 ,1,2
,1,2Comparative Transcriptome Analysis Among the Three Line of Cytoplasmic Male Sterility in Maize
XUE YaDong1, YANG Lu1, YANG HuiLi1, LI Bing1, LIN YaNan1, ZHANG HuaiSheng1, GUO ZhanYong1, TANG JiHua ,1,2
,1,2通讯作者:
收稿日期:2018-12-10接受日期:2019-02-14网络出版日期:2019-04-16
| 基金资助: |
Received:2018-12-10Accepted:2019-02-14Online:2019-04-16
作者简介 About authors
薛亚东,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (2081KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
薛亚东, 杨露, 杨慧丽, 李冰, 林亚楠, 张怀胜, 郭战勇, 汤继华. 玉米C型细胞质雄性不育花药不同发育时期的转录组分析[J]. 中国农业科学, 2019, 52(8): 1308-1323 doi:10.3864/j.issn.0578-1752.2019.08.002
XUE YaDong, YANG Lu, YANG HuiLi, LI Bing, LIN YaNan, ZHANG HuaiSheng, GUO ZhanYong, TANG JiHua.
0 引言
【研究意义】玉米是中国第一大粮食作物,常年播种面积333万hm2以上,年用种量7.5亿kg左右,需要制种23万hm2左右(全国农业技术推广服务中心 www.natesc.org.cn)。由于玉米种子生产多数采用人工去雄的方式,人工去雄的直接费用约6亿元,导致种子生产成本增加,在一定程度上制约着中国种子企业的国际竞争力,而不育化制种是当前及今后种子企业提高竞争力的一个重要途径。在玉米的质核互作不育类型中,C型细胞质雄性不育因其具有不育性稳定、恢复彻底等特点,得到了玉米育种家及相关种子企业的高度关注。由于玉米C型胞质雄性不育及恢复机制目前仍不清楚,从而在一定程度上限制了其在种子生产上的大面积推广应用。因此,明确玉米C型细胞质不育和恢复的分子机制及其调控网络,将对玉米C型胞质雄性不育的应用具有一定的促进作用。【前人研究进展】植物尤其是作物中细胞质雄性不育材料是杂交种子生产的一种重要种质资源,同时也是研究核质互作的重要遗传材料,因而得到了遗传学家和育种家的广泛关注。前人曾经对细胞质雄性不育开展了大量的研究,玉米T型细胞质的不育基因T-urf13是第一个被鉴定的细胞质雄性不育基因[1]。水稻先后克隆了CMS-HL不育基因orfH79[2]、CMS-BT的不育基因orf79[3]以及CMS-WA的不育基因WA352[4]。油菜中应用较为广泛的CMS-pol、CMS-nap与CMS-ogu 3种类型的细胞质雄性不育的不育基因orf224-atp6、orf222和orf138已经克隆[5,6,7]。萝卜、油菜、小麦等其他作物的细胞质雄性不育相关的部分基因也已被鉴定。目前,已经克隆的植物不育基因均与线粒体基因相关,且均为重组形成的嵌合基因。与之相应的恢复基因除玉米的Rf2[8]、水稻Rf17[9]和Rf2[10]、甜菜的Rf1[11]外,均为编码PPR基序蛋白的基因[3,12-17]。根据已有的研究可将细胞质雄性不育机理归纳为3个假说:毒性假说、能量亏缺假说及细胞异常凋亡假说;其相应的恢复基因则通过转录后水平的编辑、剪接、多聚腺苷酸化、剪切等方式,或翻译及翻译后水平的修饰来补偿或逆转不育基因的危害,进而恢复植株的育性[18]。玉米C型细胞质雄性不育有2个重叠效应的恢复基因Rf4和Rf5[19],分别定位于第8染色体短臂和第5染色体长臂上[20]。玉米C型胞质线粒体基因组中具有多个区域重复并伴有重组,造成与线粒体呼吸链复合体相关基因具有多个拷贝,如atp9、cox2或形成嵌合基因,如atp6,推测其中可能包含不育基因[21,22]。光学及电子显微镜观察结果表明,C型胞质不育系小孢子的败育发生在单核早期[23,24],而在二分体时期绒毡层细胞出现U氏小体异常[24],及至四分体绒毡层出现大量不规则液泡[23,24]。全基因组转录分析能够鉴定到与育性恢复及不育相关的功能基因及其调控与代谢的网络。已报道的雄性不育转录组分析结果表明,洋葱细胞质雄性不育与线粒体氧化磷酸化有关[25],辣椒细胞质雄性不育主要涉及ATP合酶、NADH脱氢酶及细胞色素氧化酶[26]。对玉米C型胞质不育系与保持系的花粉母细胞时期及单核期花药转录组分析发现MYB转录因子、氨基酸代谢和脂肪酸合成途径可能对育性产生重要影响[27]。【本研究切入点】尽管前人确立了C型胞质花粉败育时期以及分析了花粉母细胞时期与单核时期在不育系与保持系之间的差异基因,但C型胞质雄性不育的分子机制尚未解析;由于未能同时对同基因型的恢复系进行分析,无法全面解析不育与恢复可能的代谢和调控机制;而对于孢子体不育材料,在花粉败育时期取样,无法分析败育形成的基因。【拟解决的关键问题】本研究拟通过细化取样时期,比较玉米C型胞质三系材料在减数分裂前期Ⅰ、中期Ⅰ及四分体时期花药的转录组数据,在转录水平上分析玉米C型胞质雄性不育及恢复的机制,明确玉米C型胞质雄性不育发育过程中基因表达规律,以期为玉米C型雄性不育及恢复分子机制研究提供依据,同时为恢复基因及不育基因的克隆及功能分析奠定基础。1 材料与方法
1.1 试验材料
选用以优良玉米自交系豫自87-1为背景构建的玉米细胞质雄性不育系CMS-Es87-1(rf4rf4rf5rf5)、保持系N87-1(rf4rf4rf5rf5)及恢复系CMS-Es87-1(Rf4Rf4rf5rf5)为试验材料。试验材料于2016年春分期种植于河南农业大学科教园区。播种40 d后,参考MA等[28]方法每隔2 d检查玉米雄穗的花药发育进程,待花药发育至减数分裂时期将玉米整株取回实验室。对雄穗局部区域进行三点(区域两头及中部)醋酸洋红染色镜检,选取减数分裂前期(P1)、减数分裂中期Ⅰ(M1)及四分体时期(T2)的花药,液氮速冻后-80℃保存备用。每一个材料每一个时期各取3个生物学重复进行转录组测序,每个生物学材料由多个植株的花药组成,以消除个体及环境的差异。1.2 花药RNA提取
采用TRIzol(Invitrogen,Carlsbad,CA,USA)法提取玉米花药总RNA。利用NanoDrop One检测RNA浓度,用Agilent 2100检测28S/18S以及RIN值,同时用1%琼脂糖凝胶电泳检测所提取RNA的质量及完整性。1.3 转录组建库及测序
利用Oligo(dT)磁珠富集每个玉米花药样品的mRNA(Illumina,San Diego,CA,USA),加入片段化缓冲液将mRNA随机打断成短片段。将打断后的mRNA反转录成第一链cDNA,随后合成第二链。利用QiaQuick PCR提取试剂盒纯化后进行末端修复并在3'末端加上碱基A,并连接测序接头。筛选大小在300—500 bp的片段进行PCR扩增。文库经Agilent 2100 Bioanalyzer和ABI StepOnePlus Real-Time PCR System质检合格后用Illumina HiSeq 2500进行双末端测序(北京贝瑞和康生物科技有限公司)。1.4 序列分析
