摘要/Abstract
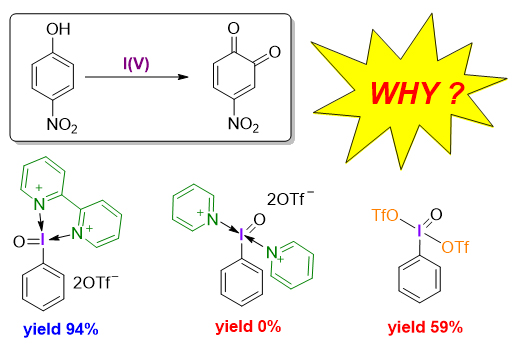
采用密度泛函理论方法研究了不同配体螯合五价碘试剂介导的苯酚氧化去芳构化反应机理以及配体对试剂活性的影响机制. 揭示了双齿氮配体具有双重作用: 一方面通过配位螯合作用增强五价碘试剂2-碘酰苯甲酸(2-iodoxybenzoic acid, IBX)的反应活性, 另一方面作为碱捕获反应中生成的强酸, 避免强酸与邻醌产物发生进一步反应, 从而提高反应的总收率. 研究结果将增加和深化对配体调控高价碘试剂反应性的认识和理解, 为理性设计与开发新配体和反应提供理论依据.
关键词: 双齿氮配体, 五价碘试剂, 氧化去芳构化, 邻醌, 密度泛函理论计算
Hypervalent iodine reagents, as a type of environmentally friendly and economical oxidants, have received extensive attention from synthetic chemistry in recent years. There are two kinds of common hypervalent iodine reagents: iodine(III) and iodine(V) reagents. Among various iodine(V) based reagents, 2-iodoxybenzoic acid (IBX) is one of the most commonly used oxidants in organic chemistry and has been widely used in selective oxidation of phenols, alcohols, thioethers and many other compounds. Although the IBX can efficiently oxidize electron-rich phenols to obtain o-quinones, which have important applications in catalysis and materials science, the synthesis of electron-deficient o-quinones is still challenging and remains an unsolved problem. Recently, a novel bidentate nitrogen-ligated iodine(V) reagent has been found to efficiently and selectively promote the oxidative dearomatization of electron-deficient phenols to o-quinones. The bidentate-nitrogen ligand has been demonstrated to play a crucial role in the successful oxidative dearomatization of electron-deficient phenols. To understand the origin of the effect of bidentate-nitrogen ligand on oxidative dearomatization, we herein conducted a detailed mechanistic study on the oxidative dearomatization of phenols promoted by iodine(V) reagents with different ligands by using density functional theory (DFT). Geometry optimizations and frequency calculations were carried out at the M06-2X/[6-31G(d,p)+LANL2DZ(I)] level of theory. To obtain more accurate electronic energies, single-point energy were calculated at the M06-2X/[6-311+G(2d,p)+def2-TZVPP(I)] level of theory. The solvation model based on density (SMD) was used to account for the solvation effect of chloroform, the solvents used in the experiment. Calculations revealed that the bidentate-nitrogen ligand not only enhances the reactivity of iodine(V) reagent but also acts as a base to neutralize the strong acid generated in the reaction to prevent product degradation. The insights into the effect of the bidentate-nitrogen ligand on the reactivity of hypervalent iodine reagent obtained from this study would facilitate the future design of novel ligand- regulated hypervalent iodine reagents for new reactions.
Key words: bidentate-nitrogen ligand, iodine(V) reagent, oxidative dearomatization, o-quinone, DFT calculation
PDF全文下载地址:
点我下载PDF
