 ,重庆师范大学生命科学学院,重庆401331
,重庆师范大学生命科学学院,重庆401331Cloning and Expression Analysis of Jasmonic Acid Carboxyl Methyltransferase Gene from Perilla frutescens
BAI HuiYang, LU Geng, LU JunXing, GUAN Li, TANG Xin, ZHANG Tao ,College of Life Sciences, Chongqing Normal University, Chongqing 401331
,College of Life Sciences, Chongqing Normal University, Chongqing 401331通讯作者:
收稿日期:2019-01-2接受日期:2019-03-12网络出版日期:2019-05-01
| 基金资助: |
Received:2019-01-2Accepted:2019-03-12Online:2019-05-01
作者简介 About authors
白辉扬,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (2537KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
白辉扬, 鲁庚, 陆俊杏, 管丽, 唐鑫, 张涛. 紫苏茉莉酸羟基甲基转移酶基因PfJMT的克隆及表达分析[J]. 中国农业科学, 2019, 52(9): 1657-1666 doi:10.3864/j.issn.0578-1752.2019.09.016
BAI HuiYang, LU Geng, LU JunXing, GUAN Li, TANG Xin, ZHANG Tao.
0 引言
【研究意义】JA及其衍生物MeJA是植物防御以及多种发育过程(如种子萌发、开花、果实发育和衰老)的重要细胞调节剂[1,2,3]。JMT是MeJA生物合成的关键酶,负责将JA甲基化为MeJA[4]。通过对JMT表达模式的研究,对植物防御和植物发育的调控,以及进一步利用JMT改良作物品种具有重要意义。【前人研究进展】茉莉酸起源于α-亚麻酸,在质体中经脂氧合酶(lipoxygenase,LOX)氧化,然后通过丙二烯氧化物合酶(allene oxide synthase,AOS)和丙二烯氧化物环化酶(allene oxide cyclase,AOC)转化为12-氧代植二烯酸(12-OPDA),再转运到过氧化物酶体,最后经过3次β氧化形成茉莉酸[5,6,7]。JA有多种代谢途径,通常经JMT甲基化产生MeJA或者通过茉莉酸共轭合酶(JA conjugate synthase,JAR1)与氨基酸(如异亮氨酸)缀合,形成高生物活性的茉莉酸异亮氨酸共轭物(jasmonoyl-isoleucine,JA-Ile)[8]。茉莉酸及其衍生物是拟南芥[9]和番茄[10]花发育的最后阶段所必需的,STITZ等[11]对烟草开花的研究中也发现茉莉酸及其衍生物对开花起调控作用,JMT可以通过调节茉莉酸相关代谢物的水平参与此过程。SEO等[4]从拟南芥中克隆获得AtJMT,发现过表达AtJMT增强了拟南芥对灰霉病菌的抗性。WU等[12]研究发现,MeJA处理引发的抗性不是由MeJA直接引起,而是由其去甲基化产物JA所引起。STITZ等[13]在烟草中过表达AtJMT并沉默了烟草甲基茉莉酯酶基因(methyl jasmonate esterase,MJE),发现在伤口处理后,这种突变体(35S-jmt/ir-mje)未能出现和野生型一样的JA,JA-Ile的上调,而是被一系列内源性MeJA取代,结果导致在总茉莉酸(Jas)水平没有差异的情况下,苏氨酸脱氨酶和胰蛋白酶抑制剂(2种JA诱导的防御基因)的表达均显着降低。QI等[14]对水稻OsJMT的研究发现,OsJMT通过介导JA和相关代谢物的水平,在水稻的生长和防御中起作用,MeJA可能是调节植物生长发育而非防御的信号,而JA和其他信号,如JA-Ile可能主要介导防御。并且发现过表达OsJMT会显著降低穗长、每穗颖花数和结实率,导致单株产量减少,但千粒重有所提高。在过去对JMT的研究报道中,过表达JMT通常会导致植物种子减产。如在拟南芥中,CIPOLLINI等[15]过表达AtJMT导致拟南芥种子重量和数量的减少。KIM等[16]在水稻中过表达AtJMT,也导致了谷粒产量和幼苗高度的降低。但有趣的是,过表达JMT却会使块茎类作物增产,如SOHN等[17]在马铃薯中过表达AtJMT,导致马铃薯的块茎产量和大小的增加,并且JMT的表达水平越高,块茎的产量也越高。而KIM等[18]在人参中过表达AtJMT也导致人参根系生长和人参皂苷异质性的刺激。NAM等[19]对过表达AtJMT大豆种子进行评估,发现转基因大豆的各成分均在商业上可获得的大豆参考范围内,从而证明了这些转基因和非转基因大豆种子的实质等同性。以上研究表明,JMT可能间接参与植物防御反应以及在种子和块茎发育过程中起着重要作用,且具有一定的商业应用前景。【本研究切入点】紫苏含有丰富的MeJA合成前体α-亚麻酸,是研究α-亚麻酸代谢途径中JMT功能较好的植物[20,21]。之前均是对JMT在植物防御中作用的研究,而关于JMT在种子发育中的研究鲜有报道。【拟解决的关键问题】本文通过对紫苏各组织包括不同发育时期种子中JMT表达的研究,揭示PfJMT在紫苏种子发育中的表达模式。并使用外源MeJA和SA处理植物,研究PfJM在不同胁迫中的表达模式。为解决过表达JMT对植物种子产量的影响提供参考,验证JMT在植物防御反应中的作用。1 材料与方法
1.1 材料
紫苏种子由重庆师范大学油用牡丹种质资源创新与利用重点实验室保存。大肠杆菌DH5α、Premix Taq酶、DL2000Maker、T/A克隆载体pGEM-T、T4 DNA连接酶、逆转录试剂盒均购于TaKaRa公司。EASYspin植物RNA快速提取试剂盒(DNaseⅠ)购于北京博迈德公司。荧光定量试剂盒购于成都百乐公司。2018年4月将紫苏种子播种于重庆师范大学生命科学学院实验基地,取开花期的根、茎、叶、花等组织,并取开花后5、10、15、20、25和30 d的种子,液氮速冻后,-80℃保存备用。2018年7月,用25 μmol·L-1 MeJA[22]和1 mmol·L-1 SA[23]喷洒处理种植于花盆中具有4片真叶的紫苏幼苗,并浇灌根部,分别取处理0、2、4、8、16、24和48 h后的紫苏根、叶组织,液氮速冻后,-80℃保存备用。
1.2 PfJMT的克隆
采用CTAB法提取紫苏种子DNA,利用植物总RNA试剂盒提取紫苏各组织RNA,并反转录为cDNA,-20℃保存备用(所有操作都严格按照说明书进行)。根据紫苏种子转录组测序结果[24],设计PfJMT引物(PfJMT-F:5′-ATGGAAGTAGTACAAGTACT -3′,PfJMT-R:5′-TTATCTCCTGGTCAACGAAACG -3′)。分别以紫苏DNA和种子cDNA为模板进行PCR扩增,将胶回收产物与pGEM-T载体连接,转化,筛选阳性单克隆测序。引物合成和测序均由英潍捷基有限公司完成。1.3 PfJMT的生物信息学分析