测序获得的原始数据首先过滤掉低质量(Q<30)、接头污染以及位置碱基N含量过高(>5%)的reads,得到clean reads用于后续分析。利用HiSat2软件[29]将过滤后的数据比对到玉米参考基因组B73第四版上(B73 AGPv4,http://ensembl.gramene.org/Zea_mays/ Info/Index),用stringtie软件[30]进行转录本拼接并进行基因表达量估算。基因表达值导入R软件包DESeq2中进行差异基因分析[29]。padj小于0.05及表达差异大于2的基因认为是差异表达的基因(DEG)。根据NCBI的玉米基因注释数据提取差异表达基因的功能信息,利用内部perl脚本从maizeGDB网站上获取差异基因的GO注释。从KAAS网站获取差异表达基因的KEGG途径,利用R软件的clusterProfiler包进行差异基因的GO富集及代谢途径的富集,并对线粒体相关途径进行了分析[32]。1.5 差异表达基因的半定量验证
分别以87-1“三系”材料的减数分裂前期Ⅰ、四分体期和单核期的花药总RNA为模板,反转录合成cDNA第一链。利用TB GreenTM Premix Ex TaqTM Ⅱ(TaKaRa)荧光定量试剂盒在CFX96实时定量PCR仪上对转录组数据差异表达的基因Zm00001d043834、Zm00001d009222、Zm00001d007966和Zm00001d009727进行定量分析。使用Primer3 plus在线设计所有qRT-PCR反应特异引物(表1)。cDNA反转录和qRT-PCR反应体系及反应程序按照PrimeScriptTMRT reagent Kit with gDNA Eraser(TaKaRa)和TB GreenTM Premix Ex TaqTM Ⅱ(TaKaRa)试剂盒里的说明书的操作进行。每个试验均设3次生物学重复和3次技术重复,利用(2-ΔΔCt)法[33]分析基因表达水平。Table 1
表1
表1差异基因表达分析所用引物
Table 1
| 引物名称 Primer name | 引物序列 Primer sequence (5′-3′) | 作用 Function |
|---|---|---|
| Zm00001d043834-F | GAGCAAGCTACAGAGCAGCA | Zm00001d043834表达分析 |
| Zm00001d043834-R | GCACCACCAAAGAGACCAAT | For the expression of Zm00001d043834 |
| Zm00001d009222-F | GAGATCCAGAGCGCCATTT | Zm00001d009222表达分析 |
| Zm00001d009222-R | GAGCCCAGGAAGAGGAAGAT | For the expression of Zm00001d009222 |
| Zm00001d007966-F | GTGCATCACCAAGCTCTTCC | Zm00001d007966表达分析 |
| Zm00001d007966-R | GTGCCACCTCCAATCATCTT | For the expression of Zm00001d007966 |
| Zm00001d009727-F | TTGTCTGCACGAGGAATCAG | Zm00001d009727表达表达 |
| Zm00001d009727-R | ACCAGACGACATCGTGTTCA | For the expression of Zm00001d009727 |
新窗口打开|下载CSV
1.6 ATP酶活性测定
根据北京索莱宝科技有限公司(Solarbio)钙镁离子ATP酶检测试剂盒说明书测定C型胞质雄性不育三系减数分裂前期花药的ATP酶活性。称取新鲜样品约0.1 g,加入1 mL试剂一进行冰浴匀浆,9 200 r/min 4℃离心10 min,取上清置于冰上待测。按照手册设置ATP酶酶促反应,水浴10 min,取上清液100 μL,分别加入定磷反应的对照管和测定管,加入定磷剂1 000 μL,混匀,40℃水浴10 min,冷却至室温后利用分光光度计于660 nm处比色。ATP酶活力(U·g-1)=7.5×(A测定管-A对照管)÷(A标准管-A空白管)÷W(样本鲜重,g),计算钙镁离子ATP酶活性。每个材料测定3次。2 结果
2.1 转录组测序与比对分析
为减少分期播种的环境影响及不同个体间的差异,同一时期至少混合3个不同播期材料及3个单株上的样品用于RNA提取及后续测序分析。所测数据经过严格的质控后,产生5.6亿对双末端序列,共计156.59 Gb的序列数据(表2)。除样品CrT23和CRT23外,Q30比例都超过了90%。PCA分析显示三系前期各有一个样品与相同时期的样品差异较大,在后续比对分析中被剔除;其余不同样品间差异明显,不同样品的生物学重复可聚类在一起。Hisat2比对到参考基因组的序列经过stringtie组装,共得到53 035个Unigene,其中20 017(37.74%)个Unigene为B73基因组中未注释的新基因。Table 2
表2
表2测序数据统计分析
Table 2
| 样品 Sample | 总序列 Total read pairs | 总碱基数 Total bases (Billion) | GC含量 GC percentage (%) | Q30比例 Q30 percentage (%) | 比对序列比例 Total mapping reads percentage (%) |
|---|---|---|---|---|---|
| CrP11 | 18374016 | 5.51 | 54.5 | 94.3 | 90.0 |
| CrP12 | 16560680 | 4.96 | 54.5 | 93.7 | 91.1 |
| CrP13 | 27650293 | 6.91 | 52.5 | 100.0 | 91.3 |
| CrM11 | 17989607 | 5.39 | 54.5 | 93.3 | 90.6 |
| CrM12 | 16908690 | 5.07 | 53.5 | 95.0 | 90.7 |
| CrM13 | 26782609 | 6.69 | 52.0 | 100.0 | 91.5 |
| CrT21 | 18705720 | 5.61 | 54.5 | 93.7 | 90.2 |
| CrT22 | 17698741 | 5.31 | 54.5 | 95.0 | 90.1 |
| CrT23 | 13798055 | 2.48 | 55.0 | 80.5 | 83.6 |
| CRP11 | 22127378 | 6.63 | 54.0 | 100.0 | 90.6 |
| CRP12 | 24978644 | 7.49 | 54.0 | 100.0 | 90.8 |
| CRP13 | 27365104 | 6.84 | 52.0 | 100.0 | 91.7 |
| CRM11 | 28575880 | 8.57 | 54.0 | 100.0 | 90.4 |
