs in accordance with OIE guidelines. In 2013, calibration was completed through an inter-laboratory test involving five OIE Reference Laboratories for Rabies with the Second World Health Organization (WHO) International Standard for Anti-Rabies Immunoglobulin being used as a reference standard in this calibration. After statistical analysis of the results, the consensus titre was established as 5.59 IU/ml. The technical and statistical data were submitted to the OIE for assessment. In February 2014, the OIE Biological Standards Commission adopted this serum as an OIE-approved standard reagent for rabies serology.
DOI:10.4269/ajtmh.1958.7.561URLPMID:13571577 [本文引用: 1]
[本文引用: 2]
[本文引用: 2]