利用NCBI在线工具BLAST(https://blast.ncbi.nlm. nih.gov/Blast.cgi)对PfJMT的氨基酸序列进行比对分析。使用Vector NTI系列软件进行序列分析,查找ORF、翻译成氨基酸序列等。使用ExPASy(https:// www.expasy.org/)分析PfJMT蛋白的分子量、等电点pI、疏水性等。用SignalP4.1(http://www.cbs.dtu.dk/ services/SignalP/)预测信号肽,用TMHMM Server v.2.0(http://www.cbs.dtu.dk/ services/TMHMM/)分析跨膜区,利用Softberry(http://linux1.softberry.com/ berry.phtml)预测亚细胞定位。使用CDD(www.ncbi. nlm.nih.gov/Structure/cdd/ wrpsb.cgi)分析其保守结构域。使用软件DNAMAN进行多序列比对分析。采用邻接法,利用MEGA7.0软件对不同物种的JMT蛋白构建系统进化树。1.4 PfJMT的表达分析
根据已克隆获得的紫苏PfJMT的ORF序列,设计qRT-PCR引物(PfJMT-RT-F:5′-CAACGTGGCGAA ATGCATGA-3′,PfJMT-RT-R:5′-TCTCCTGGTCAA CGAAACGG-3′),扩增产物大小为166 bp。分别以紫苏各组织cDNA为模版,以紫苏18S为内参(18S-F:5′-CGGCGACGCATTCAAA-3′,18S-R:5′-GCTGCCT TCCTTGGATGTGG-3′)。qRT-PCR检测PfJMT在不同组织器官中的表达水平,重复3次。试验结果用SPSS 13软件进行统计分析,当P<0.05时,统计结果具有统计学意义。柱形图用Origin7.0进行绘制。2 结果
2.1 PfJMT的克隆和生物信息学分析
在紫苏种子转录组测序结果中发现一个紫苏JMT,经分析发现该序列具有完整的ORF。根据紫苏JMT序列设计引物,分别以紫苏DNA和种子cDNA为模板,克隆获得紫苏JMT序列,将其命名为PfJMT(图1-A)。PfJMT具有3个外显子和2个内含子(图1-B),ORF为1 050 bp,编码349个氨基酸。PfJMT预测蛋白分子量为39.4 kD,pI为5.79,带负电荷的氨基酸(Asp+Glu)为43个,带正电荷的氨基酸(Arg+Lys)为36个,不稳定系数为53.95,属于不稳定蛋白。平均亲水性(GRAVY)为-0.137,属于亲水性蛋白。预测PfJMT蛋白无信号肽,无跨膜域,定位于细胞质。PfJMT含有一个甲基转移酶-7(Methyltransf-7)结构域(图1-C)。图1
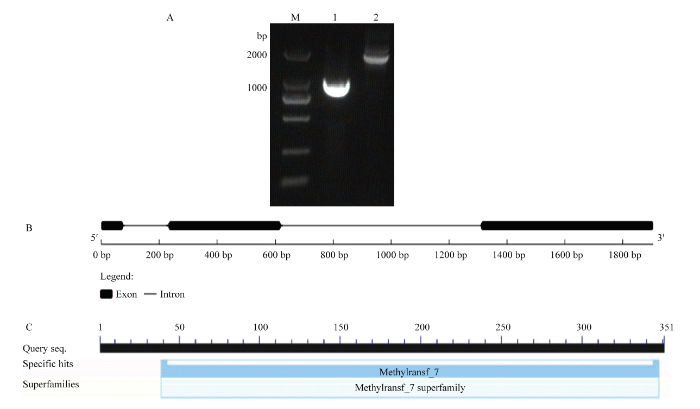 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1PfJMT的克隆与分析
A:PfJMT的PCR扩增产物,M:DL2000;1:PfJMT ORF扩增产物;2:PfJMT的DNA扩增产物;B:PfJMT的结构;C:保守结构域的预测
Fig. 1Cloning and analysis of the PfJMT
A: PCR amplification product of PfJMT, M: DL2000; 1: PfJMT ORF amplification product; 2: PfJMT DNA amplification product; B: Structure of PfJMT; C: Prediction of conserved domain
2.2 PfJMT的聚类分析
通过比对紫苏、棉花Gossypium hirsutum(AJQ31847.1)、可可Theobroma cacao(EOY14822.1)、蓖麻Ricinus communis(XP 002510424.1)、拟南芥Arabidopsis thaliana(AEE29876.1)、向日葵Helianthus annuus(OTG00826.1)、青蒿Artemisia annua(AIN76708.1)、建兰Cymbidium ensifolium(AFH89623.1)、丹参Salvia miltiorrhiza(APC65704.1)、蒺藜苜蓿Medicago truncatula(XP 003638267.1)和水稻Oryza sativa Japonica Group(XP_015639512.1)的JMT序列。发现PfJMT与丹参的氨基酸序列相似度最高,为80.5%;与水稻的序列相似度最低,为36.8%(表1)。使用DNAMAN对其中5个物种的JMT进行多序列比对,发现1个保守基序的部分位置用方框标注(图2),这段基序存在于甲基转移酶-7结构域中,可能与JMT的功能密切相关。Table 1
表1
表1不同植物JMT蛋白一致率
Table 1
| 名称 Name | 水稻Oryza sativa | 紫苏 Perilla frutescens | 丹参 Salvia miltiorrhiza | 建兰Cymbidium ensifolium | 苜蓿Medicago truncatula | 向日葵Helianthus annuus | 青蒿Artemisia annua | 拟南芥Arabidopsis thaliana | 蓖麻 Ricinus communis | 棉花Gossypium hirsutum | 可可 Theobroma cacao |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 水稻 Oryza sativa | 36.80 | 35.88 | 41.57 | 36.62 | 37.87 | 37.54 | 38.29 | 40.41 | 36.84 | 38.08 | |
| 紫苏Perilla frutescens | 36.80 | 80.52 | 48.70 | 45.27 | 45.93 | 46.97 | 46.84 | 49.86 | 49.13 | 51.30 | |
| 丹参 Salvia miltiorrhiza | 35.88 | 80.52 | 46.57 | 43.73 | 46.18 | 46.20 | 44.97 | 48.31 | 48.02 | 48.03 | |
| 建兰 Cymbidium ensifolium | 41.57 | 48.70 | 46.57 | 44.08 | 46.20 | 45.81 | 45.80 | 50.55 | 47.37 | 47.25 | |
| 苜蓿 Medicago truncatula | 36.62 | 45.27 | 43.73 | 44.08 | 53.19 | 54.40 | 49.47 | 55.56 | 57.49 | 57.72 | |
| 向日葵Helianthus annuus | 37.87 | 45.93 | 46.18 | 46.20 | 53.19 | 75.76 | 55.40 | 56.94 | 56.91 | 56.63 | |
| 青蒿 Artemisia annua | 37.54 | 46.97 | 46.20 | 45.81 | 54.40 | 75.76 | 55.22 | 58.68 | 58.29 | 57.26 | |
| 拟南芥Arabidopsis thaliana | 38.29 | 46.84 | 44.97 | 45.80 | 49.47 | 55.40 | 55.22 | 60.11 | 60.87 | 59.03 | |
| 蓖麻 Ricinus communis | 40.41 | 49.86 | 48.31 | 50.55 | 55.56 | 56.94 | 58.68 | 60.11 | 67.03 | 68.65 | |
| 棉花 Gossypium hirsutum | 36.84 | 49.13 | 48.02 | 47.37 | 57.49 | 56.91 | 58.29 | 60.87 | 67.03 | 76.22 | |