| CRM12 | 18086263 | 5.42 | 55.0 | 97.6 | 89.8 |
| CRM13 | 29768530 | 7.44 | 52.5 | 100.0 | 91.3 |
| CRT21 | 27996156 | 8.39 | 55.5 | 93.3 | 90.3 |
| CRT22 | 20498349 | 6.14 | 55.5 | 94.7 | 90.1 |
| CRT23 | 12902671 | 2.32 | 55.0 | 78.9 | 83.1 |
| NrP11 | 17959776 | 5.38 | 54.0 | 94.0 | 91.0 |
| NrP12 | 18272442 | 5.48 | 55.0 | 94.7 | 90.4 |
| NrP13 | 28541683 | 7.13 | 52.0 | 100.0 | 90.8 |
| NrM11 | 19324515 | 5.79 | 55.5 | 94.0 | 89.6 |
| NrM12 | 18245189 | 5.47 | 55.5 | 94.0 | 89.8 |
| NrM13 | 29194399 | 7.29 | 52.5 | 100.0 | 92.1 |
| NrT21 | 21652022 | 6.49 | 57.0 | 100.0 | 89.4 |
| NrT22 | 20912786 | 6.27 | 56.0 | 100.0 | 90.0 |
新窗口打开|下载CSV
2.2 差异表达基因分析
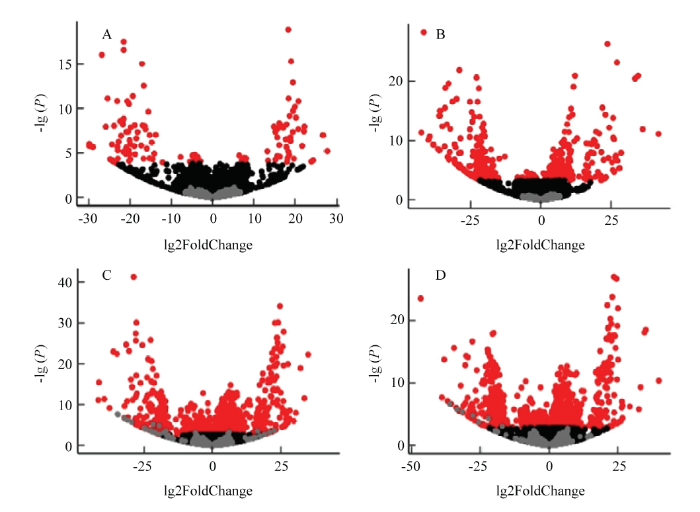
将差异基因定义为FDR(false discovery rate)<0.05且表达差异倍数在2倍以上的基因(图1)。通过比较87-1三系不同时期及相同时期不同系间的表达谱,获得在三系不同时期或同一材料不同时期之间的显著性差异基因(表3)。87-1不育系在减数分裂中期Ⅰ与前期Ⅰ相比共有121个差异表达基因,其中48个基因个上调,73个基因下调;在四分体时期有329个基因相比中期Ⅰ表现为下调,而与前期Ⅰ相比,差异基因之间表现出显著的变化。87-1保持系在不同发育时期之间差异表达基因的变化趋势与不育系相似,但上调表达的基因数量更多。87-1恢复系在不同发育时期中差异基因数目的变化趋势与保持系和不育系明显不同,中期Ⅰ和前期Ⅰ的差异基因数量与四分体和中期Ⅰ间的差异数量相比变化不大。保持系与不育系的四分体时期出现大量差异表达基因(723上调/409下调),而恢复系与不育系相比在相同时期也有较多差异表达的基因。在87-1三系材料的整个发育时期中有135个基因均存在差异表达。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1差异基因火山图
A:不育系中期Ⅰ与前期Ⅰ比较;B:恢复系中期Ⅰ与前期Ⅰ比较;C:四分体时期恢复系与不育系比较;D:四分体时期保持系与不育系比较
Fig. 1Volcano plot of differentially expressed genes
A: Comparison between prophaseⅠand metaphaseⅠin the sterile lines; B: Comparison between prophaseⅠand metaphaseⅠin the restorer lines; C: Comparison between the restorer lines and the sterile lines at tetrad stage; D: Comparison between the maintainer lines and the sterile lines at tetrad stage
Table 3
表3
表3差异基因统计分析
Table 3
| 差异比较 Comparison | 表达趋势 Up-/Down regulated | 差异基因数 DE gene numbers | 差异比较 Comparison | 表达趋势 Up-/Down regulated | 差异基因数 DE gene numbers |
|---|---|---|---|---|---|
| CrM1/CrP1 | 上调Up | 48 | CRP1/CrP1 | 上调Up | 57 |
| 下调Down | 73 | 下调Down | 160 | ||
| CrT2/CrM1 | 上调Up | 85 | CRM1/CrM1 | 上调Up | 273 |
| 下调Down | 329 | 下调Down | 380 | ||
| CrT2/CrP1 | 上调Up | 230 | CRT2/CrT2 | 上调Up | 975 |
| 下调Down | 1088 | 下调Down | 454 | ||
| CRM1/CRP1 | 上调Up | 233 | NrP1/CrP1 | 上调Up | 50 |
| 下调Down | 192 | 下调Down | 53 | ||
| CRT2/CRM1 | 上调Up | 142 | NrM1/CrM1 | 上调Up | 99 |
| 下调Down | 188 | 下调Down | 64 | ||
| CRT2/CRP1 | 上调Up | 732 | NrT2/CrT2 | 上调Up | 723 |
| 下调Down | 775 | 下调Down | 409 | ||
| NrM1/NrP1 | 上调Up | 52 | 发育时序 Time Series | NA/无 | 135 |
| 下调Down | 54 | ||||
| NrT2/NrM1 | 上调Up | 356 | NrT2/NrP1 | 上调Up | 786 |
| 下调Down | 560 | 下调Down | 1078 |
新窗口打开|下载CSV
2.3 差异表达基因GO功能分析
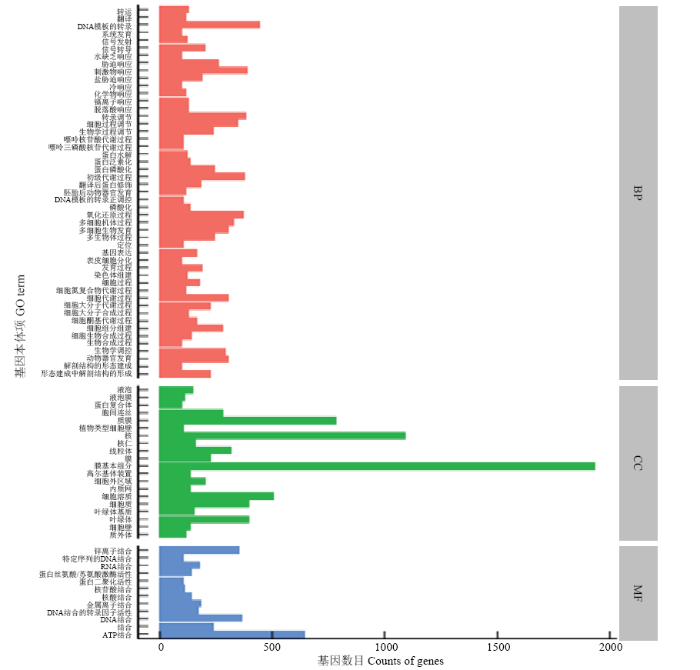
将时间轴差异分析结果及两两对比的结果取并集进行分析,共得到5 676个显著的差异表达基因。通过GO分析,有4 273个基因获得4 946个GO注释条目,其中具有3个及以上Unigene的GO条目的基因有2 128个。差异表达基因富集在生物学过程中的有4 231个,细胞组分有4 082个,分子功能有4 085个。富集最多的前81个GO分类如图2所示,细胞组分中富集基因最多的部位是膜的组分(integral component of membrane),其次是核内(nucleus)和质膜上(plasma membrane)的基因,而线粒体中也富集到较多的差异基因;分子功能中富集较多的3个亚类分别是ATP结合、DNA结合及锌离子结合。与转录有关的基因在生物学过程中富集最多,其次还有响应刺激有关的基因以及氧化还原反应有关的基因。时间轴分析的差异基因多富集在与磷酸酶活性相关的GO条目中。87-1恢复系与不育系在减数分裂中期Ⅰ差异表达的基因多富集在花粉发育、配子体发育等生物学过程中。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2差异表达GO分类
