摘要/Abstract
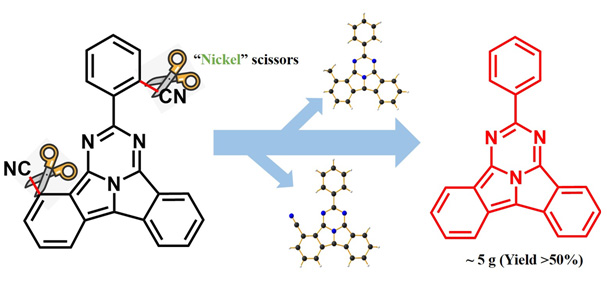
酞荭具有独特的稠环结构、光谱性质及多样的配位方式, 是一类颇具潜力的新兴材料. 在酞荭研究起步阶段, 无取代酞荭 Pr-2是研究反应与探索应用最重要的化合物. 然而目前制备 Pr-2的方法仅能基于 Pr-1的脱氰基反应. 该方法成本高昂并难以放大, 限制了酞荭类材料的后续研究与发展. 本工作利用Ni(II)催化剂替代昂贵的Rh(I), 高效制备 Pr-2, 且该方法可放大至十克级别, 产率超过50%. 同时, 在调整反应条件的过程中, 新生成氰基( Pr-1.5)与甲基( Pr-1.6)取代的酞荭化合物, 都展现出良好的平面性与π-π相互作用, 而不同取代基对酞荭的荧光性质有着重要的影响. 本论文所报道的工作, 尤其是便捷的合成方法对于酞荭的推广和应用具有极为重要而深远的意义.
关键词: 酞荭, 稠环, 脱氰基, 染料, 杂环
Phthalorubines ( Prs), a new series of fused-ring dyes, with novel planar structure, interesting synthetic mechanism, characteristic spectra properties and multiple coordinated patterns, have potential applications in different fields. During the start-up stage for phthalorubine research, a molecule without any substituents, such as Pr-2,is the most important compound for both synthetic studies and application exploration. Reported method to obtain Pr-2 is via decyanation of Pr-1, the first phthalorubine compound easily synthesized from phthalonitrile. However, the process of decyanation requires expensive Rh(I) complex as catalyst, which also make the reaction difficult to amplify. These disadvantages greatly limit follow-up studies of phthalorubines. A facile method to synthesize Pr-2could effectively accelerate the development and subsequent studies of phthalorubines. Herein, we reported a facile method to synthesize Pr-2via Ni(II) catalyzed process with high yield of 58%, while this method could be amplified to more than 10 grams. Moreover, during the process of tuning reaction conditions, two new kinds of phthalorubines were detected, with cyano group ( Pr-1.5) and methyl group ( Pr-1.6), respectively. According to the results of different conditions, Pr-1.5 is the key intermediate product from Pr-1 to Pr-2. Methyl group in molecule Pr-1.6is derived from trimethylaluminum, which also works as Lewis acid in the process of decyanation. The structures of Pr-1.5 and Pr-1.6 were further confirmed by X-ray single crystal diffraction, exhibiting planar structures with strong π-π interaction. In addition, different substituents greatly influence the packing patterns of phthalorubines, while molecules Pr-1.5 pack as “head-tail” pattern and Pr-1.6as “head-head” pattern. We also studied the photophysical properties of these two new phthalorubines. Pr-1.5and Pr-1.6 exhibit similar absorption spectra to reported compounds but totally different emission efficiencies. It suggests electron donating groups is beneficial to high quantum yield. The facile synthetic method and properties reported in this work would greatly help the development of phthalorubines.
Key words: phthalorubines, fused rings, decyanation, dyes, heterocycles
PDF全文下载地址:
点我下载PDF