| 可可 Theobroma cacao | 38.08 | 51.30 | 48.03 | 47.25 | 57.72 | 56.63 | 57.26 | 59.03 | 68.65 | 76.22 |
新窗口打开|下载CSV
图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2不同植物JMT的多序列比对分析
Fig. 2Multiple sequence alignment analysis of different plant JMT
使用DNAMAN对其中5个物种的JMT进行多序列比对,发现1个保守基序的部分位置用方框标注(图2),这段基序存在于甲基转移酶-7结构域中,可能与JMT的功能密切相关。
2.3 PfJMT与其他物种的系统进化树分析
使用MEGA7.0对这11个物种的JMT构建进化树(图3),发现紫苏和丹参亲缘关系最近,其次与棉花、可可、蓖麻、拟南芥等双子叶植物亲缘关系较近,与建兰和水稻2个单子叶植物的亲缘关系较远。表明JMT在单子叶和双子叶植物的进化过程中可能存在较大的差异。图3
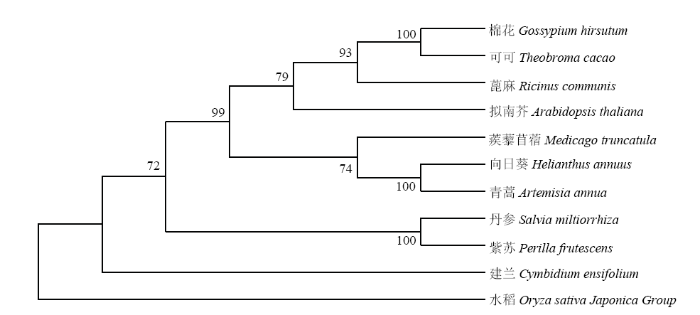 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3不同植物基于JMT氨基酸序列的系统进化树
分支上的数字表示Bootstrap验证中基于1 000次重复该节点的可信度
Fig. 3Phylogenetic tree of different plants based on amino acid of JMT
The number on the branches represent the reliability percent of bootstraps values based on1000 replications
2.4 PfJMT的实时荧光定量分析
通过对紫苏不同组织和不同处理中PfJMT的qRT-PCR分析,结果表明PfJMT在紫苏各组织中均有表达。其中,根和茎中的相对表达量最低,叶和花中的表达量比根和茎中略高,幼嫩种子中的表达量最高。在不同发育时期的种子中,开花后5 d的种子表达量最高,且随着种子的发育,PfJMT的表达量逐渐下调(图4)。在用外源MeJA、SA处理后的紫苏根、叶中,PfJMT的表达和对照相比,均显著下调(图5)。图4
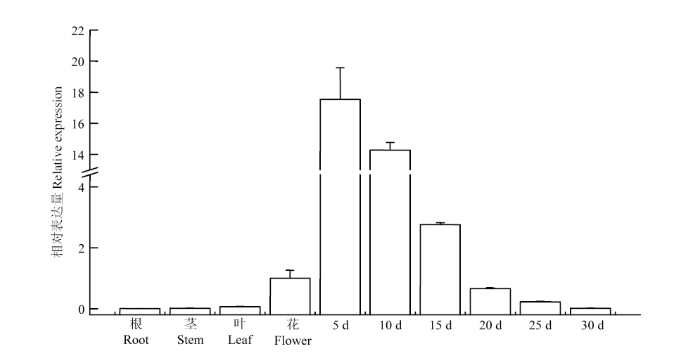 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4PfJMT在不同组织和种子不同发育时期的相对表达量
Fig. 4The relative expression of PfJMT in different tissues and seeds at different developmental stages
图5
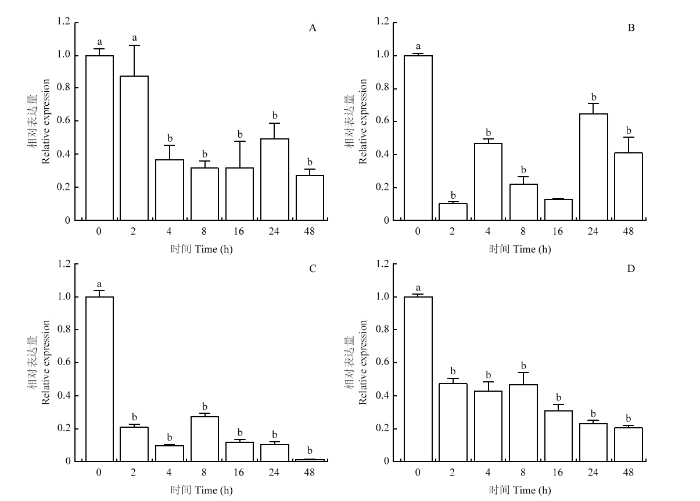 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5MeJA和SA处理对PfJMT表达的影响
A、B:MeJA处理的紫苏叶片(A)和根(B)中PfJMT的表达;C、D:SA处理后的紫苏叶片(C)和根(D)中PfJMT的表达。不同字母表示在P<0.05水平差异显著
Fig. 5Effect of MeJA and SA treatment on PfJMT expression
A,B: Expression of PfJMT in leaves (A) and roots (B) of Perilla frutescens treated with MeJA; C,D: Expression of PfJMT in leaves (C) and roots (D) of Perilla frutescens treated with SA. Different letters indicate statistical difference(P<0.05)
3 讨论
3.1 JMT在不同植物中的保守性较低
本试验根据紫苏种子转录组测序结果设计引物克隆获得PfJMT。生物信息学分析表明,PfJMT蛋白由349个氨基酸组成,pI为5.79,属于不稳定的亲水蛋白,定位于细胞质,与水稻、棉花JMT的亚细胞定位一致[14,25]。保守功能结构域分析发现,PfJMT含有一个甲基转移酶-7结构域,是一种依赖S-腺苷-蛋氨酸(S-adenosyladenyl-L-methionine,SAM)的甲基转移酶,属于SABATH甲基转移酶家族[26,27]。序列比对分析发现,JMT在不同植物中序列差异较大(表1)。系统进化树分析发现,来自单子叶植物和双子叶植物的JMT亲缘关系较远。因此,来自不同植物的JMT在功能上保守,在序列上不保守。3.2 JTM主要在植物发育而非防御反应中发挥作用
组织特异性表达分析发现,PfJMT在紫苏开花期各组织中均有表达,在花和叶中的相对表达量高于根和茎,在开花后5 d的种子中具有最高的表达量,并且随着种子的发育表达量逐渐降低,这与转录组测序的结果相一致[24]。而在拟南芥中,AtJMT只在莲座叶、茎生叶和花中表达,而在根、茎、角果中不表达[4]。茉莉酸途径和水杨酸途径在植物防御反应中发挥着重要的作用,通常JA途径主要介导对食草动物以及坏死性病原体的抗性,SA途径主要介导对生物营养型病原体的抗性[28,29,30]。JA途径和SA途径存在拮抗作用,这是植物长期在各种生物和非生物胁迫中,为了生存而精确分配有限的资源来适应环境的结果[31]。先前研究证明了MeJA处理引起的抗性不是由MeJA直接引起的,而是由其去甲基化产物JA引起的[12]。本研究在使用外源MeJA和SA处理后的紫苏叶和根中,发现JMT表达均显著下调,该结果与MeJA不直接参与防御反应的理论相符,但不同于使用SA处理导致水稻OsJMT表达既有上调又有下调的结果,这可能是由于我们使用过量的MeJA和SA处理所致。MeJA处理后JMT表达下调可能是一种反馈调节,也可能是MeJA作为一种信号分子,在接受到这种信号后,植物为了响应JA途径而下调JMT的表达,并将MeJA去甲基化形成JA参与到后续的防御反应中。而SA处理,抑制了JA途径,JMT的表达也随之降低。以上结果表明,JMT主要在植物发育(如种子发育)中发挥重要作用,而非防御反应。
3.3 JMT在基因工程中的应用
有研究表明,过表达JMT通常会使植物的抗性有所提高,这是由于过表达JMT会导致茉莉酮酸酯水平上升而不影响茉莉酮酸水平的结果[4]。虽然过表达JMT会使块茎类作物的产量提升,但会造成种子类作物产量的降低。在水稻中,过表达AtJMT导致MeJA积累水平增加,介导了胁迫信号导致ABA水平上升,影响了水稻穗的分化,最终导致产量降低[16]。并且,利用水稻自身的JMT在水稻中过表达,也出现了类似情况[14]。本研究发现,PfJMT在种子发育过程中呈现出逐渐降低的表达模式,因此过表达JMT除了会对穗分化产生不良影响,还可能对种子的发育本身带来不利的结果,要将JMT用于改良种子类作物具有较高的难度,但由于过表达JMT会使块茎类作物增加,因此将JMT用于改良块茎类作物将会是简单有效的。4 结论
从紫苏中克隆得到PfJMT的ORF,编码349个氨基酸,该氨基酸序列具有一个甲基转移酶-7结构域,与拟南芥、丹参等双子叶植物亲缘关系较近,而与建兰、水稻等单子叶植物亲缘关系较远。PfJMT在开花后5d的种子中表达量最高,并随着种子的发育表达量逐渐降低。外源MeJA和SA处理,均导致PfJMT的表达量显著下调。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1073/pnas.92.10.4114URLPMID:11607536 [本文引用: 1]