转运:Transport;翻译:Translation;DNA模板的转录:Transcription, DNA-templated;系统发育:System development;信号发射:Signaling;信号转导:Signal transduction;水缺乏响应:Response to water deprivation;胁迫响应:Response to stress;刺激物响应:Response to stimulus;盐胁迫响应:Response to salt stress;冷响应:Response to cold;化学物响应:Response to chemical;镉离子响应:Response to cadmium ion;脱落酸响应:Response to abscisic acid;转录调节:Regulation of transcription;细胞过程调节:Regulation of cellular process;生物学过程调节:Regulation of biological process;嘌呤核苷酸代谢过程:Purine ribonucleotide catabolic process;嘌呤三磷酸核苷代谢过程:Purine ribonucleoside triphosphate catabolic process;蛋白水解:Proteolysis;蛋白泛素化:Protein ubiquitination;蛋白磷酸化:Protein phosphorylation;初级代谢过程:Primary metabolic process;翻译后蛋白修饰:Post-translational protein modification;胚胎后动物器官发育:Post-embryonic animal organ development;DNA模板的转录正调控:Positive regulation of transcription, DNA-templated;磷酸化:Phosphorylation;氧化还原过程:Oxidation-reduction process;多细胞机体过程:Multicellular organismal process;多细胞生物发育:Multicellular organism development;多生物体过程:Multi-organism process;定位:Localization;基因表达:Gene expression;表皮细胞分化:Epidermal cell differentiation;发育过程:Developmental process;染色体组建:Chromosome organization;细胞过程:Cellular process;细胞氮复合物代谢过程:Cellular nitrogen compound metabolic process;细胞代谢过程:Cellular metabolic process;细胞大分子代谢过程:Cellular macromolecule metabolic process;细胞大分子生物合成过程:Cellular macromolecule biosynthetic process;细胞酮基代谢过程:Cellular ketone metabolic process;细胞组分组建:Cellular component organization;细胞生物合成过程:Cellular biosynthetic process;生物合成过程:Biosynthetic process;生物学调控:Biological regulation;动物器官发育:Animal organ development;解剖结构的形态建成:Anatomical structure morphogenesis;形态建成中解剖结构的形成:Anatomical structure formation involved in morphogenesis;液泡:Vacuole;液泡膜:Vacuolar membrane;蛋白复合体:Protein complex;胞间连丝:Plasmodesma;质膜:Plasma membrane;植物类型细胞壁:Plant-type cell wall;核:Nucleus;核仁:Nucleolus;线粒体:Mitochondrion;膜:Membrane;膜基本组分:Integral component of membrane;高尔基体装置:Golgi apparatus;细胞外区域:Extracellular region;内质网:Endoplasmic reticulum;细胞溶质:Cytosol;细胞质:Cytoplasm;叶绿体基质:Chloroplast stroma;叶绿体:Chloroplast;细胞壁:Cell wall;质外体:Apoplast;锌离子结合:Zinc ion binding;特定序列的DNA结合:Sequence-specific DNA binding;RNA结合:RNA binding;蛋白丝氨酸/苏氨酸激酶活性:Protein serine/threonine kinase activity;蛋白二聚化活性:Protein dimerization activity;核苷酸结合:Nucleotide binding;核酸结合:Nucleic acid binding;金属离子结合:Metal ion binding;DNA结合的转录因子活性:DNA binding transcription factor activity;DNA结合:DNA binding;结合:Binding;ATP结合:ATP binding。BP:生物过程 Biological processes;CC:细胞组分 Cell components;MF:分子功能 Molecular function
Fig. 2GO classification of differentially expressed genes
2.4 差异表达基因的KEGG通路分析
通过对87-1三系不同时期 两两对比及时间轴差异基因分别进行KEGG分析,共获得37个不同的代谢通路(表4)。在两两对比分析中出现最多次数的途径是角质、软木脂和蜡质合成途径,共出现12次;其次是辅酶Q及其他萜-醌合成、脂肪酸降解和苯丙烷合成途径。在所有途径中,富集基因最多的是碳代谢途径,其次是糖酵解途径和DNA复制途径。Table 4
表4
表4差异表达基因数量最多的15个代谢通路
Table 4
| 代谢通路 Pathway ID | 通路名称 Name of pathways | 通路出现次数 Occurrences of pathways | 基因数目 Counts of genes |
|---|---|---|---|
| zma01200 | 碳代谢 Carbon metabolism | 1 | 28 |
| zma00010 | 糖酵解 Glycolysis/Gluconeogenesis | 1 | 20 |
| zma03030 | DNA复制 DNA replication | 1 | 19 |
| zma00620 | 丙酮酸盐代谢 Pyruvate metabolism | 2 | 16 |
| zma03440 | 同源重组 Homologous recombination | 1 | 15 |
