 ,*西南大学农学与生物科技学院, 重庆 400716
,*西南大学农学与生物科技学院, 重庆 400716Chemical compositions of cuticular waxes on stems and leaves of three legume green manure crops
LI Yang, YAO Lu-Hua, GUO Xin, ZHAO Xiao, HUANG Lei, WANG Deng-Ke, ZHANG Xue-Feng, XIAO Qian-Lin, YANG Rui-Ji, GUO Yan-Jun ,*College of Agronomy and Biotechnology, Southwest University, Chongqing 400716, China
,*College of Agronomy and Biotechnology, Southwest University, Chongqing 400716, China通讯作者:
收稿日期:2019-03-24接受日期:2019-05-12网络出版日期:2019-07-22
| 基金资助: |
Received:2019-03-24Accepted:2019-05-12Online:2019-07-22
| Fund supported: |
作者简介 About authors
E-mail:286923599@qq.com。

摘要
关键词:
Abstract
Keywords:
PDF (624KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
栗扬, 姚露花, 郭欣, 赵晓, 黄蕾, 王登科, 张学风, 肖前林, 杨瑞吉, 郭彦军. 三种豆科绿肥作物茎和叶角质层蜡质化学组成分析[J]. 作物学报, 2020, 46(1): 131-139. doi:10.3724/SP.J.1006.2020.94048
LI Yang, YAO Lu-Hua, GUO Xin, ZHAO Xiao, HUANG Lei, WANG Deng-Ke, ZHANG Xue-Feng, XIAO Qian-Lin, YANG Rui-Ji, GUO Yan-Jun.
绿肥作物种植于主要作物种植间隙, 是一类以改良土壤质量为目的的植物, 其中以豆科绿肥作物利用较广[1]。豆科绿肥作物既可通过生物固氮增加土壤中的氮素, 也可通过翻埋入土, 以绿肥的形式改良土壤质量。然而, 相较于粮食作物, 对夏季绿肥作物基本理化特性的认识尚不足, 相关研究文献也少, 制约了研究者对这些绿肥作物的开发利用。因此, 了解夏季绿肥作物的抗性生理指标, 可为提高这类作物的抗逆性、降低田间管理成本提供理论依据。
植物角质层蜡质是植物与外界环境的第一接触面, 在保护植物免受生物及非生物胁迫的侵害、提高抗逆性方面发挥着非常重要的作用[2]。如干旱胁迫下, 植物叶角质层蜡质含量的增加被认为与提高植物的抗旱性有关[3]。随着紫外线辐射强度的变化, 角质层蜡质晶体结构也随之发生变化, 以通过反射减少紫外线伤害[4]。角质层蜡质中的醛类物质被认为与病原菌的侵染有关[5]。此外, 朱命炜等[6]和韦存虚等[7]应用扫描电镜观察发现成熟芦荟(Aloe vera)和星星草(Puccinellia tenuiflora)叶表面分布有密集的瘤状蜡质, 能有效地减少水分散失和增强叶片反射光辐射的能力。目前的研究结果表明, 植物角质层蜡质主要由长链脂肪酸及其衍生物组成, 如醛类、初级醇、酯类、烷烃、次级醇、酮, 及支链醇、支链烷烃、三萜醇、双酮、烯烃、二醇和酮醇等物质[8]。植物种类不同, 其所含的角质层蜡质组分也不同, 如油菜(Brassica napus)和番茄(Lycopersicon esculentum)以烷类物质为优势成分, 紫花苜蓿(Medicago sativa)、玉米(Zea mays)以初级醇为优势成分[9], 羊草(Leymus chinensis)以二酮为优势成分[10]。蜡质组分的品种差异说明参与角质层蜡质生物合成及转运的途径也可能存在差异[11]。角质层蜡质的合成, 首先在细胞质中由可溶性的脂肪酸合成酶催化从头合成16C/18C的饱和脂肪酸, 然后在内质网中由脂肪酰-CoA延长酶催化16C/18C的脂肪酸延长产生不同链长的长链脂肪酸。脂肪酰-CoA延长酶是一个多酶复合体, 包括β-酮脂酰-CoA合酶(3-ketoacyl- CoA synthase, KCS)、β-酮脂酰-CoA还原酶(3-ketoacyl-CoA reductase, KCR)、β-羟脂酰-CoA脱水酶(3-hydroxacyl-CoA dehydratase, HCD)和反式烯脂酰-CoA还原酶(trans-2,3-enoyl-CoA reductase, ECR)。基于蜡质组分分析及基因功能验证, 目前已证实, 角质层蜡质生物合成主要包括脱羰基途径(烷合成途径)、酰基还原途径(初级醇合成途径)和聚酮合酶途径(polyketide synthase, PKS)[12]。如在拟南芥(Arabidopsis thaliana)中已鉴定出参与蜡质合成的21个KCS基因、参与合成次级醇和酮的MAH1基因及参与烷基酯合成的WSD1基因[12], 在小麦(Triticum aestivum)与大麦(Hordeum vulgare)中鉴定出参与β-二酮合成的基因[13]。挖掘蜡质合成基因已被作为选育抗逆作物材料的方法之一[9]。如WXP1在紫花苜蓿中的超表达, 显著增加了烷烃含量, 提高了植株的抗旱性[13], OsGL1-6在水稻(Oryza sativa)中参与了初级醇的合成, 并可提高水稻抗旱性[15]。
柽麻(Crotalaria juncea)、竹豆(Phaseolus calcaratus)、田菁(Sesbania cannabina)是生产中常用的几种夏季豆科绿肥作物。柽麻与玉米间作, 可显著提高玉米地上部产量[16]。在松嫩平原盐碱土, 随着田菁生物量增加, 土壤pH、可溶性盐含量逐年降低, 有机质含量、碱解氮含量逐年升高[17]。幼龄果园套种竹豆绿肥与清耕相比, 可减少径流70%、泥沙80%以上, 对改善园地土壤水肥, 促进幼树生长有明显的促进作用[18]。目前尚无这些绿肥作物角质层蜡质方面的研究报道。鉴于此, 本试验从田间采集柽麻、田菁和竹豆叶片及茎秆, 利用色谱质谱联用仪鉴定了蜡质组分, 用气相色谱对蜡质组分进行了定量分析, 并计算了各组分相对含量及碳链分布特征, 为进一步挖掘这些植物的蜡质合成基因, 提高夏季豆科绿肥作物抗逆性提供理论基础。
1 材料与方法
1.1 试验地点
试验地位于西南大学农学与生物科技学院实验农场(29°48′N, 106°24′E), 属亚热带季风性湿润气候, 年平均气温为18.2°C, 降水量为1084.6 mm。夏季绿肥作物生长季(4月至9月)月平均气温分别为18.9°C、22.5°C、25.3°C、28.6°C、28.5°C和24.3°C; 平均降水量分别为94.6、144.2、206.3、169.4、128.6和126.6 mm; 月平均日照时数分别为96.8、103.8、97.2、162.5、164.3和104.7 h (1988—2017年)。供试土壤为粘质黄壤, 0~20 cm土壤耕层pH 6.37, 含有机质 12.4 g kg-1、全氮0.73 g kg-1、全磷0.12 g kg-1、全钾3.32 g kg-1、碱解氮67.4 mg kg-1、速效磷8.6 mg kg-1、速效钾82.5 mg kg-1。1.2 绿肥作物
供试绿肥作物包括柽麻(Crotalaria juncea)、竹豆(Phaseolus calcaratus)和田菁(Sesbania cannabina)。种子经消毒(10% H2O2)后, 直接播在田间。设置9个小区, 小区面积10 m2 (2 m×5 m), 每个品种3个小区(重复), 随机区组排列。播种时, 行距40 cm, 株距30 cm。等幼苗出土1周后, 开始间苗, 每小区留苗76株。定期除杂, 不浇水(靠雨水)。待植物进入现蕾期开始采样分析。因竹豆在重庆地区开花较迟且少, 采样时只有少数植株有花蕾。1.3 蜡质提取