Jasmonic acid (JA) is a naturally occurring growth regulator found in higher plants. Several physiological roles have been described for this compound (or a related compound, methyl jasmonate) during plant development and in response to biotic and abiotic stress. To accurately determine JA levels in plant tissue, we have synthesized JA containing13C for use as an internal standard with an isotopic composition of [225]:[224] 0.98:0.02 compared with [225]:[224] 0.15:0.85 for natural material. GC analysis (flame ionization detection and MS) indicate that the internal standard is composed of 92% 2-( )-[13C]JA and 8% 2-( )-7-iso-[13C]JA. In soybean plants, JA levels were highest in young leaves, flowers, and fruit (highest in the pericarp). In soybean seeds and seedlings, JA levels were highest in the youngest organs including the hypocotyl hook, plumule, and 12-h axis. In soybean leaves that had been dehydrated to cause a 15% decrease in fresh weight, JA levels increased 5-fold within 2 h and declined to approximately control levels by 4 h. In contrast, a lag time of 1-2 h occurred before abscisic acid accumulation reached a maximum. These results will be discussed in the context of multiple pathways for JA biosynthesis and the role of JA in plant development and responses to environmental signals.
DOI:10.1093/aob/mct067URLPMID:23558912 [本文引用: 1]

Jasmonates are important regulators in plant responses to biotic and abiotic stresses as well as in development. Synthesized from lipid-constituents, the initially formed jasmonic acid is converted to different metabolites including the conjugate with isoleucine. Important new components of jasmonate signalling including its receptor were identified, providing deeper insight into the role of jasmonate signalling pathways in stress responses and development. The present review is an update of the review on jasmonates published in this journal in 2007. New data of the last five years are described with emphasis on metabolites of jasmonates, on jasmonate perception and signalling, on cross-talk to other plant hormones and on jasmonate signalling in response to herbivores and pathogens, in symbiotic interactions, in flower development, in root growth and in light perception. The last few years have seen breakthroughs in the identification of JASMONATE ZIM DOMAIN (JAZ) proteins and their interactors such as transcription factors and co-repressors, and the crystallization of the jasmonate receptor as well as of the enzyme conjugating jasmonate to amino acids. Now, the complex nature of networks of jasmonate signalling in stress responses and development including hormone cross-talk can be addressed.
DOI:10.1016/S0168-9525(03)00138-0URLPMID:12850447 [本文引用: 1]