| zma00071 | 脂肪酸降解 Fatty acid degradation | 5 | 14 |
| zma00130 | 辅酶Q及其他萜-醌合成 Ubiquinone and other terpenoid-quinone biosynthesis | 6 | 13 |
| zma00040 | 戊糖与葡萄糖醛酸相互转换 Pentose and glucuronate interconversions | 1 | 9 |
| zma00073 | 角质、软木脂及蜡质合成 Cutin, suberine and wax biosynthesis | 12 | 8 |
| zma00940 | 苯丙烷合成 Phenylpropanoid biosynthesis | 5 | 6 |
| zma00290 | 缬氨酸、亮氨酸和异亮氨酸合成 Valine, leucine and isoleucine biosynthesis | 2 | 5 |
| zma02010 | ABC运输 ABC transporters | 1 | 5 |
| zma00500 | 淀粉和蔗糖代谢 Starch and sucrose metabolism | 1 | 4 |
| zma00770 | 泛酸酯和辅酶A合成 Pantothenate and CoA biosynthesis | 1 | 4 |
| zma04016 | 植物MAPK信号途径 MAPK signaling pathway-plant | 1 | 4 |
新窗口打开|下载CSV
2.5 线粒体氧化磷酸化途径相关基因的表达分析
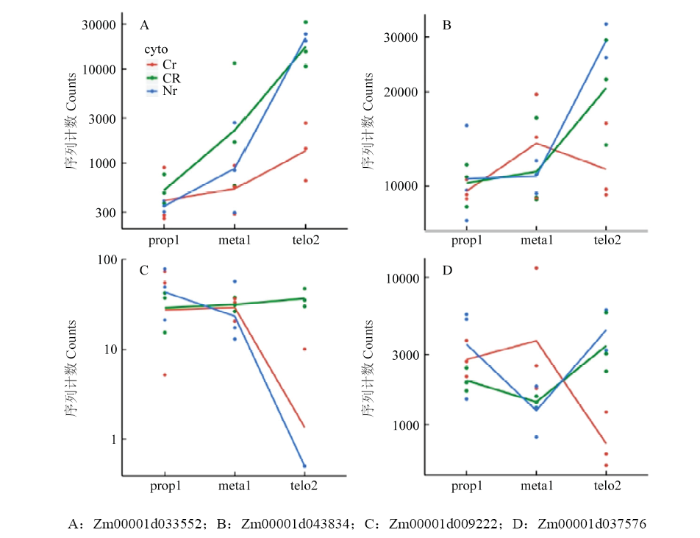
线粒体是细胞的能量工厂,而细胞质雄性不育多与线粒体相关,尤其与线粒体内氧化磷酸化途径相关[18,34-35]。玉米中共有187个基因与氧化磷酸化途径有关(KEGG途径zma00190)。将该基因集与差异表达基因取交集,共有13个基因在三系不同发育时期中表达呈显著差异(表5),其中基因Zm00001d033552和Zm00001d043834在87-1保持系花药不同发育时期持续上调表达,而在87-1不育系中表达则不存在差异;而在87-1恢复系的花药不同发育时期的表达量与保持系相似(图3-A和图3-B)。87-1保持系与不育系的花药从减数分裂前期到四分体时期的差异表达相比,有4个基因(Zm00001d033552、Zm00001d043834、 Zm00001d045122和Zm00001d037576)显著上调表达;在87-1恢复系中3个基因(Zm00001d033552、 Zm00001d045122和Zm00001d037576)的表达趋势与保持系相同,同时有另外3个基因(Zm00001d041214、Zm00001d015569和Zm00001d009222)的表达水平显著提高(表5,图3-C)。值得注意的是,基因Zm00001d037576在87-1三系的花药发育时期的表达波动较大(图3-D)。上述这些差异表达基因主要富集在氧化磷酸化途径的复合体Ⅱ、复合体Ⅳ和复合体Ⅴ中(图4)。Table 5
表5
表5线粒体氧化磷酸化途径相关基因的表达差异
Table 5
| 基因编号 ID number of gene | 中期Ⅰ恢复系比不育系 CR/Cr-m1 | 四分体恢复系比不育系 CR/Cr-t2 | 四分体保持系比不育系 Nr/Cr-t2 | 不育系四分体比前期Ⅰ Cr-t2/p1 | 保持系四分体比中期Ⅰ Nr-t2/m1 | 保持系四分体比前期Ⅰ Nr-t2/p1 | 恢复系四分体比前期Ⅰ CR-t2/p1 | 染色体 Chr. | 基因功能描述 Description |
|---|---|---|---|---|---|---|---|---|---|
| Zm00001d033552 | 2.94±0.83 | 3.60±0.83 | 3.78±0.93 | 4.09±0.93 | 5.95±0.93 | 5.15±0.83 | 1 | 腺苷三磷酸酶4, 质膜类型 ATPase 4 plasma membrane-type | |
| Zm00001d043834 | 1.33±0.40 | 1.44±0.40 | 1.42±0.40 | 1.07±0.35 | 3 | 类线粒体腺苷三磷酸合成酶贝塔亚基 ATP synthase subunit beta, mitochondrial-like | |||
| Zm00001d045122 | 4.60±1.08 | 4.04±1.19 | 9 | 腺苷三磷酸酶8, 质膜类型 ATPase 8 plasma membrane-type | |||||
| Zm00001d041214 | 1.74±0.54 | 3 | 腺苷三磷酸酶4, 质膜类型 ATPase 4 plasma membrane-type | ||||||
| Zm00001d015569 | 1.64±0.41 | 5 | 液泡质子泵同系物1 Vacuolar proton pump homolog 1 | ||||||
| Zm00001d037576 | 2.22±0.6 | 2.54±0.67 | -1.85±0.60 | 6 | 焦磷酸供能的液泡质膜质子泵 Pyrophosphate-energized vacuolar membrane proton pump | ||||
| Zm00001d009222 | 3.38±1.07 | -3.61±1.06 | -7.12±1.77 | -7.90±1.77 | 8 | 焦磷酸供能的液泡质膜质子泵 Pyrophosphate-energized vacuolar membrane proton pump | |||
| Zm00001d052022 | -5.78±1.58 | 4 | 腺苷三磷酸酶8, 质膜类型 ATPase 8 plasma membrane-type | ||||||
| Zm00001d045497 | -2.04±0.59 | 9 | V型质子偶联腺苷三磷酸亚基e1 V-type proton ATPase subunit e1 | ||||||
| Zm00001d027304 | -3.2±0.90 | -2.94±1.00 | -3.17±0.90 | 1 | 腺苷三磷酸酶9, 质膜类型 ATPase 9, plasma membrane-type | ||||
| Zm00001d007966 | -1.18±0.30 | -1.17±0.30 | 2 | 琥珀酸脱氢酶4 Succinate dehydrogenase4 | |||||
| Zm00001d036728 | -4.01±0.95 | 6 | 腺苷三磷酸酶2, 质膜类型 ATPase 2, plasma membrane-type | ||||||
| Zm00001d009727 | -8.63±2.86 | 8 | 线粒体细胞色素c氧化酶亚基5b-2 Cytochrome c oxidase subunit 5b-2 mitochondrial |
新窗口打开|下载CSV
图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3发育过程中基因表达分析
