摘要/Abstract
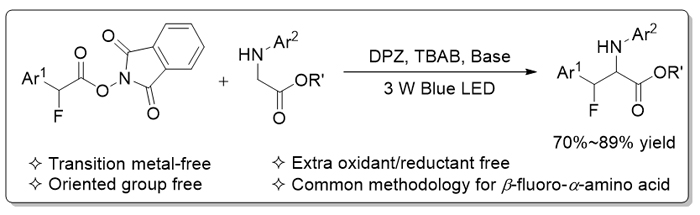
通过可见光驱动光氧化还原催化, 发展了一种新颖、便利的β-氟代-α-氨基酸衍生物的合成方法. 以非金属的二氰基吡嗪衍生物(DPZ)为光催化剂, 以易于制备的N-芳基甘氨酸酯和芳基乙酸氧化还原酯为原料, 通过单电子氧化还原分别生成酯基取代α-氨烷基自由基及α-氟代苄基自由基. 经过高反应活性自由基的交叉偶联, 高产率地得到目标产物. 该方法由于氧化还原中性反应途径而无需额外的氧化剂或还原剂, 且属于绿色、可持续的有机催化合成策略.
关键词: 光氧化还原催化, 二氰基吡嗪衍生物(DPZ), 自由基偶联, N-芳基甘氨酸酯, 芳基乙酸氧化还原酯, β-氟代-α-氨基酸
Photoredox catalysis is a practical and efficient synthetic technique that enables previously challenging or even impossible chemical transformations proceeding effectively. As one of the most prominent contribution to green chemistry, it provides a sustainable platform to the generation of high reactive radical species under mild reaction conditions. On the other hand, β-fluoro α-amino acids are significant structural units present in enzyme inhibitors, drugs and probes. Catalytic β-C(sp3)―H fluorination of α-amino acid represents a direct synthetic approach, but the harsh reaction conditions and the limited substrate scopes lead to high difficulty for these methodologies to being generalized to industrial application. Here, we report a novel and modular protocol that is via photoredox catalytic radical coupling. By using a dicyanopyrazine-derived chromophore (DPZ) as the photoredox catalyst, two readily accessible starting substrates, that are N-aryl glycine esters and α-fluoro-aryl acetic acid-derived redox-active esters (RAEs), can undergo single-electron oxidation and reduction, respectively. The resulting ester-substituted α-amino radicals and α-fluoro benzylic radicals then experience cross coupling, a highly reactive process in radical chemistry. As a result, a series of β-fluoro-α-amino acid derivatives were obtained in high yields. In this transition metal-free catalytic system, no extra oxidant or reductant is required, representing a redox neutral platform. General procedure for the synthesis of β-fluoro-α-amino acid derivatives is: to a flame dried Schlenk tube was sequentially added N-aryl substituted glycine esters 1 (0.4 mmol), RAEs 2 (0.2 mmol), DPZ (0.004 mmol, 1.42 mg), tetra-n-butyl- ammonium bromide (0.04 mmol, 12.9 mg), sodium dihydrogen phosphate (0.40 mmol, 48 mg) and cyclopentyl methyl ester (CPME) (4 mL). Then degassed three times by freeze-pump-thaw method. The reaction mixture was stirred under an argon atmosphere at 25 ℃ irradiated by a 3 W blue LED for 48 h. After completion of the reaction, the reaction mixture was directly loaded onto a short basified silica gel column, followed by gradient elution with petroleum ether/ethyl acetate (V/V, 8/1). Removing the solvent in vacuo, afforded products.
Key words: photoredox catalysis, dicyanopyrazine-derived chromophore (DPZ), radical coupling, N-aryl glycine esters, redox-active esters (RAEs), β-fluoro-α-amino acid derivatives
PDF全文下载地址:
点我下载PDF