分别采集每个小区各植物不同植株相同叶位叶片5片, 同时按留茬5 cm刈割后, 剪取茎段。提蜡前, 利用WinFOLIA专业组织图像分析系统(Regent Instrument Inc, Canada)和数字化扫描仪(EPSON V750, Japan)测定茎、叶的正投影面积, 而3种豆科绿肥作物的茎可近似看作圆柱体, 故茎、叶面积可通过以下公式进行计算。叶片面积 = 2×投影面积
茎秆表面积 = π×投影面积
每个小区叶片和茎秆样品, 在室温条件下分别用含5 μg内标(24烷)的氯仿(5 mL)涡旋浸提3次, 每次30 s。提取液混合后经40°C氮气吹干, 加入20 μL BSTFA [N,O-Bis (三甲基硅烷基)三氟乙酰胺])和20 μL吡啶, 于70°C衍生反应45 min, 多余衍生剂用氮吹仪再次吹干。将所得提取物溶于1 mL氯仿, 进行气相色谱和质谱分析。
1.4 气相色谱–质谱(GC-MS)分析
采用9790II气相色谱仪(浙江福立)分析蜡质含量。色谱柱为DM-5 (5%二苯基 + 95%二甲基聚硅氧烷固定液), 毛细管柱长30 m, 直径0.32 mm, 液膜厚度0.25 μm。氮气作为载气; 进样量为1.0 μL; 柱膜和FID检测器的温度分别为300°C和320°C; 分流比为10︰3。初始温度为80°C, 以每分钟15°C的速度升温至260°C, 并保持10 min, 之后以每分钟2°C升温至290°C, 保持1 min; 再以每分钟5°C的速度升温至320°C, 并保持10 min。利用气相色谱—质谱联用仪(GCMS-AOC-5000 Plus)鉴定蜡质组分, 程序升温方式同色谱分析。鉴定成分时主要根据离子峰质荷比(m/z)结合已发表相关成分质谱图, 解析化合物结构。基于FID峰值量化蜡质, 根据质谱所鉴定的标准确定色谱中各种化学成分的出峰位置, 用内标计算出植物单位面积的实际蜡质含量, 单位为μg cm-2。1.5 数据分析
试验数据为3个重复的平均值±标准误。采用SPSS(17.0)统计软件, 利用单因素方差分析(One- Way ANOVA)比较不同作物茎、叶的蜡质总含量、蜡质各组分含量及链长分布特征, 显著性检验水平为P = 0.05 (l.s.d.)。2 结果与分析
2.1 蜡质组分鉴定
以质谱分析获得化合物离子峰质荷比(m/z), 再结合文献中对蜡质成分的鉴定质谱图, 从柽麻茎中鉴定出5类物质, 包括脂肪酸、初级醇、醛类、烷烃和萜类化合物, 叶片中鉴定出4类物质, 包括初级醇、醛类、烷烃和萜类化合物。田菁茎中发现7类物质, 包括脂肪酸、初级醇、烷基酯、醛类、烷烃、二醇、萜类和固醇类化合物, 叶片中鉴定出5类物质, 包括脂肪酸、初级醇、醛类、烷烃和萜类。其中二醇为1,18-30烷醇和1,16-30烷醇, 其中1,16-30烷醇离子峰构成为m/z 73(100%)、103(38%)、149(59%)、285(23%)、299(63%)、313(25%)、387(8%)、401(15%)、415(6%)、598(质量峰)(图1)。竹豆茎和叶中均鉴定出5类物质, 包括脂肪酸、初级醇、醛类、烷烃和萜类。柽麻和田菁茎和叶均有低于10%左右的成分未能被鉴定, 而竹豆茎和叶中未鉴定成分高达30%左右。图1
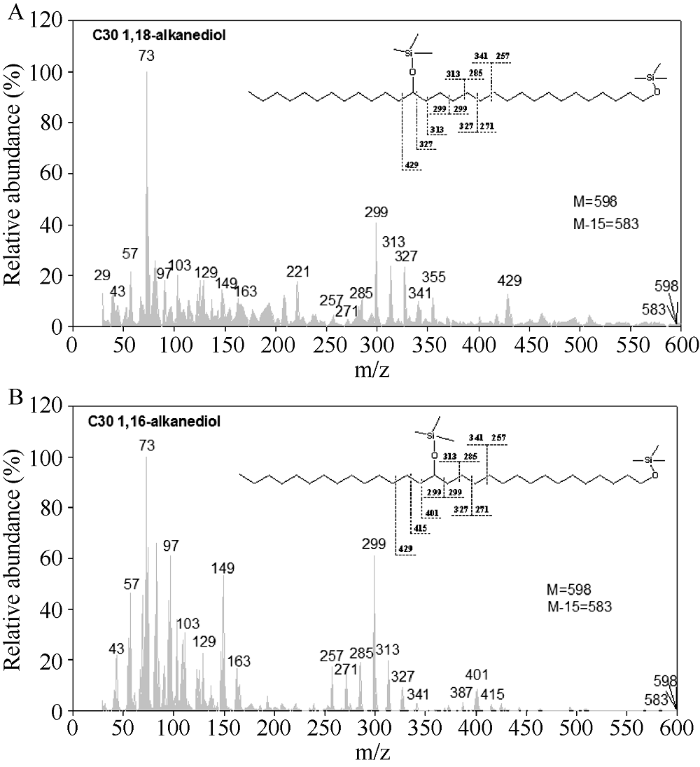 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1田菁茎蜡质中双醇化学结构分析
A: 1,18 -30双醇; B: 1,16 30-双醇。
Fig. 1Chemical structure of diols identified in Sesbania cannabina stem wax
A: C30 1,18-alkanediol; B: C30 1,16-alkanediol.
2.2 茎、叶蜡质总量及组分相对含量分析
柽麻、田菁、竹豆3种绿肥作物的茎、叶蜡质总含量存在显著种间及部位差异, 其中柽麻茎蜡质总含量为16.33 μg cm-2, 显著高于田菁茎(6.45 μg cm-2)和竹豆茎(0.72 μg cm-2)(图2)。就茎和叶比较, 柽麻茎显著高于叶片, 其他2种植物茎和叶之间无显著差异。图2
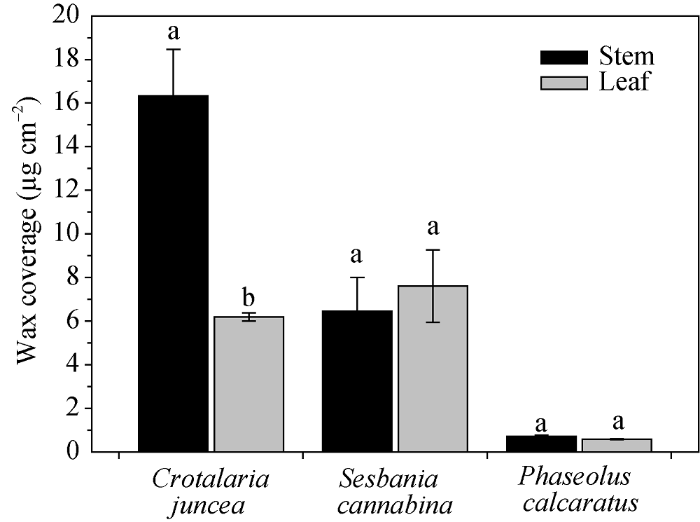 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2柽麻、田菁、竹豆的茎和叶蜡质总量
数据柱上不同小写字母表示品种内差异显著(P < 0.05)。
Fig. 2Wax coverage in stem and leaf of Crotalaria juncea, Sesbania cannabina, and Phaseolus calcaratus
Different lowercase letters above the data bar within each species represent significant difference at P < 0.05.
柽麻茎蜡质中, 烷烃为优势成分, 占蜡质总量的57.38%, 其次是初级醇, 占14.23%, 脂肪酸占11.23%, 醛类和萜类化合物均低于7%, 未鉴定蜡质组分约占2.96% (图3)。叶片中初级醇为优势成分, 占蜡质总量的50.12%, 醛类和萜类化合物分别占12.77%和15.95%, 而烷烃比例只有5.26%, 约12.45%的成分未能鉴定出来。
图3
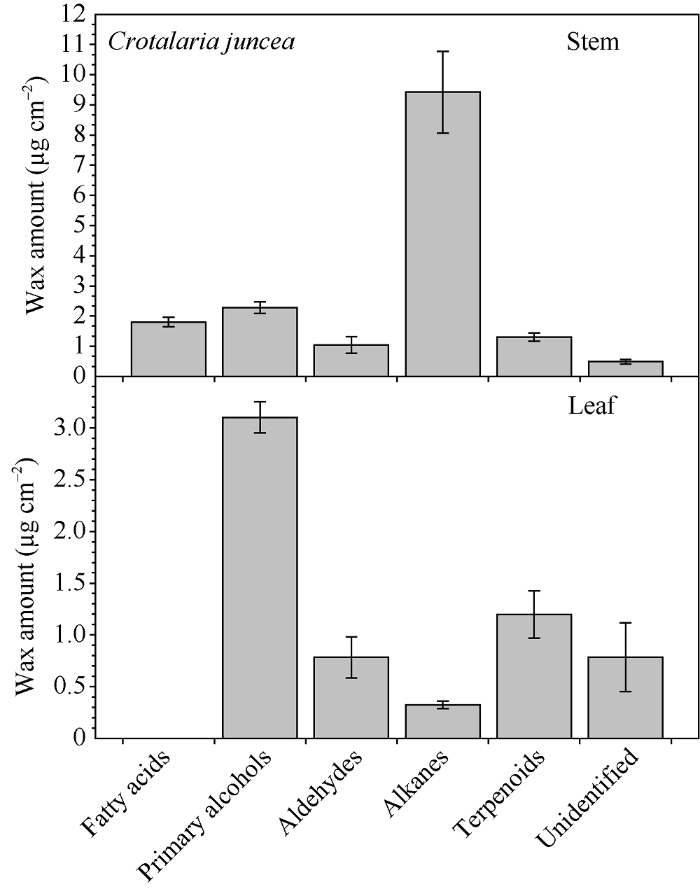 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3柽麻茎和叶中主要蜡质组分实际含量
Fig. 3Amount of wax compound class on stem and leaf of Crotalaria juncea
田菁茎、叶蜡质中的优势成分均为初级醇, 其中茎中占总蜡质的30.12%, 而叶中高达71.21%。其次是醛类, 茎和叶中分别占总蜡质的22.89%和19.39%(图4)。茎中发现的二醇, 所占比例为3.58%, 烷基酯比例为0.44%。脂肪酸、烷烃和三萜类物质在茎和叶中比例分别为7.99%和3.86%、6.59%和3.29%、14.63%和0.11%。另外, 茎和叶中均有8.55%和2.13%的未知成分。
图4
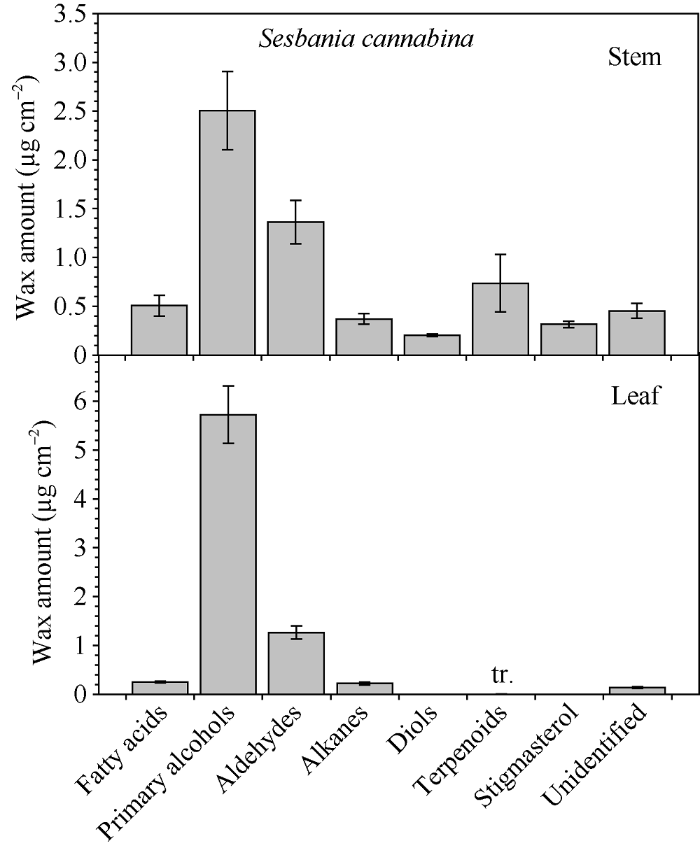 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4田菁茎和叶主要蜡质组分实际含量
Fig. 4Amount of wax compound class on stem and leaf of Sesbania cannabina
竹豆茎、叶蜡质中的优势成分均为烷烃, 分别占总蜡质的40.79%和39.27%。其次茎中分别为醛(25.28%)、初级醇(12.18%)和三萜类(2.61%), 叶中三类组分的比例分别为8.34%、3.75%和12.69% (图5)。茎和叶中均有小于2%的脂肪酸, 以及17.22%和22.66%的未知成分。
图5
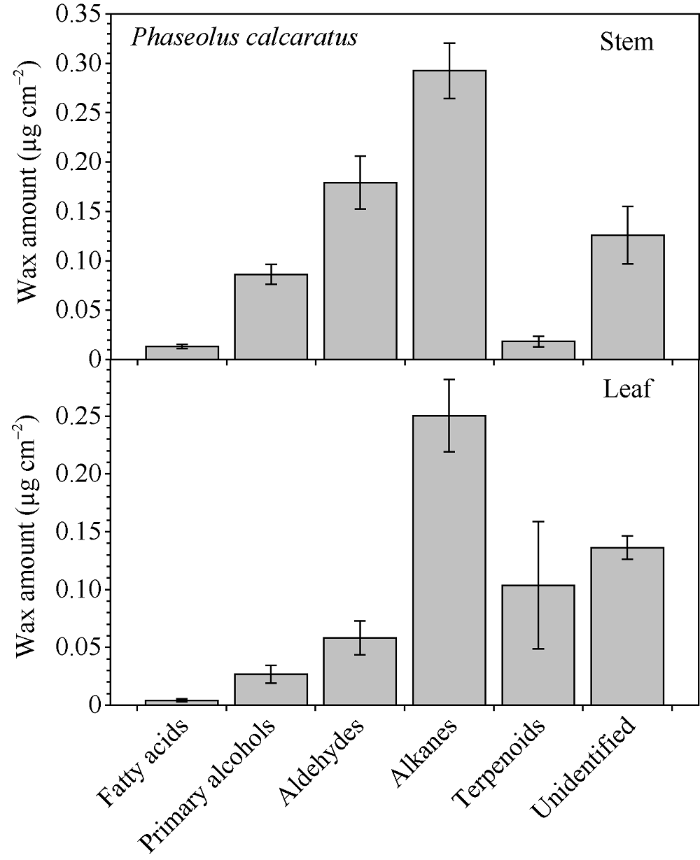 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5竹豆茎和叶主要蜡质组分实际含量
Fig. 5Amount of wax compound class on stem and leaf of Phaseolus calcaratus
2.3 碳链分布特征分析
不同绿肥作物中发现的蜡质组分多数以同系物的形式存在, 如脂肪酸和初级醇均为不同链长的偶数碳分子, 而醛和烷烃为奇数和偶数碳共存的化合物, 且烷烃表现为明显的奇数优势, 醛表现为偶数优势。2.3.1 柽麻主要蜡质组分碳链分布特征 柽麻茎中脂肪酸碳链分布在C20~C32之间, 其中以C30为优势组分, 占总脂肪酸的33.33%, 其次为C32, 占22.20% (图6)。初级醇碳链分布茎中在C22~C34之间, 叶中在C24~C32之间, 二者均以C28为优势组分, 分别占总初级醇的45.34%和68.66%; 茎中含有较高比例的C32, 达到31%, 而叶中只有0.14%。茎和叶中醛的碳链分布存在较大差异, 茎中以C32为优势组分(52.13%), 叶中以C30为优势组分(70.83%)。烷烃链长在C25~C33之间, 茎和叶均以C31为优势组分, 分别占总烷烃的79.49%和59.56%。萜类物质, 茎和叶中均以羽扇豆醇为最高(80%左右), 其中茎中还有20%左右α-香树脂醇, 叶中有15%左右的乙酰化羽扇豆醇。