The plant floral scent methyl jasmonate (MeJA) has been identified as a vital cellular regulator that mediates diverse developmental processes and defense responses against biotic and abiotic stresses. The pleiotropic effects of MeJA have raised numerous questions about its regulation for biogenesis and mode of action. Characterization of the gene encoding jasmonic acid carboxyl methyltransferase has provided basic information on the role(s) of this phytohormone in gene-activation control and systemic long-distance signaling. Recent approaches using functional genomics and bioinformatics have identified a whole set of MeJA-responsive genes, and provide insights into how plants use volatile signals to withstand diverse and variable environments.
DOI:10.1073/pnas.081557298URLPMID:11287667 [本文引用: 4]

Methyl jasmonate is a plant volatile that acts as an important cellular regulator mediating diverse developmental processes and defense responses. We have cloned the novel gene JMT encoding an S-adenosyl-L-methionine: jasmonic acid carboxyl methyltransferase (JMT) from Arabidopsis thaliana. Recombinant JMT protein expressed in Escherichia coli catalyzed the formation of methyl jasmonate from jasmonic acid with Kmvalue of 38.5 M. JMT RNA was not detected in young seedlings but was detected in rosettes, cauline leaves, and developing flowers. In addition, expression of the gene was induced both locally and systemically by wounding or methyl jasmonate treatment. This result suggests that JMT can perceive and respond to local and systemic signals generated by external stimuli, and that the signals may include methyl jasmonate itself. Transgenic Arabidopsis overexpressing JMT had a 3-fold elevated level of endogenous methyl jasmonate without altering jasmonic acid content. The transgenic plants exhibited constitutive expression of jasmonate-responsive genes, including VSP and PDF1.2. Furthermore, the transgenic plants showed enhanced level of resistance against the virulent fungus Botrytis cinerea. Thus, our data suggest that the jasmonic acid carboxyl methyltransferase is a key enzyme for jasmonate-regulated plant responses. Activation of JMT expression leads to production of methyl jasmonate that could act as an intracellular regulator, a diffusible intercellular signal transducer, and an airborne signal mediating intra- and interplant communications.
DOI:10.1007/BF02637260URL [本文引用: 1]

Jasmonates are derived from oxygenated fatty acids via the octadecanoid pathway and characterized by a pentacyclic ring structure. They have regulatory function as signaling molecules in plant development and adaptation to environmental stress. Until recently, it was the cyclopentanone jasmonic acid (JA) that attracted most attention as a plant growth regulator. It becomes increasingly clear, however, that biological activity is not limited to JA but extends to, and may even differ between its many metabolities and conjugates as well as its cyclopentenone precursors. The enzymes of jasmonate biosynthesis and metabolism may thus have a regulatory function in controlling the activity and relative levels of different signaling molecules. Such a function is supprted by both the characteration of loss of function mutants in Arabidopsis , and the biochemical characterization of the enzymes themselves.
[本文引用: 1]
DOI:10.1093/aob/mcm079URL [本文引用: 1]
DOI:10.1146/annurev-cellbio-092910-154055URLPMID:22559264 [本文引用: 1]

Abstract Plant hormones have pivotal roles in the regulation of plant growth, development, and reproduction. Additionally, they emerged as cellular signal molecules with key functions in the regulation of immune responses to microbial pathogens, insect herbivores, and beneficial microbes. Their signaling pathways are interconnected in a complex network, which provides plants with an enormous regulatory potential to rapidly adapt to their biotic environment and to utilize their limited resources for growth and survival in a cost-efficient manner. Plants activate their immune system to counteract attack by pathogens or herbivorous insects. Intriguingly, successful plant enemies evolved ingenious mechanisms to rewire the plant's hormone signaling circuitry to suppress or evade host immunity. Evidence is emerging that beneficial root-inhabiting microbes also hijack the hormone-regulated immune signaling network to establish a prolonged mutualistic association, highlighting the central role of plant hormones in the regulation of plant growth and survival.
DOI:10.1105/tpc.8.3.403URLPMID:12239389 [本文引用: 1]

The very high proportions of trienoic fatty acids found in chloroplast membranes of all higher plants suggest that these lipid structures might be essential for photosynthesis. We report here on the production of Arabidopsis triple mutants that contain negligible levels of trienoic fatty acids. Photosynthesis at 22 degrees C was barely affected, and vegetative growth of the mutants was identical with that of the wild type, demonstrating that any requirement for trienoic acyl groups in membrane structure and function is relatively subtle. Although vegetative growth and development were unaffected, the triple mutants are male sterile and produce no seed under normal conditions. Comparisons of pollen development in wild-type and triple mutant flowers established that pollen grains in the mutant developed to the tricellular stage. Exogenous applications of alpha-linolenate or jasmonate restored fertility. Taken together, the results demonstrate that the critical role of trienoic acids in the life cycle of plants is as the precursor of oxylipin, a signaling compound that regulates final maturation processes and the release of pollen.
DOI:10.1105/tpc.017954URL [本文引用: 1]
URL [本文引用: 1]
DOI:10.1007/s00425-008-0690-8URLPMID:2756367 [本文引用: 2]