Fig. 3Comparative analysis of genes along with the microspore development
A:Zm00001d033552;B:Zm00001d043834;C:Zm00001d009222;D:Zm00001d037576
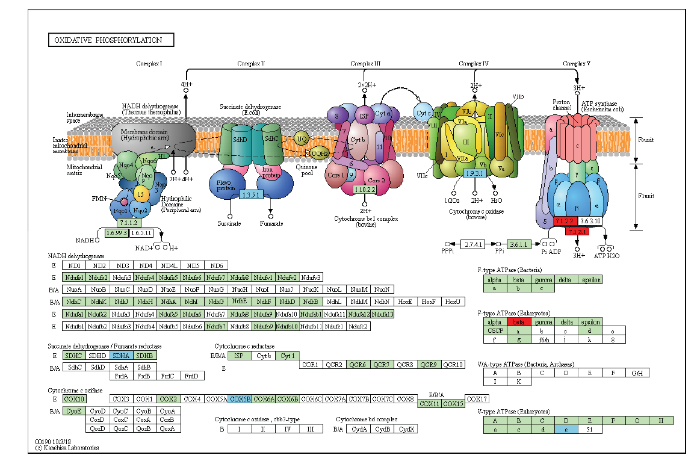
图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4氧化磷酸化途径中差异表达基因
绿底框:物种特异的基因;红底框:上调基因;天蓝底框:下调基因
Fig. 4differentially expressed genes in oxidative phosphorylation pathways
Green boxes: Enzymes specific to this species; Red boxes: Up-regulated genes; Skyblue boxes: Down-regulated genes
为验证转录组中差异基因的表达结果,挑选了氧化磷酸化途径中的4个差异表达基因Zm00001d007966(ComplexⅡ)、Zm00001d009727(ComplexⅣ)、Zm00001d043834(ComplexⅤ,F-type ATPase beta)和Zm00001d009222(ComplexⅤ,inorganic diphosphatase),以actin为内参基因,通过qRT-PCR技术分析它们在玉米C型胞质不育花药不同发育阶段的表达变化(图5)。结果表明,Zm00001d007966随着花药的发育表达量逐渐下降,在减数分裂中期Ⅰ时恢复系的表达量约为不育系的2倍;Zm00001d009727在恢复系中表达趋势与Zm00001d007966相同,在不育系及保持系中都是先下降再上升,且不育系单核期比中期Ⅰ上升了1倍多;恢复系和保持系中,Zm00001d043834和Zm00001d009222从中期Ⅰ到末期Ⅱ的表达趋势相反,从末期Ⅱ到单核期表达趋势相同;Zm00001d043834在三系材料末期Ⅱ中迅速上升,不育系上升了7倍多,但其表达量仍低于保持系和恢复系。qTR-PCR结果与转录组分析结果基本一致。
图5
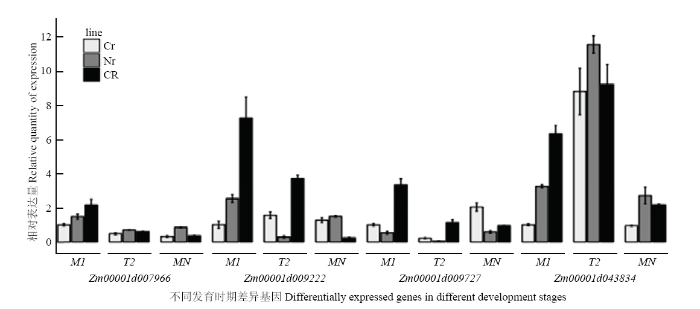 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5不同发育时期差异基因荧光定量检测
CR:恢复系;Cr:不育系;Nr:保持系,M1:中期1;T2:末期2(tetrad);MN:单核期
Fig. 5qTR-PCR expression of differentially expressed genes in different development stages
CR: Restorer lines; Cr: Sterile lines; Nr: Maintainer lines; M1: Metaphase 1; T2: Telophase 2 (tetrad); MN: Mononucleate stage
2.6 细胞色素P450基因的表达分析
细胞色素P450通过调节许多重要的细胞过程而影响植物的生长与发育,而一些P450基因参与合成植物激素如生长素、赤霉素、细胞分裂素等在植物开花成熟过程中发挥着重要的作用[36,37]。转录组分析不同时期保持系与不育系、恢复系与不育系的比较中发现12个细胞色素P450家族基因的表达存在显著差异(表6)。在减数分裂前期,保持系中Zm00001d024412与Zm00001d042814比不育系的表达量要高得多(log2值分别为19.96和17.59),表明不育系中这两个基因几乎不表达或表达量极低;在恢复系中,Zm00001d042814的表达量恢复到保持系的水平。及至减数分裂中期Ⅰ,这两个基因在不同材料的比较中没有差异,同时保持系与不育系间未出现新的差异表达细胞色素P450家族基因;而在恢复系中新增3个上调及3个下调的细胞色素P450基因。在减数分裂末期2时,前期1的2个差异基因再次出现差异,且表达趋势相同;而在不育系中下调的细胞色素基因在恢复系中都得到了恢复。Table 6
表6
表6细胞色素P450基因的表达
Table 6
| 时期 stage | 比较 Comparison | 表达 Expression | 差异倍数log2FLC | 基因ID Gene ID | 染色体 Chr. | 功能描述 Description |
|---|---|---|---|---|---|---|
| 末期2 Telo2 | Nr/Cr | 上调Up | 21.83 | Zm00001d024412 | 10 | NADPH-细胞色素P450还原酶 NADPH-cytochrome P450 reductase |
| 9.20 | Zm00001d042814 | 3 | 86A1型细胞色素P450 Cytochrome P450 86A1 | |||
| 7.31 | Zm00001d011932 | 8 | 类89A2型细胞色素P450 Cytochrome P450 89A2-like | |||
| 6.94 | Zm00001d013862 | 5 | 细胞色素P450大家族蛋白 Cytochrome P450 superfamily protein | |||
| 6.92 | Zm00001d029526 | 1 | 78A5型细胞色素P450 Cytochrome P450 78A5 | |||
| 下调Down | -17.05 | Zm00001d039697 | 3 | 类711A1型细胞色素P450 Cytochrome P450 711A1-like | ||
| -20.91 | Zm00001d004486 | 2 | 71D7型细胞色素P450 Cytochrome P450 71D7 | |||
| CR/Cr | 上调Up | 18.54 | Zm00001d024412 | 10 | 细胞色素P450还原酶 Cytochrome P450 reductase | |
| 8.54 | Zm00001d042814 | 3 | 86A1型细胞色素P450 Cytochrome P450 86A1 | |||
| 7.29 | Zm00001d013862 | 5 | 推断的细胞色素P450大家族蛋白 Putative cytochrome P450 superfamily protein | |||
| 7.23 | Zm00001d011932 | 8 | 类89A2型细胞色素P450 Cytochrome P450 89A2-like | |||
| 6.46 | Zm00001d012326 | 8 | 细胞色素P450大家族蛋白 Cytochrome P450 superfamily protein | |||
| 5.82 | Zm00001d029526 | 1 | 78A5型细胞色素P450 Cytochrome P450 78A5 | |||