图6
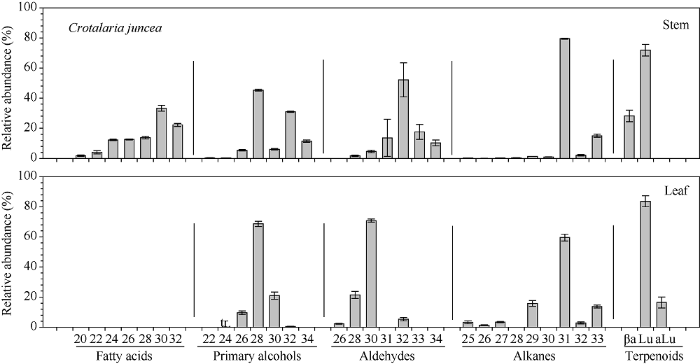 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6柽麻茎和叶蜡质各组分同系物相对含量
Fig. 6Relative abundance of compound homologs in Crotalaria juncea
2.3.2 田菁主要蜡质组分碳链分布特征 田菁茎和叶中的脂肪酸碳链分布范围在C20~C32, 其中茎中以C30为优势组分(68.35%), 而叶中以C26为优势组分(30.15%)(图7)。初级醇碳链分布在茎和叶中差异较大, 其中茎中在C26~C32, 叶中在C22~C32, 但2个部位均以C30为优势组分, 分别占83.05%和73.25%。醛碳链分布茎中在C28~C34, 叶中在C28~C32, 2个部位均以C30为优势组分, 分别占46.96%和52.53%。烷碳链分布茎中在C25~C33, 叶中在C25~C31, 2个部位均以C29为优势组分, 分别占41.77%和51.18%。茎中所鉴定的二醇为C30同分异构体, 其中1,18-30烷醇占27.91%, 1,16-30烷醇占72.09%。茎中发现的萜类物质为β-香树脂醇和羽扇豆醇, 而叶中只有β-香树脂醇。
图7
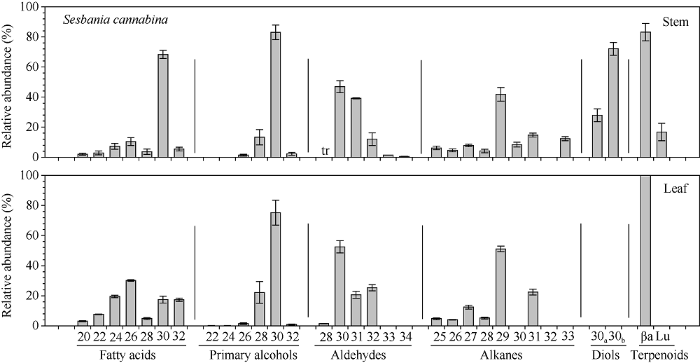 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7田菁茎和叶蜡质各组分同系物相对含量
Fig. 7Relative abundance of compound homologs in Sesbania cannabina
2.3.3 竹豆主要蜡质组分碳链分布特征 竹豆茎和叶中脂肪酸碳链分布均在C22~C30之间, 且均以C28为优势组分(60%左右)(图8)。初级醇碳链分布, 茎中在C24~C30, 叶中在C24~C34, 其中茎中以C28为优势组分(68.75%), 叶中以C32为优势组分(60.38%)。茎中醛碳链在C28~C32, 其中C30为优势组分(88.75%), 而叶中只有C28醛。茎和叶中烷烃碳链分布均在C25~ C33之间, 且均以C31为优势组分, 分别占40.75%和60.26%。茎和叶中的萜类物质均为β-香树脂醇和羽扇豆醇, 其中茎以β-香树脂醇为优势组分(73.69%), 叶以羽扇豆醇为优势组分(72.97%)。
图8
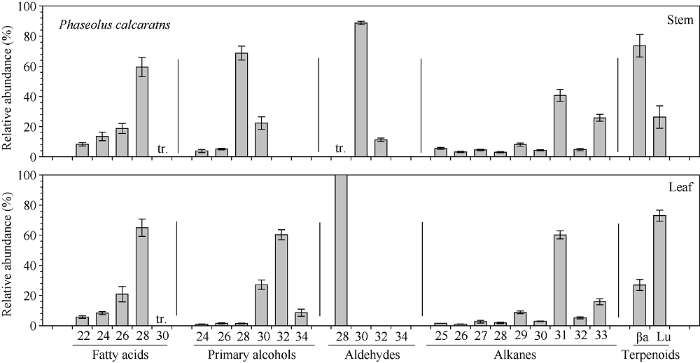 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图8竹豆茎和叶蜡质各组分同系物相对含量
Fig. 8Relative abundance of compound homologs in Phaseolus calcaratus
3 讨论
3种豆科绿肥作物的茎和叶中均含有脂肪酸、初级醇、醛类和烷烃, 且这些组分含量总和占总蜡质含量的比例超过60%。说明烷合成途径和醇合成途径是这些植物合成角质层蜡质的主要途径。这与在其他主要作物上的发现是一致的[9]。然而, 蕨类植物叶角质层蜡质以烷基酯为优势成分[19], 羊草以二酮为优势成分[10]。这些结果暗示了, 在长期进化过程中, 不同植物为适应不同的生存环境其角质层蜡质生物合成产生了不同的进化方向。此外, 我们还发现田菁茎和叶中的蜡质组分存在较大差异, 有些成分只出现在叶片, 如二醇和固醇类物质, 其中二醇为1,18-30烷醇和1,16-30烷醇。在高山罂粟(Papaver alpinum)叶片中也鉴定出C-30的二醇同系物, 但其羟基位置在1,11[20], 在蓖麻(Ricinus communis)叶片中, 鉴定出链长C-22到C-28、羟基位置在1,3的二醇同系物[21]。Busta和Jetter[22]认为这些碳链一头为初级醇、碳链中间存在另一个羟基的二元醇结构, 其生物合成过程可能是由P450加氧酶参与完成的。在拟南芥(A. thaliana)中, MAH1作为羟化酶参与了二醇的合成[23]。田菁叶中二醇物质的发现, 为今后研究二醇生物合成途径提供了新的材料。3种豆科绿肥作物茎和叶中蜡质含量存在较大差异, 其中茎蜡质含量最高的是柽麻, 其次是田菁, 竹豆蜡质含量最低。一般情况下, 植物蜡质含量的增加与其抗旱性的提高相联系[2]。尽管都是夏季绿肥作物, 蜡质含量较低的竹豆为藤本植物, 群体高度低、叶片数量多、地表覆盖度高, 在进化过程中其生长环境可能相对湿润, 不需要过高的蜡质沉积就能适应生长环境。而柽麻与田菁株型直立, 地表覆盖度低, 生长环境相对干燥, 叶片可能需要沉积较多的蜡质来抵御逆境胁迫。当然, 影响植物抗逆性的因素较多, 今后有必要进一步分析几种植物蜡质含量与其抗旱性的关系。就茎和叶角质层蜡质含量比较, 拟南芥茎秆高于叶片[24], 马铃薯(Solanum tuberosum)无显著差异[25], 蒲公英(Taraxacum officinale)叶片高于茎秆[26], 本试验中柽麻茎高于叶片, 而田菁和竹豆茎和叶蜡质无显著差异。这些结果说明不同植物茎和叶角质层蜡质的沉积存在器官特异性, 且这可能与各自不同的生理功能有关。对12个大麦品种的比较发现, 蜡质沉积较多的品种具有较高的抗旱性[3]。转录因子MYB96在亚麻荠(Camelina sativa)中的超量表达可显著增加蜡质沉积, 并提高植物抗旱性[27]。
在茎和叶蜡质组分方面, 柽麻茎以烷烃为优势组分, 而叶以初级醇为优势组分, 说明参与蜡质合成的主效基因在2个器官的表达存在差异, 如拟南芥参与初级醇合成的CER4在叶中较高表达, 而参与烷合成的CER1在茎中高表达[28]。田菁茎和叶中的优势组分均为初级醇(30.12%和71.21%), 且烷烃组分比例不足10%, 说明田菁角质层蜡质以醇合成途径为主。竹豆茎和叶中的优势成分均为烷烃, 说明其角质层蜡质合成以烷合成途径为主。在其他作物上也有类似的研究结果, 如紫花苜蓿叶以初级醇为主, 茎以烷为主[14]; 亚麻荠(C. sativa)叶以烷基酯为主, 茎以三萜类化合物为主[29]; 而玉米叶以初级醇为主, 茎以醛为主[30]。尽管目前尚无法确定这些优势组分是否在植物抗逆方面起主要作用, 然而干旱胁迫下烷类含量的增加被认为与多数植物的抗旱性提高有关[2]; 植物叶表长链醛类的缺失可抑制病原真菌孢子的萌发[31]。
3种绿肥作物茎和叶中主要的蜡质组分均以同系物形式存在, 且整体表现出脂肪酸、初级醇和醛以偶数碳占优势、而烷烃以奇数碳占优势的碳链分布特征。这与绝大多数植物上的研究结果是一致的[32]。就蜡质组分内同系物优势化合物碳链长度分析, 柽麻和竹豆茎和叶均以C31为优势化合物, 而田菁以C29为优势化合物。说明参与烷烃生物合成的同源基因在茎和叶中是一致的。茎和叶初级醇优势化合物在柽麻和田菁是一致的, 分别为C28和C30; 而竹豆茎中以C28为优势化合物, 叶中以C32为优势化合物。出现这一结果的原因可能在于链长延长酶(FAE)及参与初级醇合成的CER4在竹豆不同器官存在差异[33]。醛被认为是烷合成途径和醇合成途径的中间产物[9]。几种绿肥作物茎和叶中醛的优势化合物存在较大差异, 暗示不同器官的醛可能来自不同的合成途径。
4 结论
从3种夏季绿肥作物茎和叶中共鉴定出8类化合物, 包括脂肪酸、初级醇、烷基酯、醛类、烷烃、二醇、萜类和固醇类化合物。尽管主要蜡质组分在品种及部位间一致, 但不同作物及部位又有各自独特的组分存在, 如柽麻茎中未检测出脂肪酸, 田菁茎中鉴定出固醇类和二醇化合物。其中田菁茎中的二醇化合物初步结构解析为1,18-30烷醇和1,16-30烷醇。此外, 不同物种、不同部位所积累的优势组分及优势化合物也存在一定差异, 说明参与蜡质合成的基因在物种、器官间有所不同。这为今后从分子水平上揭示角质层蜡质参与夏季绿肥作物抗逆机制提供了理论基础。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.4141/S00-034URL [本文引用: 1]
DOI:10.1104/pp.113.222737URL [本文引用: 3]

