摘要/Abstract
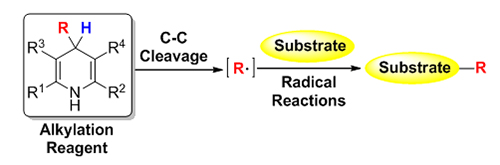
汉斯酯(Hantzsch Esters)在1881年被合成以来一直被用作还原剂参与加氢还原反应, 近年来科学家发现4-取代的汉斯酯可以发生碳碳键断裂而发生烷基迁移反应. 随后大量以4-取代的汉斯酯类化合物作为烷基化试剂的反应被报道出来, 科学家发现这类烷基化反应的历程主要是烷基自由基的迁移历程. 随着近年自由基化学的快速发展, 为这类新型烷基化试剂的应用发展提供了有力基础. 本文按照该烷基化试剂参与的反应类型分类, 进行简单介绍.
关键词: 4-取代汉斯酯, 1,4-二氢吡啶, 自由基, 烷基化
Hantzsch Esters were first synthesized by Arthur Rudolf Hantzsch in 1881, and widely used in pharmaceutical chemistry. The application of Hantsch Esters in organic synthesis in the early time was mainly focused on the dehydrogenation of 1,4-dihydrogen pyridines (DHPs) in the synthesis of functional pyridines. In 1955, Mauzerall and Westheimer found that Malachite Green could be reduced by Hantzsch Esters to generate the hydrogenated product. Then these DHPs were extensively used as a reductant for decades due to their electron and hydrogen donating properties. In recent years, scientist found that C—C bond cleavage at 4-position of 4-substituted Hantzsch Esters would lead alkyl transfer, and the alkylation process was a radical process. With the rapid development of free radical chemistry, various alkylation reactions using 4-substituted Hantzsch Esters as alkylation reagent have been developed, such as addition reactions of imines and alkenes; cross-coupling reactions with aryl halides; substitution reactions with functional aromatics; Tsuji-Trost reaction; radical insertion with sulfur dioxide; and asymmetric alkylation etc. The advantages in alkylation transfer by using 4-substituted Hantzsch Esters as alkyl source in the past five years were witnessed dramatically: (1) Highly toxic alkyl metal reagents could be avoided in the alkylation reactions; (2) Compared with the moisture sensitivity of alkyl metal reagents Hantzsch Esters are easily handling; (3) 1,4-Dihydrogen pyridines (DHPs) are biologically-inspired model molecular of reduced nicotinamide adenine dinucleotide (NADH), which would expand the application in biosynthesis. A brief summary in this field is presented in this review, and the advances are classified according to different reaction types. Although these creativity works were developed, there are still some challenges: (1) Could aromatic groups at 4-position of 4-substituted Hantzsch Esters serve as arylation reagents? (2) How to recover the rest pyridine part of Hantzsch Esters after alkylation; (3) New type reactions need to be developed for the asymmetric synthesis.
Key words: 4-substituted Hantzsch Ester, 1,4-dihydropyridine, free radical, alkylation
PDF全文下载地址:
点我下载PDF