Treatment with methyl jasmonate (MeJA) elicits herbivore resistance in many plant species and over-expression of JA carboxyl methyltransferase (JMT) constitutively increases JA-induced responses in Arabidopsis. When wild-type (WT) Nicotiana attenuata plants are treated with MeJA, a rapid transient endogenous JA burst is elicited, which in turn increases levels of nicotine and trypsin proteinase inhibitors (TPIs) and resistance to larvae of the specialist herbivore, Manduca sexta. All of these responses are impaired in plants silenced in lipoxygenase 3 expression (asLOX3) but are restored to WT levels by MeJA treatment. Whether these MeJA-induced responses are directly elicited by MeJA or by its cleavage product, JA, is unknown. Using virus-induced gene silencing (VIGS), we silenced MeJA-esterase (NaMJE) expression and found this gene responsible for most of the MeJA-cleaving activity in N. attenuata protein extracts. Silencing NaMJE in asLOX3, but not in WT plants, significantly reduced MeJA-induced nicotine levels and resistance to M. sexta, but not TPI levels. MeJA-induced transcript levels of threonine deaminase (NaTD) and phenylalanine ammonia lyase (NaPAL1) were also decreased in VIGS MJE (asLOX3) plants. Finally the performance of M. sexta larvae that fed on plants treated with JA or MeJA demonstrated that silencing NaMJE inhibited MeJA-induced but not JA-induced resistance in asLOX3 plants. From these results, we conclude that the resistance elicited by MeJA treatment is directly elicited not by MeJA but by its de-methylated product, JA.
[本文引用: 1]
DOI:10.1111/jipb.v58.6URL [本文引用: 3]

Jasmonic acid(JA) and related metabolites play a key role in plant defense and growth. JA carboxyl methyltransferase(JMT) may be involved in plant defense and development by methylating JA to methyl jasmonate(Me JA) and thus influencing the concentrations of JA and related metabolites. However, no JMT gene has been well characterized in monocotyledon defense and development at the molecular level. After we cloned a rice JMT gene,Os JMT1, whose encoding protein was localized in the cytosol, we found that the recombinant Os JMT1 protein catalyzed JA to Me JA. Os JMT1 is up-regulated in response to infestation with the brown planthopper(BPH; Nilaparvata lugens). Plants in which Os JMT1 had been overexpressed(oeJMT plants) showed reduced height and yield. These oe-JMT plants also exhibited increased Me JA levels but reduced levels of herbivore-induced JA and jasmonoyl-isoleucine(JAIle). The oe-JMT plants were more attractive to BPH female adults but showed increased resistance to BPH nymphs,probably owing to the different responses of BPH female adults and nymphs to the changes in levels of H_2O_2 and Me JA in oe-JMT plants. These results indicate that Os JMT1,by altering levels of JA and related metabolites, plays a role in regulating plant development and herbivore-induced defense responses in rice.
[本文引用: 1]
DOI:10.4161/psb.4.4.8199URL [本文引用: 2]

Drought is one of the major constraints to rice production worldwide. The development of rice panicle and spikelet meristem is repressed under the drought conditions, resulting in a reduction in the numbers of panicles and spikelets. In our recent report, we demonstrated that methyl jasmonate (MeJA) plays an important role in drought-induced loss of grain yield. Transgenic overexpression of the Arabidopsis gene jasmonic acid carboxyl methyltransferase (AtJMT) in rice resulted in a large reduction in grain yield through increased MeJA and ABA levels in young panicles. Exposure of nontransgenic plants to drought conditions also increased MeJA and ABA levels in young panicles and significantly reduced grain yield. In both cases, the reduction in grain yield was due to lower numbers of spikelets and lower filling rates than were observed for nontransgenic (NT) controls. The ABA increase in AtJMT transgenic panicles grown in non-drought conditions suggests that MeJA, rather than drought stress, induces ABA biosynthesis under drought conditions. These results led us postulate that plants produce MeJA during drought stress, which in turn stimulates the production of ABA, together leading to a loss of grain yield.
DOI:10.1007/s11816-010-0153-0URL [本文引用: 1]

Jasmonates control diverse plant developmental processes, such as seed germination, flower, fruit and seed development, senescence and tuberization in potato. To understand the role of methyl jasmonate (MeJA) in potato tuberization, the Arabidopsis JMT gene encoding jasmonic acid carboxyl methyltransferase was constitutively overexpressed in transgenic potato plants. Increases in tuber yield and size as well as in vitro tuberization frequency were observed in transgenic plants. These were correlated with JMT mRNA level he higher expression level, the higher the tuber yield and size. The levels of jasmonic acid (JA), MeJA and tuberonic acid (TA) were also higher than those in control plants. Transgenic plants also exhibited higher expression of jasmonate-responsive genes such as those for allene oxide cyclase (AOC) and proteinase inhibitor II (PINII). These results indicate that JMT overexpression induces jasmonate biosynthesis genes and thus JA and TA pools in transgenic potatoes. This results in enhanced tuber yield and size in transgenic potato plants.
DOI:10.1080/17550874.2010.498062URL [本文引用: 1]

Methyl jasmonate (MeJA) triggers the production of secondary metabolites in plants and participates in a diverse range of plant developmental processes. MeJA is derived from jasmonic acid (JA) via the octadecanoid pathway and the reaction is catalyzed by jasmonic acid carboxyl methyltransferase (JMT). In this study, transgenic Panax ginseng roots were constructed to express an Arabidopsis jasmo...
DOI:10.1016/j.foodchem.2015.09.046URLPMID:26593488 [本文引用: 1]

Transgenic overexpression of theArabidopsisgene for jasmonic acid carboxyl methyltransferase (AtJMT) is involved in regulating jasmonate-related plant responses. To examine its role in the compositional profile of soybean (Glycine max), we compared the seeds from field-grown plants that over-expressAtJMTwith those of the non-transgenic, wild-type (WT) counterpart. Our analysis of chemical compositions included proximates, amino acids, fatty acids, isoflavones, and antinutrients. Overexpression ofAtJMTin the seeds resulted in decreased amounts of tryptophan, palmitic acid, linolenic acid, and stachyose, but increased levels of gadoleic acid and genistein. In particular, seeds from the transgenic soybeans contained 120.0–130.5% more genistein and 60.5–82.1% less stachyose than the WT. A separate evaluation of ingredient values showed that all were within the reference ranges reported for commercially available soybeans, thereby demonstrating the substantial equivalence of these transgenic and non-transgenic seeds.
DOI:10.1186/s12864-016-2805-0PMID:4920993 [本文引用: 1]