The plant cuticle is an extracellular hydrophobic layer that covers the aerial epidermis of all land plants, providing protection against desiccation and external environmental stresses. The past decade has seen considerable progress in assembling models for the biosynthesis of its two major components, the polymer cutin and cuticular waxes. Most recently, two breakthroughs in the long-sought molecular bases of alkane formation and polyester synthesis have allowed construction of nearly complete biosynthetic pathways for both waxes and cutin. Concurrently, a complex regulatory network controlling the synthesis of the cuticle is emerging. It has also become clear that the physiological role of the cuticle extends well beyond its primary function as a transpiration barrier, playing important roles in processes ranging from development to interaction with microbes. Here, we review recent progress in the biochemistry and molecular biology of cuticle synthesis and function and highlight some of the major questions that will drive future research in this field.
DOI:10.1007/s10681-009-0027-0URL [本文引用: 2]

The effect of drought on barley leaf epicuticular wax load (EWL), residual transpiration rates (RTR) and grain yield was examined by subjecting 12 barley genotypes to controlled irrigation and terminal water stress conditions. The mean leaf epicuticular wax load was found to be 9% greater in the plants subjected to terminal water stress than in those provided irrigation, while the mean residual transpiration rate of the irrigated plants was 20% higher than in those subjected to water stress. Under these stress conditions, the correlation between grain yield and the epicuticular wax load was positive (P<0.01), while that between the grain yield and the residual transpiration rate was negative (P<0.05). Under the water stress conditions, the breeding lines studied showed a greater mean epicuticular wax load than the commercial varieties, while the residual transpiration rate was greater in these varieties than in the breeding lines. The greater epicuticular wax load of the breeding lines favoured their tolerance of drought, improving their yields over those of the commercial varieties.
DOI:10.1007/s10535-007-0038-4URL [本文引用: 1]
DOI:10.1016/j.funbio.2017.05.003URLPMID:28705398 [本文引用: 1]