| 下调Down | -1.22 | Zm00001d002937 | 2 | 细胞色素P450,72家族,A亚族,多肽8 Cytochrome P450 family 72 subfamily A polypeptide 8 | ||
| -20.04 | Zm00001d039697 | 3 | 类711A1型细胞色素P450 Cytochrome P450 711A1-like | |||
| -23.94 | Zm00001d004486 | 2 | 71D7型细胞色素P450 Cytochrome P450 71D7 | |||
| 中期1 Meta1 | Nr/Cr | 上调Up | - | - | - | - |
| 下调Down | - | - | - | - | ||
| CR/Cr | 上调Up | 7.46 | Zm00001d011932 | 8 | 类89A2型细胞色素P450 Cytochrome P450 89A2-like | |
| 6.79 | Zm00001d013862 | 5 | 推断的细胞色素P450大家族蛋白 Putative cytochrome P450 superfamily protein | |||
| 3.88 | Zm00001d020673 | 7 | 推断的细胞色素P450大家族蛋白 Putative cytochrome P450 superfamily protein | |||
| 下调Down | -3.58 | Zm00001d049573 | 4 | 推断的细胞色素P450大家族蛋白 Putative cytochrome P450 superfamily protein | ||
| -4.16 | Zm00001d037701 | 6 | 推断的细胞色素P450大家族蛋白 Putative cytochrome P450 superfamily protein | |||
| -16.87 | Zm00001d039697 | 3 | 类711A1型细胞色素P450 Cytochrome P450 711A1-like | |||
| 前期1 Prop1 | Nr/Cr | 上调Up | 19.96 | Zm00001d024412 | 10 | NADPH-细胞色素P450还原酶 NADPH--cytochrome P450 reductase |
| 17.59 | Zm00001d042814 | 3 | 86A1型细胞色素P450 Cytochrome P450 86A1 | |||
| 下调Down | - | - | - | - | ||
| CR/Cr | 上调Up | 17.44 | Zm00001d042814 | 3 | 86A1型细胞色素P450 Cytochrome P450 86A1 | |
| 下调Down | - | - | - | - |
新窗口打开|下载CSV
2.7 ATP酶活性变化
氧化磷酸化途径是线粒体内重要的代谢途径。玉米C型胞质不育线粒体基因组内发生重复或嵌合的基因(atp6c、atp9-2和cox2-2)均与该途径相关,其中cox2(-2)位于复合体Ⅳ中,atp6c和atp9(-2)位于复合体Ⅴ中。重复的基因打破原有基因间的剂量比例[38,39,40],而嵌合基因又干扰了正常atp6的生成,并进一步干扰了复合体Ⅴ的功能行使[18],最终引起线粒体ATP酶的活性变化。对“三系”材料减数分裂期间花药ATP酶活性测定结果显示,不育系的ATP酶活性相较于保持系明显低了1个酶活单位(图6),而在恢复系中,ATP酶的活性不但得到恢复,并且比保持系提高了一个酶活单位。图6
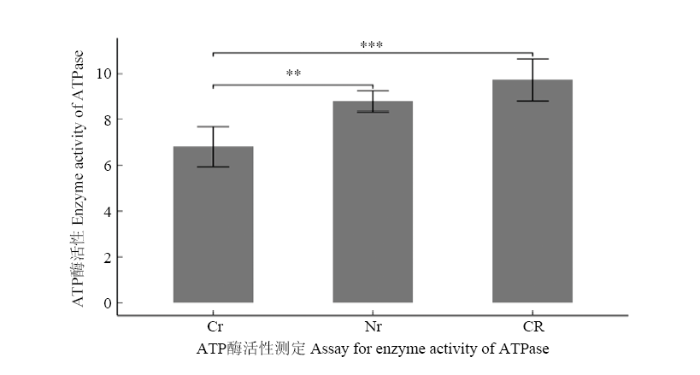 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6三系材料花药ATP酶活性测定与比较
CR:恢复系;Cr:不育系;Nr:保持系;**表示0.01水平差异显著;***表示0.001水平差异显著
Fig. 6Assay for Activity of ATPase of anthers from the three lines
CR: Restorer lines; Cr: Sterile lines; Nr: Maintainer lines; ** indicates difference at the 0.01 level; *** indicates difference at the 0.001 level
3 讨论
3.1 玉米C型胞质雄性不育系败育时期
LEE等[23]通过电镜观察发现玉米C型胞质雄性不育系的花药在四分体时期出现差异,主要为绒毡层细胞出现大量小液泡(类型Ⅰ)或高度液泡化(类型Ⅱ),细胞器如线粒体等正常。进一步观察发现,不育系绒毡层细胞在二分体时期未观察到U小体(Ubisch body),四分体时除出现液泡外,胞质的电子密度亦有降低,小孢子初期开始败育[24]。本研究中发现87-1保持系与不育系在前期仅有103个差异表达基因;而87-1保持系与不育系相比前期Ⅰ至中期Ⅰ的差异表达基因也相对较少,而同一时期,保持系与不育系小孢子发育也没有明显区别,说明这些差异基因可能与后期的小孢子败育无直接关系。87-1不育系的花药从中期Ⅰ到四分体,以及不同材料的花药四分体之间则存在大量的差异表达基因,而前人利用电镜观察到不育系败育的表型相吻合[23,24],因此可以推测引起玉米C胞质雄性不育小孢子败育的关键时期应在减数分裂中期Ⅰ到减数分裂后期Ⅱ之间,而这一阶段正是玉米花药绒毡层细胞二核化的高峰期[41]。LI等[27]曾经对玉米C型胞质雄性不育系及保持系的花粉母细胞及单核期进行了转录组测序,虽然鉴定到一些差异表达的基因,但是这些时期由于不是引起小孢子败育的关键时期,鉴定到的差异表达基因可能与小孢子的后期败育有关。为了筛选出与玉米C型胞质雄性不育小孢子败育与恢复的关键基因,本研究选择了减数分裂前期、减数分裂中期Ⅰ及四分体3个花药发育的关键时期进行了转录组分析[23,24],并从差异不同时期差异基因的变化趋势中推断出基因表达改变的主要时期,为后续表达分析、分子鉴定的取样提供了依据。3.2 玉米C型胞质不育恢复系通过能量补偿恢复育性
前人通过RNA杂交与C型胞质不育系和保持系的线粒体测序分析,发现玉米N胞质与C胞质的线粒体基因组在atp6、atp9和coxⅡ等基因序列间存在差异,其中C胞质线粒体中atp6c为嵌合体基因,atp9和coxⅡ具有2个拷贝[21]。线粒体全基因组分析中也发现有其它的嵌合体基因,但与已知的线粒体基因均不同源[22]。尽管这3个基因可能与C胞质不育相关,迄今为止,尚没有分子证据能证明其中的基因为不育基因。CHEN等[18]总结了引起细胞质雄性不育的4个模型,分别是毒性假说、能量亏损假说、异常PCD假说及反向调节假说。本研究通过对线粒体基因进行分析,发现上述3个基因组间存在差异的基因在不育系与保持系及恢复系之间的差异表达无显著差异(图7)。由于atp6及atp9是氧化磷酸化途径中复合体Ⅴ的组成部分,coxⅡ为复合体Ⅳ的成分,这些基因微小的表达差异也可引起能量供应的波动,进而造成花粉不育。转录组数据分析结果表明,氧化磷酸化相关基因在87-1不育系中表达较低,而在87-1保持系与恢复系中上调表达(图4,红框基因);而在87-1恢复系中同时还抑制了该途径中另外几个基因的表达(图4,蓝框基因),从而恢复线粒体的能量供求;ATP酶活性测定结果显示ATP酶活性波动趋势(图6)与由转录组数据中相应基因的表达趋势推导的结论一致。氧化磷酸化途径复合体Ⅴ与ATP的合成直接有关,qRT-PCR分析显示,复合体Ⅴ亚基基因Zm00001d043834在四分体时期达到高峰,且中期Ⅰ中恢复系和保持系中的表达量比不育系高出数倍(图5)。据此推断,玉米C型胞质雄性不育可能是由线粒体能量不足引起的,而恢复基因则可以对不育基因引起的能量亏损进行补偿;同时恢复基因可能通过协调氧化磷酸化途径中不同的复合体亚基,促进整个途径中的反应有序运行,弥补不育基因的有害作用,进而恢复育性。图7