Perilla (Perilla frutescens(L.) varfrutescens) produces high levels of 伪-linolenic acid (ALA), a -3 fatty acid important to health and development. To uncover key genes involved in fatty acid (FA) and triacylglycerol (TAG) synthesis in perilla, we conducted deep sequencing of cDNAs from developing seeds and leaves for understanding the mechanism underlying ALA and seed TAG biosynthesis. Perilla cultivar Dayudeulkkae contains 66.0 and 56.2 % ALA in seeds and leaves, respectively. Using Illumina HiSeq 2000, we have generated a total of 392 megabases of raw sequences from four mRNA samples of seeds at different developmental stages and one mature leaf sample of Dayudeulkkae.De novoassembly of these sequences revealed 54,079 unique transcripts, of which 32,237 belong to previously annotated genes. Among the annotated genes, 66.5 % (21,429 out of 32,237) showed highest sequences homology with the genes fromMimulus guttatus, a species placed under the same Lamiales order as perilla. Using Arabidopsis acyl-lipid genes as queries, we searched the transcriptome and identified 540 unique perilla genes involved in all known pathways of acyl-lipid metabolism. We characterized the expression profiles of 43 genes involved in FA and TAG synthesis using quantitative PCR. Key genes were identified through sequence and gene expression analyses. This work is the first report on building transcriptomes from perilla seeds. The work also provides the first comprehensive expression profiles for genes involved in seed oil biosynthesis. Bioinformatic analysis indicated that our sequence collection represented a major transcriptomic resource for perilla that added valuable genetic information in order Lamiales. Our results provide critical information not only for studies of the mechanisms involved in ALA synthesis, but also for biotechnological production of ALA in other oilseeds. The online version of this article (doi:10.1186/s12864-016-2805-0) contains supplementary material, which is available to authorized users.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.3724/SP.J.1006.2015.00725URLMagsci [本文引用: 1]

<p>植物防御素具有广谱抗菌活性,不仅具有抗真菌、抗细菌、蛋白酶抑制和昆虫淀粉酶抑制等活性,而且参与调节植物的生长和发育。本研究根据白菜防御素基因序列设计引物,从甘蓝型油菜中克隆获得5个防御素基因,其cDNA全长325~461 bp,含有177~243 bp开放阅读框,编码58~80个氨基酸,含有8个保守Cys残基,具备Knot1功能域。系统进化分析表明,BnPDF2.1、BnPDF2.3、BnPDF2.5与拟南芥PDF2亲缘关系较近,可能具有蛋白酶抑制活性。荧光定量分析表明,防御素基因具有组织表达特异性,在花蕾和叶中表达量较高,角果中次之;经1 mmol L<sup>–1</sup> 水杨酸处理开花期油菜2 h后,防御素基因在茎、花蕾、角果中的表达量均有不同程度的上调,但在叶中表达有所下调,在根中表达无明显变化。</p>
DOI:10.3724/SP.J.1006.2015.00725URLMagsci [本文引用: 1]

<p>植物防御素具有广谱抗菌活性,不仅具有抗真菌、抗细菌、蛋白酶抑制和昆虫淀粉酶抑制等活性,而且参与调节植物的生长和发育。本研究根据白菜防御素基因序列设计引物,从甘蓝型油菜中克隆获得5个防御素基因,其cDNA全长325~461 bp,含有177~243 bp开放阅读框,编码58~80个氨基酸,含有8个保守Cys残基,具备Knot1功能域。系统进化分析表明,BnPDF2.1、BnPDF2.3、BnPDF2.5与拟南芥PDF2亲缘关系较近,可能具有蛋白酶抑制活性。荧光定量分析表明,防御素基因具有组织表达特异性,在花蕾和叶中表达量较高,角果中次之;经1 mmol L<sup>–1</sup> 水杨酸处理开花期油菜2 h后,防御素基因在茎、花蕾、角果中的表达量均有不同程度的上调,但在叶中表达有所下调,在根中表达无明显变化。</p>
DOI:10.1186/s12864-018-4595-zURL [本文引用: 2]

Perilla frutescensis well known for its high -linolenic acid (ALA) accumulation in seeds and medicinal values as well as a source of edible and general-purpose oils. However, the regulatory mechanisms of the biosynthesis of fatty acid in its seeds remain poorly understood due to the lacking of sequenced genome. For better understanding the regulation of lipid metabolism and further increase its oil content or modify oil composition, time-course transcriptome and lipid composition analyses were performed. Analysis of fatty acid content and composition showed that the -linolenic acid and oleic acid accumulated rapidly from 5 DAF to 15 DAF and then kept relatively stable. However, the amount of palmitic acid and linoleic acid decreased quickly from 5 DAF to 15DAF. No significant variation of stearic acid content was observed from 5 DAF to 25DAF. Our transcriptome data analyses revealed that 110,176 unigenes were generated from six seed libraries at 5, 10, 20 DAF. Of these, 53 (31 up, 22 down) and 653 (259 up, 394 down) genes showed temporal and differentially expression during the seed development in 5 DAF vs 10 DAF, 20 vs 10 DAF, respectively. The differentially expressed genes were annotated and found to be involved in distinct functional categories and metabolic pathways. Deep mining of transcriptome data led to the identification of key genes involved in fatty acid and triacylglycerol biosynthesis and metabolism. Thirty seven members of transcription factor familyAP2,B3andNFYBputatively involved in oil synthesis and deposition were differentially expressed during seed development. The results of qRT-PCR for selected genes showed a strong positive correlation with the expression abundance measured in RNA-seq analysis. The present study provides valuable genomic resources for characterizingPerillaseed gene expression at the transcriptional level and will extend our understanding of the complex molecular and cellular events of oil biosynthesis and accumulation in oilseed crops. The online version of this article (10.1186/s12864-018-4595-z) contains supplementary material, which is available to authorized users.
DOI:10.7606/j.issn.1004-1389.2015.08.009URL [本文引用: 1]

According to the transcriptome of Asian cotton Shixiya I(GhJMT, GenBank accession: KJ856913). Sequence analysis indicated that GhJMT contains an open reading frame(ORF) of 1 116 bp, which encodes 371 amino acids. Conserved domain analysis showed that GhJMT has a conserved domain of methyltransferase-7. Phylogenetic tree analysis indicated that GhJMT was closer to the counterpart in Theobroma cacao, which was an unstable hydrophilic proteins sub-cellularly cytoplasm. Real time quantitative PCR results showed that the expression of GhJMT gene in roots after drought stress for 12 h was rapidly up-regulated and reached the highest, which was 2.6-fold of the control. While in stems the expression of GhJMT reached the highest level at 9 h and about 2.3-fold of the control, but at 3 h the expression of GhJMT gene in leaves reached the highest, which was 2.1-fold of the control. Base on the experimental results, the expression of GhJMT gene could be induced by drought stress. The results would help to clarify the correlation between GhJMT gene expression and drought resistance of plants.
DOI:10.7606/j.issn.1004-1389.2015.08.009URL [本文引用: 1]