Asexually produced conidia of the wheat powdery mildew fungus Blumeria graminis f. sp. tritici (Bgt) are known to perceive cuticular very-long-chain aldehydes as signal substances strongly stimulating germination and differentiation of infection structures in a concentration- and chain-length-dependent manner. Conidial germination and appressorium formation are widely prevented by the presence of free water on the host surface. However, sexually produced ascospores can differentiate immersed in water. Applying a Formvar?-based in?vitro-system showed that ascospore appressorium formation was strongly induced by the presence of wheat leaf cuticular wax. Similar to conidia, ascospore appressorium formation is triggered by the presence of very-long-chain aldehydes in a chain-length-dependent manner with n-octacosanal as the most inducing aldehyde. Surface hydrophobicity positively affected ascospore germination but not appressorium formation. Ascospores required significantly more time to complete the differentiation of appressoria and exhibited a more distinct dependence on the availability of free water than their conidial counterparts. Unlike conidia, ascospores showed a more variable germination and differentiation pattern even with a single germ tube differentiating an appressorium. Despite these differences our results demonstrate that a host surface recognition principle based on cuticular very-long-chain aldehydes is a common feature of B. graminis f. sp. tritici ascospores and conidia.
[本文引用: 1]
[本文引用: 1]
URL [本文引用: 1]

利用扫描电镜和 X射线电子探针研究了星星草 (Puccinellia tenuiflora)的叶表皮及其与生境高盐的关系。结果表明 ,叶表皮由表皮细胞和气孔器组成 ,下表皮气孔器多于上表皮 ,且常下陷 ,表皮具表皮毛。表皮细胞外存在丰富的蜡质纹饰和蜡质颗粒 ,这些蜡质包含盐离子 ,具有泌盐的功能。这些特征表明星星草受外界生态因素的影响 ,而演化出具有泌盐功能的蜡质层来适应所生长的高盐生境。
URL [本文引用: 1]

利用扫描电镜和 X射线电子探针研究了星星草 (Puccinellia tenuiflora)的叶表皮及其与生境高盐的关系。结果表明 ,叶表皮由表皮细胞和气孔器组成 ,下表皮气孔器多于上表皮 ,且常下陷 ,表皮具表皮毛。表皮细胞外存在丰富的蜡质纹饰和蜡质颗粒 ,这些蜡质包含盐离子 ,具有泌盐的功能。这些特征表明星星草受外界生态因素的影响 ,而演化出具有泌盐功能的蜡质层来适应所生长的高盐生境。
DOI:10.1023/A:1005409405771URL [本文引用: 1]

The chemical composition of rodlet-shaped wax crystals on fronds of Osmunda regalis was analyzed. In all, 139 compounds belonging to 14 homologous series were detected in the surface extract. They included typical plant wax constituents: alkanes (C25–C33), alkyl esters (C38–C50), primary alcohols (C22–C32), secondary alcohols (C27 and C29), ketones (C27–C33), aldehydes (C24–C34), fatty acids (C24–C32), and
 -sitosterol. Additionally, bifunctional C29 compounds (
-sitosterol. Additionally, bifunctional C29 compounds ( -diketone, ketols, diols) were identified. Nonacosan-10-one as the most abundant single compound, together with its bifunctional derivatives, is likely to form the wax crystals found on O. regalis fronds. Hence, a new type of wax crystals is defined morphologically and chemically. The occurrence of comparable wax aggregates on surfaces of higher plants is discussed.
-diketone, ketols, diols) were identified. Nonacosan-10-one as the most abundant single compound, together with its bifunctional derivatives, is likely to form the wax crystals found on O. regalis fronds. Hence, a new type of wax crystals is defined morphologically and chemically. The occurrence of comparable wax aggregates on surfaces of higher plants is discussed.DOI:10.1007/s00299-015-1772-2URLPMID:25693495 [本文引用: 4]

The aerial parts of plants are covered with a cuticle, a hydrophobic layer consisting of cutin polyester and cuticular waxes that protects them from various environmental stresses. Cuticular waxes mainly comprise very long chain fatty acids and their derivatives such as aldehydes, alkanes, secondary alcohols, ketones, primary alcohols, and wax esters that are also important raw materials for the production of lubricants, adhesives, cosmetics, and biofuels. The major function of cuticular waxes is to control non-stomatal water loss and gas exchange. In recent years, the in planta roles of many genes involved in cuticular wax biosynthesis have been characterized not only from model organisms like Arabidopsis thaliana and saltwater cress (Eutrema salsugineum), but also crop plants including maize, rice, wheat, tomato, petunia, Medicago sativa, Medicago truncatula, rapeseed, and Camelina sativa through genetic, biochemical, molecular, genomic, and cell biological approaches. In this review, we discuss recent advances in the understanding of the biological functions of genes involved in cuticular wax biosynthesis, transport, and regulation of wax deposition from Arabidopsis and crop species, provide information on cuticular wax amounts and composition in various organs of nine representative plant species, and suggest the important issues that need to be investigated in this field of study.
[本文引用: 2]
[本文引用: 2]
DOI:10.1016/j.plipres.2012.10.002URL [本文引用: 1]