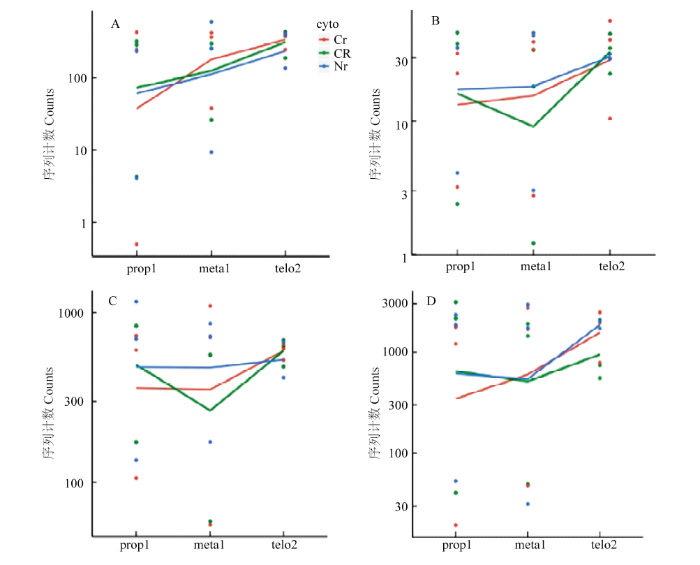 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7线粒体基因atp6c、atp9-1、cox2-1及nad4时序表达分析
Fig. 7Timeline expression analysis of the genes atp6c, atp9-1, cox2-1 and nad4 located in mitochondrial genome
A:atp6c;B:atp9-1;C:cox2-1;D:nad4
3.3 细胞色素P450基因的调控与玉米C型胞质雄性不育相关
植物细胞色素P450基因家族参与多个代谢途径,影响植物的生长发育及开花[37]。过表达CYP78A6的拟南芥植株与野生型相比具有更大的荚果、较短的雄蕊以及较低的育性等[42]。敲除CYP78A5的拟南芥具有更多的叶片[43]。而CYP78A9则参与拟南芥生殖发育[44]。水稻中CYP704B2与花药角质的合成及花粉外壁的形成相关[45]。玉米核不育基因MS26为细胞色素P450类基因[46]。前人研究表明细胞色素P450家族的基因与雄性器官的发育和育性有关。本研究发现减数分裂前期Ⅰ时不育系相比保持系,细胞色素P450基因Zm00001d024412与Zm00001d042814表达量极低,恢复系通过恢复其中一个细胞色素P450基因Zm00001d042814使得减数分裂正常向后进行。随后恢复系中通过恢复更多的细胞色素P450基因并抑制该家族中不需要的基因保证绒毡层细胞的正常功能最终恢复花粉的育性。由此推测,不育基因可能影响到细胞色素P450基因的表达,而恢复基因通过早期恢复相应细胞色素P450基因的表达,进而级联恢复后续发育所需的该家族基因,保证了细胞功能的正常行使,从而使育性得以恢复。4 结论
玉米C型胞质不育形成的关键时期可能为减数分裂中期Ⅰ与减数分裂后期Ⅱ之间;玉米C型胞质不育机理可能是能量亏损假说,恢复基因通过直接或间接作用进行能量补偿而恢复育性。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1016/0092-8674(86)90465-4URL [本文引用: 1]
DOI:10.1186/1471-2229-10-125URL [本文引用: 1]
DOI:10.1105/tpc.105.038240URL [本文引用: 2]
[本文引用: 1]
DOI:10.1105/tpc.3.12.1349URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1073/pnas.0901860106URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1073/pnas.102301599URL [本文引用: 1]
DOI:10.1073/pnas.1511748112URL [本文引用: 1]
DOI:10.1146/annurev-arplant-050213-040119URL [本文引用: 4]
[本文引用: 1]
[本文引用: 1]
Magsci [本文引用: 1]

通过对恢复系凤可 1、A619与 Cms-C237、Cms-CMo17组配的 F2和 BC1分离群体的研究结果表明:凤可 1有两对重叠恢复基因 Rf4和Rf5。用微卫星标记(SSR)将凤可1中的恢复主基因Rf5定位在第 5染色体长臂上,与引物 bnlg1711、bnlg1346和 phi058紧密连锁,距 3个引物的遗传距离分别为 7.51cM、1.68cM、9.87cM;恢复主基因 Rf4与第8染色体短臂上的引物bnlg2307连锁。
Magsci [本文引用: 1]

通过对恢复系凤可 1、A619与 Cms-C237、Cms-CMo17组配的 F2和 BC1分离群体的研究结果表明:凤可 1有两对重叠恢复基因 Rf4和Rf5。用微卫星标记(SSR)将凤可1中的恢复主基因Rf5定位在第 5染色体长臂上,与引物 bnlg1711、bnlg1346和 phi058紧密连锁,距 3个引物的遗传距离分别为 7.51cM、1.68cM、9.87cM;恢复主基因 Rf4与第8染色体短臂上的引物bnlg2307连锁。
DOI:10.1007/BF00334775URL [本文引用: 2]
DOI:10.1534/genetics.107.073312URL [本文引用: 2]
[本文引用: 5]
[本文引用: 6]

利用光学和电子显微镜对玉米C型胞质雄性不育系和保持系的花药与小孢子发育过程进行对比观察.结果表明,不育系小孢子初始败育发生在幼龄小孢子时期,单核中期基本上完全败育,绒毡层到开花前崩溃.在小孢子初始发育时期,不育系绒毡层细胞的质体呈“杯状”变态.导致雄性不育主要的细胞学原因是绒毡层细胞结构、功能的失常.
[本文引用: 6]

利用光学和电子显微镜对玉米C型胞质雄性不育系和保持系的花药与小孢子发育过程进行对比观察.结果表明,不育系小孢子初始败育发生在幼龄小孢子时期,单核中期基本上完全败育,绒毡层到开花前崩溃.在小孢子初始发育时期,不育系绒毡层细胞的质体呈“杯状”变态.导致雄性不育主要的细胞学原因是绒毡层细胞结构、功能的失常.
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
DOI:10.1186/gb-2008-9-12-r181URL [本文引用: 1]
[本文引用: 2]
DOI:10.1038/nbt.3122URL [本文引用: 1]
DOI:10.1186/s13059-014-0550-8URL
DOI:10.1089/omi.2011.0118URL [本文引用: 1]
DOI:10.1006/meth.2001.1262URL [本文引用: 1]
DOI:10.1016/j.mito.2014.02.008URL [本文引用: 1]
DOI:10.1016/j.mito.2014.04.009URL [本文引用: 1]
[本文引用: 1]
DOI:10.1016/S2095-3119(14)60980-1URL [本文引用: 2]
DOI:10.1534/genetics.108.090936URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1105/tpc.109.070326URL [本文引用: 1]
DOI:10.1111/tpj.12335URL [本文引用: 1]