According to the transcriptome of Asian cotton Shixiya I(GhJMT, GenBank accession: KJ856913). Sequence analysis indicated that GhJMT contains an open reading frame(ORF) of 1 116 bp, which encodes 371 amino acids. Conserved domain analysis showed that GhJMT has a conserved domain of methyltransferase-7. Phylogenetic tree analysis indicated that GhJMT was closer to the counterpart in Theobroma cacao, which was an unstable hydrophilic proteins sub-cellularly cytoplasm. Real time quantitative PCR results showed that the expression of GhJMT gene in roots after drought stress for 12 h was rapidly up-regulated and reached the highest, which was 2.6-fold of the control. While in stems the expression of GhJMT reached the highest level at 9 h and about 2.3-fold of the control, but at 3 h the expression of GhJMT gene in leaves reached the highest, which was 2.1-fold of the control. Base on the experimental results, the expression of GhJMT gene could be induced by drought stress. The results would help to clarify the correlation between GhJMT gene expression and drought resistance of plants.
URL [本文引用: 1]

近年来对植物甲基转移酶(methyltransferases,MTs)的研究发现了新一类成员,并用最初发现的3个酶将其命名为SA-BATH甲基转移酶(SABATHMTs),这3个酶分别是水杨酸羧基甲基转移酶(salicylic acid carboxyl methyltransferases,SAMT)、苯甲酸羧基甲基转移酶(benzoic acid carboxyl methyltransferases,BAMT)和可可碱合酶(theobromine synthase)。SABATHMTs能对植物激素和其他一些小分子物质进行N位或O位甲基化形成相应的甲基化产物,在植物次生代谢、发育及防御中起重要作用。本文从SABATHMTs潜在底物、进化及调控等方面综述了近年来对该家族的研究。
URL [本文引用: 1]

近年来对植物甲基转移酶(methyltransferases,MTs)的研究发现了新一类成员,并用最初发现的3个酶将其命名为SA-BATH甲基转移酶(SABATHMTs),这3个酶分别是水杨酸羧基甲基转移酶(salicylic acid carboxyl methyltransferases,SAMT)、苯甲酸羧基甲基转移酶(benzoic acid carboxyl methyltransferases,BAMT)和可可碱合酶(theobromine synthase)。SABATHMTs能对植物激素和其他一些小分子物质进行N位或O位甲基化形成相应的甲基化产物,在植物次生代谢、发育及防御中起重要作用。本文从SABATHMTs潜在底物、进化及调控等方面综述了近年来对该家族的研究。
[本文引用: 1]
DOI:10.1016/j.chom.2008.05.009URLPMID:18541211 [本文引用: 1]

In response to biotic stress, crosstalk between plant hormonal signaling pathways prioritizes defense over other cellular functions. Some plant pathogens take advantage of this regulatory system by mimicking hormones that interfere with host immune responses to promote virulence. Here we discuss the various roles that crosstalk may play in response to pathogens with different infection strategies.
DOI:10.1111/j.1365-313X.2009.03875.xURLPMID:19392690 [本文引用: 1]

Summary The importance of phytohormone balance is increasingly recognized as central to the outcome of plant athogen interactions. Recently it has been demonstrated that abscisic acid signalling pathways are utilized by the bacterial phytopathogen Pseudomonas syringae to promote pathogenesis. In this study, we examined the dynamics, inter-relationship and impact of three key acidic phytohormones, salicylic acid, abscisic acid and jasmonic acid, and the bacterial virulence factor, coronatine, during progression of P.syringae infection of Arabidopsis thaliana . We show that levels of SA and ABA, but not JA, appear to play important early roles in determining the outcome of the infection process. SA is required in order to mount a full innate immune responses, while bacterial effectors act rapidly to activate ABA biosynthesis. ABA suppresses inducible innate immune responses by down-regulating SA biosynthesis and SA-mediated defences. Mutant analyses indicated that endogenous ABA levels represent an important reservoir that is necessary for effector suppression of plant-inducible innate defence responses and SA synthesis prior to subsequent pathogen-induced increases in ABA. Enhanced susceptibility due to loss of SA-mediated basal resistance is epistatically dominant over acquired resistance due to ABA deficiency, although ABA also contributes to symptom development. We conclude that pathogen-modulated ABA signalling rapidly antagonizes SA-mediated defences. We predict that hormonal perturbations, either induced or as a result of environmental stress, have a marked impact on pathological outcomes, and we provide a mechanistic basis for understanding priming events in plant defence.
DOI:10.1016/j.chom.2017.01.007URLPMID:28182949 [本文引用: 1]

The heavy metal copper is an essential microelement required for normal plant growth and development, but it inhibits primary root growth when in excess. The mechanism underlying how excess Cu functions in this process remains to be further elucidated. Here, we reported that higher concentration of CuSO(4) inhibited primary root elongation of Arabidopsis seedlings by affecting both elongation... [Show full abstract]
DOI:10.1146/annurev-arplant-050213-040145URLPMID:24471835 [本文引用: 1]

Abstract Precise allocation of limited resources between growth and defense is critical for plant survival. In shade-intolerant species, perception of competition signals by informational photoreceptors activates shade-avoidance responses and reduces the expression of defenses against pathogens and insects. The main mechanism underlying defense suppression is the simultaneous downregulation of jasmonate and salicylic acid signaling by low ratios of red:far-red radiation. Inactivation of phytochrome B by low red:far-red ratios appears to suppress jasmonate responses by altering the balance between DELLA and JASMONATE ZIM DOMAIN (JAZ) proteins in favor of the latter. Solar UVB radiation is a positive modulator of plant defense, signaling through jasmonate-dependent and jasmonate-independent pathways. Light, perceived by phytochrome B and presumably other photoreceptors, helps plants concentrate their defensive arsenals in photosynthetically valuable leaves. The discovery of connections between photoreceptors and defense signaling is revealing novel mechanisms that control key resource allocation decisions in plant canopies.