Cuticular waxes and cutin form the cuticle, a hydrophobic layer covering the aerial surfaces of land plants and acting as a protective barrier against environmental stresses. Very-long-chain fatty acid derived compounds that compose the cuticular waxes are produced in the endoplasmic reticulum of epidermal cells before being exported to the environmental face of the epidermis. Twenty years of genetic studies on Arabidopsis thaliana have led to the molecular characterization of enzymes catalyzing major steps in fatty acid elongation and wax biosynthesis. Although transporters required for wax export from the plasma membrane have been identified, intracellular and extracellular traffic remains largely unknown. In accordance with its major function in producing an active waterproof barrier, wax metabolism is up-regulated at the transcriptional level in response to water deficiency. However its developmental regulation is still poorly described. Here, we discuss the present knowledge of wax functions, biosynthesis and transport as well as the regulation of these processes. (C) 2012 Elsevier Ltd.
DOI:10.1016/j.pbi.2009.09.009URLPMID:19864175 [本文引用: 2]

The plant cuticle is an extracellular lipid structure deposited over the aerial surfaces of land plants, which seals the shoot and protects it from biotic and abiotic stresses. It is composed of cutin polymer matrix and waxes, produced and secreted by epidermal cells. The use of forward and reverse genetic approaches in Arabidopsis has led to the identification of enzymes involved in fatty acid elongation and biosynthesis of wax components, as well as transporters required for lipid delivery to the cuticle. However, major questions concerning alkane formation, intracellular and extracellular wax transport, regulation of wax deposition, and assembly of cuticular components into a functional cuticle remain to be resolved.
DOI:10.1093/jxb/erw144URLPMID:27162275 [本文引用: 2]
DOI:10.1111/j.1365-313X.2005.02405.xURLPMID:15918883 [本文引用: 1]

The identification of leaf wax genes involved in stress tolerance is expected to have great potential for crop improvement. Here we report the characterization of a novel AP2 domain-containing putative transcription factor gene from the model legume Medicago truncatula. The gene, designated WXP1, is able to activate wax production and confer drought tolerance in alfalfa (Medicago sativa), the most important forage legume species in the world and a close relative of M. truncatula. The predicted protein of WXP1 has 371 aa; it is one of the longest peptides of all the single AP2 domain proteins in M. truncatula. WXP1 is distinctly different from the most studied genes in the AP2/ERF transcription factor family such as AP2s, CBF/DREB1s, DREB2s, WIN1/SHN1 and GL15. Transcript level of WXP1 is inducible by cold, abscisic acid and drought treatment mainly in shoot tissues in M. truncatula. Overexpression of WXP1 under the control of the CaMV35S promoter led to a significant increase in cuticular wax loading on leaves of transgenic alfalfa. Scanning electron microscopy revealed earlier accumulation of wax crystals on the adaxial surface of newly expanded leaves and higher densities of wax crystalline structures on both adaxial and abaxial surfaces of mature leaves. Gas chromatography-mass spectrometry analysis revealed that total leaf wax accumulation per surface area increased 29.6-37.7% in the transgenic lines, and the increase was mainly contributed by C30 primary alcohol. WXP1 overexpression induced a number of wax-related genes. Transgenic leaves showed reduced water loss and chlorophyll leaching. Transgenic alfalfa plants with increased cuticular waxes showed enhanced drought tolerance demonstrated by delayed wilting after watering was ceased and quicker and better recovery when the dehydrated plants were re-watered.
DOI:10.1371/journal.pone.0065139URLPMID:23741473 [本文引用: 1]

Cuticular wax is a class of organic compounds that comprises the outermost layer of plant surfaces. Plant cuticular wax, the last barrier of self-defense, plays an important role in plant growth and development. The OsGL1-6 gene, a member of the fatty aldehyde decarbonylase gene family, is highly homologous to Arabidopsis CER1, which is involved in cuticular wax biosynthesis. However, whether OsGL1-6 participates in cuticular wax biosynthesis remains unknown. In this study, an OsGL1-6 antisense-RNA vector driven by its own promoter was constructed and introduced into the rice variety Zhonghua11 by Agrobacterium-mediated transformation to obtain several independent transgenic plants with decreased OsGL1-6 expression. These OsGL1-6 antisense-RNA transgenic plants showed droopy leaves at the booting stage, significantly decreased leaf cuticular wax deposition, thinner cuticle membrane, increased chlorophyll leaching and water loss rates, and enhanced drought sensitivity. The OsGL1-6 gene was constitutively expressed in all examined organs and was very highly expressed in leaf epidermal cells and vascular bundles. The transient expression of OsGL1-6-GFP fusion indicated that OsGL1-6 is localized in the endoplasmic reticulum. Qualitative and quantitative analysis of the wax composition using gas chromatography-mass spectrometry revealed a significantly reduced total cuticular wax load on the leaf blades of the OsGL1-6 antisense-RNA transgenic plants as well as markedly decreased alkane and aldehyde contents. Their primary alcohol contents increased significantly compared with those in the wild type plants, suggesting that OsGL1-6 is associated with the decarbonylation pathways in wax biosynthesis. We propose that OsGL1-6 is involved in the accumulation of leaf cuticular wax and directly impacts drought resistance in rice.
DOI:10.11686/cyxb2015483URL [本文引用: 1]

本试验在重庆冬油菜种植区,于油菜收获后,选择竹豆,田菁和柽麻3种夏季绿肥作物,通过与夏玉米间作,分析了间作模式下土壤当季速效养分,植物养分吸收性能及地上部生物产量变化情况.结果表明,间作柽麻后玉米地上部产量显著提高35%,间作田菁后玉米产量显著下降21%,而间作竹豆无显著影响.单作绿肥除田菁地上部全氮含量显著低于间作田菁外,其余两个品种的全氮,全磷含量与间作绿肥无显著差异;而单作绿肥全钾含量均显著高于间作绿肥.与单作玉米比较,间作竹豆和田菁时玉米全氮含量,全磷含量显著增加,而间作柽麻时无显著变化;玉米全钾含量整体呈增加趋势.绿肥周围土壤的硝态氮含量显著高于间作玉米周围土壤.间作玉米周围土壤硝态氮含量,速效磷含量和速效钾含量呈现出整体高于单作玉米土壤的趋势,且相关分析结果表明土壤硝态氮含量与植物全氮含量呈显著正相关关系.综合分析认为,柽麻植株有较高的全氮含量和生物产量,且与柽麻间作的玉米地上部产量也最高,适宜作为玉米夏季间作的豆科绿肥.
DOI:10.11686/cyxb2015483URL [本文引用: 1]

本试验在重庆冬油菜种植区,于油菜收获后,选择竹豆,田菁和柽麻3种夏季绿肥作物,通过与夏玉米间作,分析了间作模式下土壤当季速效养分,植物养分吸收性能及地上部生物产量变化情况.结果表明,间作柽麻后玉米地上部产量显著提高35%,间作田菁后玉米产量显著下降21%,而间作竹豆无显著影响.单作绿肥除田菁地上部全氮含量显著低于间作田菁外,其余两个品种的全氮,全磷含量与间作绿肥无显著差异;而单作绿肥全钾含量均显著高于间作绿肥.与单作玉米比较,间作竹豆和田菁时玉米全氮含量,全磷含量显著增加,而间作柽麻时无显著变化;玉米全钾含量整体呈增加趋势.绿肥周围土壤的硝态氮含量显著高于间作玉米周围土壤.间作玉米周围土壤硝态氮含量,速效磷含量和速效钾含量呈现出整体高于单作玉米土壤的趋势,且相关分析结果表明土壤硝态氮含量与植物全氮含量呈显著正相关关系.综合分析认为,柽麻植株有较高的全氮含量和生物产量,且与柽麻间作的玉米地上部产量也最高,适宜作为玉米夏季间作的豆科绿肥.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1093/aob/mcy078URLPMID:30252045 [本文引用: 1]

The cuticular waxes sealing plant surfaces against excessive water loss are complex mixtures of very-long-chain aliphatics, with compositions that vary widely between plant species. To help fill the gap in our knowledge about waxes of non-flowering plant taxa, and thus about the cuticle of ancestral land plants, this study provides comprehensive analyses of waxes on temperate fern species from five different families.
DOI:10.1016/0031-9422(96)00180-XURL [本文引用: 1]
DOI:10.1016/s0031-9422(02)00560-5URLPMID:12620356 [本文引用: 1]

Surface extracts from primary leaves of Castor bean were found to contain 1.8 microg cm(-2) of cuticular waxes. The mixture comprised alkanes (C(26)-C(29)), primary alcohols (C(22)-C(38)), aldehydes (C(26) and C(28)), fatty acids (C(20)-C(34)) and triterpenoids (lupeol, beta- and alpha-amyrin). Besides, a series of n-alkane-1,3-diols was detected, with chain lengths ranging from C(22) to C(28), a strong predominance of even-numbered homologs, and a maximum for hexacosane-1,3-diol. Seven other compounds were assigned to a novel class of wax constituents and identified as homologous unbranched 3-hydroxyaldehydes ranging from C(22) to C(28). As the chain length distribution of this series closely paralleled the homolog pattern of 1,3-diols, it seems likely that both compound classes are biosynthetically related.
DOI:10.1007/s11101-017-9542-0URL [本文引用: 1]
DOI:10.1093/jxb/erp061URLPMID:19346242 [本文引用: 1]

Arabidopsis wax components containing secondary functional groups were examined (i) to test the biosynthetic relationship between secondary alcohols and ketols and (ii) to determine the regiospecificity and substrate preference of the enzyme involved in ketol biosynthesis. The stem wax of Arabidopsis wild type contained homologous series of C(27) to C(31) secondary alcohols (2.4 microg cm(-2)) and C(28) to C(30) ketones (6.0 microg cm(-2)) dominated by C(29) homologues. In addition, compound classes containing two secondary functional groups were identified as C(29) diols (approximately 0.05 microg cm(-2)) and ketols (approximately 0.16 microg cm(-2)). All four compound classes showed characteristic isomer distributions, with functional groups located between C-14 and C-16. In the mah1 mutant stem wax, diols and ketols could not be detected, while the amounts of secondary alcohols and ketones were drastically reduced. In two MAH1-overexpressing lines, equal amounts of C(29) and C(31) secondary alcohols were detected. Based on the comparison of homologue and isomer compositions between the different genotypes, it can be concluded that biosynthetic pathways lead from alkanes to secondary alcohols, and via ketones or diols to ketols. It seems plausible that MAH1 is the hydroxylase enzyme involved in all these conversions in Arabidopsis thaliana.
DOI:10.1111/tpj.13294URLPMID:27496682 [本文引用: 1]

To protect plants against biotic and abiotic stress, the waxy cuticle must coat all epidermis cells. Here, two independent approaches addressed whether cell-type-specific differences exist between wax compositions on trichomes and other epidermal cells of Arabidopsis thaliana, possibly with different protection roles. First, the total waxes from a mutant lacking trichomes (gl1) were compared to waxes from wild type and a trichome-rich mutant (cpc tcl1 etc1 etc3). In the stem wax, compounds with aliphatic chains longer than 31 carbons (derived from C32 precursors) increased in relative abundance in cpc tcl1 etc1 etc3 over gl1. Similarly, the leaf wax from the trichome-rich mutant contained higher amounts of C32+ compounds as compared to gl1. Second, leaf trichomes were isolated, and their waxes were analyzed. The wax mixtures of the trichome-rich mutant and the wild type were similar, comprising alkanes and alkenes as well as branched and unbranched primary alcohols. The direct analyses of trichome waxes confirmed that they contained relatively high concentrations of C32+ compounds, compared with the pavement cell wax inferred from analysis of gl1 leaves. Finally, the cell-type-specific wax compositions were put into perspective with expression patterns of wax biosynthesis genes in trichomes and pavement cells. Analyses of published transcriptome data (Marks et?al., ) revealed that core enzymes involved in elongation of wax precursors to various carbon chain lengths are expressed differentially between epidermis cell types. By combining the chemical and gene expression data, we identified promising gene candidates involved in the formation of C32+ aliphatic chains.
DOI:10.1021/acs.jafc.7b00818URLPMID:28467851 [本文引用: 1]

Complex mixtures of cuticular waxes coat plant surfaces to seal them against environmental stresses, with compositions greatly varying between species and possibly organs. This paper reports comprehensive analyses of the waxes on both above- and below-ground organs of potato, where total wax coverages varied between petals (2.6 μg/cm2), leaves, stems, and tubers (1.8-1.9 μg/cm2), and rhizomes (1.1 μg/cm2). The wax mixtures on above-ground organs were dominated by alkanes, occurring in homologous series of isomeric C25-C35 n-alkanes, C25-C35 2-methylalkanes, and C26-C34 3-methylalkanes. In contrast, below-ground organs had waxes rich in monoacylglycerols (C22-C28 acyls) and C18-C30 alkyl ferulates, together with fatty acids (rhizomes) or primary alcohols (tubers). The organ-specific wax coverages, compound class distribution, and chain length profiles suggest highly regulated activities of wax biosynthesis enzymes, likely related to organ-specific ecophysiological functions.
DOI:10.1016/j.plaphy.2017.04.004URLPMID:28432976 [本文引用: 1]

Primary plant surfaces are coated with hydrophobic cuticular waxes to minimize non-stomatal water loss. Wax compositions differ greatly between plant species and, in the few species studied systematically so far, also between organs, tissues, and developmental stages. However, the wax mixtures of more species in diverse plant families must be investigated to assess overall wax variability, and ultimately to correlate organ-specific composition with local water barrier properties. Here, we present comprehensive analyses of the waxes covering five organs of Taraxacum officinale (dandelion), to help close a gap in our understanding of wax chemistry in the Asteraceae family. First, novel wax constituents of the petal wax were identified as C25 6,8- and 8,10-ketols as well as C27 6,8- and 8,10-ketols. Nine other component classes (fatty acids, primary alcohols, esters, aldehydes, alkanes, triterpenols, triterpene acetates, sterols, and tocopherols) were detected in the wax mixtures covering leaves, peduncles, and petals, as well as fruit beaks and pappi. Wax coverages varied from 5?μg/cm2 on peduncles to 37?μg/cm2 on petals. Alcohols predominated in leaf wax, while both alcohols and alkanes were found in similar amounts on peduncles and petals, and mainly alkanes on the fruit beaks and pappi. Chain length distributions within the wax compound classes were similar between organs, centered around C26 for fatty acids, alcohols, and aldehydes, and C29 for alkanes. However, the quantities of homologs with longer chain lengths varied substantially between organs, reaching well beyond C30 on all surfaces except leaves, suggesting differences in elongation enzymes determining the alkyl chain structures. The detailed wax profiles presented here will serve as basis for future investigations into wax biosynthesis in the Asteraceae and into wax functions on different dandelion organs.
DOI:10.1007/s00299-014-1636-1URL [本文引用: 1]

Camelina has been highlighted as an emerging oilseed crop. Transgenic Camelina plants overexpressing Arabidopsis MYB96 exhibited drought resistance by activating expression of Camelina wax biosynthetic genes and accumulating wax load.
Camelina (Camelina sativa L.) is an oilseed crop in the Brassicaeae family with potential to expand biofuel production to marginal land. The aerial portion of all land plants is covered with cuticular wax to protect them from desiccation. In this study, the Arabidopsis MYB96 gene was overexpressed in Camelina under the control of the CaMV35S promoter. Transgenic Camelina plants overexpressing Arabidopsis MYB96 exhibited normal growth and development and enhanced tolerance to drought. Deposition of epicuticular wax crystals and total wax loads increased significantly on the surfaces of transgenic leaves compared with that of non-transgenic plants. The levels of alkanes and primary alcohols prominently increased in transgenic Camelina plants relative to non-transgenic plants. Cuticular transpiration occurred more slowly in transgenic leaves than that in non-transgenic plants. Genome-wide identification of Camelina wax biosynthetic genes enabled us to determine that the expression levels of CsKCS2, CsKCS6, CsKCR1-1, CsKCR1-2, CsECR, and CsMAH1 were approximately two to sevenfold higher in transgenic Camelina leaves than those in non-transgenic leaves. These results indicate that MYB96-mediated transcriptional regulation of wax biosynthetic genes is an approach applicable to generating drought-resistant transgenic crops. Transgenic Camelina plants with enhanced drought tolerance could be cultivated on marginal land to produce renewable biofuels and biomaterials.
DOI:10.1104/pp.106.086785URLPMID:16980563 [本文引用: 1]

A waxy cuticle that serves as a protective barrier against uncontrolled water loss and environmental damage coats the aerial surfaces of land plants. It is composed of a cutin polymer matrix and waxes. Cuticular waxes are complex mixtures of very-long-chain fatty acids and their derivatives. We report here the molecular cloning and characterization of CER4, a wax biosynthetic gene from Arabidopsis (Arabidopsis thaliana). Arabidopsis cer4 mutants exhibit major decreases in stem primary alcohols and wax esters, and slightly elevated levels of aldehydes, alkanes, secondary alcohols, and ketones. This phenotype suggested that CER4 encoded an alcohol-forming fatty acyl-coenzyme A reductase (FAR). We identified eight FAR-like genes in Arabidopsis that are highly related to an alcohol-forming FAR expressed in seeds of jojoba (Simmondsia chinensis). Molecular characterization of CER4 alleles and genomic complementation revealed that one of these eight genes, At4g33790, encoded the FAR required for cuticular wax production. Expression of CER4 cDNA in yeast (Saccharomyces cerevisiae) resulted in the accumulation of C24:0 and C26:0 primary alcohols. Fully functional green fluorescent protein-tagged CER4 protein was localized to the endoplasmic reticulum in yeast cells by confocal microscopy. Analysis of gene expression by reverse transcription-PCR indicated that CER4 was expressed in leaves, stems, flowers, siliques, and roots. Expression of a beta-glucuronidase reporter gene driven by the CER4 promoter in transgenic plants was detected in epidermal cells of leaves and stems, consistent with a dedicated role for CER4 in cuticular wax biosynthesis. CER4 was also expressed in all cell types in the elongation zone of young roots. These data indicate that CER4 is an alcohol-forming FAR that has specificity for very-long-chain fatty acids and is responsible for the synthesis of primary alcohols in the epidermal cells of aerial tissues and in roots.
DOI:10.1016/j.phytochem.2014.06.018URL [本文引用: 1]

Camelina sativa (L.) Crantz is an emerging low input, stress tolerant crop with seed oil composition suitable for biofuel and bioproduct production. The chemical compositions and ultrastructural features of surface waxes from C sativa aerial cuticles, seeds, and roots were analyzed using gas chromatography and microscopy. Alkanes, primary fatty alcohols, and free fatty acids were common components of all analyzed organs. A particular feature of leaf waxes was the presence of alkyl esters of long-chain fatty acids and very long-chain fatty alcohols, ranging from C-38 to C-50 and dominated by C-42, C-44 and C-46 homologues. Stem waxes were mainly composed of non-sterol pentacyclic triterpenes. Flowers accumulated significant amounts of methyl-branched iso-alkanes (C-29 and C-31 total carbon number) in addition to straight-chain alkanes. Seed waxes were mostly primary fatty alcohols of up to 32 carbons in length and unbranched C-29 and C-31 alkanes. The total amount of identified wax components extracted by rapid chloroform dipping of roots was 280 mu g g(-1) (fresh weight), and included alkyl hydroxycinnamates, predominantly alkyl coumarates and alkyl caffeates. This study provides qualitative and quantitative information on the waxes of C sativa root, shoot, and seed boundary tissues, allowing the relative activities of wax biosynthetic pathways in each respective plant organ to be assessed. This detailed description of the protective surface waxes of C sativa may provide insights into its drought-tolerant and pathogen-resistant properties, and also identifies C sativa as a potential source of renewable high-value natural products. (C) 2014 Elsevier Ltd.
DOI:10.1104/pp.109.150540URLPMID:20605912 [本文引用: 1]

Transcription factors of the homeodomain-leucine zipper IV (HD-ZIP IV) family play crucial roles in epidermis-related processes. To gain further insight into the molecular function of OUTER CELL LAYER1 (OCL1), 14 target genes up- or down-regulated in transgenic maize (Zea mays) plants overexpressing OCL1 were identified. The 14 genes all showed partial coexpression with OCL1 in maize organs, and several of them shared preferential expression in the epidermis with OCL1. They encoded proteins involved in lipid metabolism, defense, envelope-related functions, or cuticle biosynthesis and include ZmWBC11a (for white brown complex 11a), an ortholog of AtWBC11 involved in the transport of wax and cutin molecules. In support of the annotations, OCL1-overexpressing plants showed quantitative and qualitative changes of cuticular wax compounds in comparison with wild-type plants. An increase in C24 to C28 alcohols was correlated with the transcriptional up-regulation of ZmFAR1, coding for a fatty acyl-coenzyme A reductase. Transcriptional activation of ZmWBC11a by OCL1 was likely direct, since transactivation in transiently transformed maize kernels was abolished by a deletion of the activation domain in OCL1 or mutations in the L1 box, a cis-element bound by HD-ZIP IV transcription factors. Our data demonstrate that, in addition to AP2/EREBP and MYB-type transcription factors, members of the HD-ZIP IV family contribute to the transcriptional regulation of genes involved in cuticle biosynthesis.
DOI:10.1016/j.funbio.2012.05.006URLPMID:22862917 [本文引用: 1]

Conidial germination and differentiation - the so-called prepenetration processes - of the barley powdery mildew fungus (Blumeria graminis f. sp. hordei) are essential prerequisites for facilitating penetration of the host cuticle. Although the cell cycle is known to be pivotal to cellular differentiation in several phytopathogenic fungi there is as yet no information available concerning the relationship between cell cycle and infection structure development in the obligate biotroph B. graminis. The timing of specific developmental events with respect to nuclear division and morphogenesis was followed on artificial and host leaf surfaces by 4',6-diamidino-2-phenylindole (DAPI) staining in combination with a pharmacological approach applying specific cell cycle inhibitors. It was found that the uninucleate conidia germinated and then underwent a single round of mitosis 5-6?h after inoculation. During primary germ tube formation the nucleus frequently migrated close to the site of primary germ tube emergence. This nuclear repositioning was distinctly promoted by very-long-chain aldehydes that are common host cuticular wax constituents known to induce conidial differentiation. The subsequent morphogenesis of the appressorial germ tube preceded mitosis that was spatially uncoupled from subsequent cytokinesis. Blocking of S-phase with hydroxyurea did not inhibit formation of the appressorial germ tube but prevented cytokinesis and appressorium maturation. Benomyl treatment that arrests the cell cycle in mitosis inhibited nuclear separation, cytokinesis, and formation of mature appressoria. Thus, we conclude that a completed mitosis is not a prerequisite for the formation and swelling of the appressorial germ tube, which normally provides the destination for one of the daughter nuclei, while appressorium maturation depends on mitosis.
In: Riederer C. Muller, eds. Biology of the Plant Cuticle.
[本文引用: 1]
DOI:10.1104/pp.112.201640URL [本文引用: 1]

Primary aerial surfaces of land plants are coated by a lipidic cuticle, which forms a barrier against transpirational water loss and protects the plant from diverse stresses. Four enzymes of a fatty acid elongase complex are required for the synthesis of very-long-chain fatty acid (VLCFA) precursors of cuticular waxes. Fatty acid elongase substrate specificity is determined by a condensing enzyme that catalyzes the first reaction carried out by the complex. In Arabidopsis (Arabidopsis thaliana), characterized condensing enzymes involved in wax synthesis can only elongate VLCFAs up to 28 carbons (C28) in length, despite the predominance of C29 to C31 monomers in Arabidopsis stem wax. This suggests additional proteins are required for elongation beyond C28. The wax-deficient mutant eceriferum2 (cer2) lacks waxes longer than C28, implying that CER2, a putative BAHD acyltransferase, is required for C28 elongation. Here, we characterize the cer2 mutant and demonstrate that green fluorescent protein-tagged CER2 localizes to the endoplasmic reticulum, the site of VLCFA biosynthesis. We use site-directed mutagenesis to show that the classification of CER2 as a BAHD acyltransferase based on sequence homology does not fit with CER2 catalytic activity. Finally, we provide evidence for the function of CER2 in C28 elongation by an assay in yeast (Saccharomyces cerevisiae).
